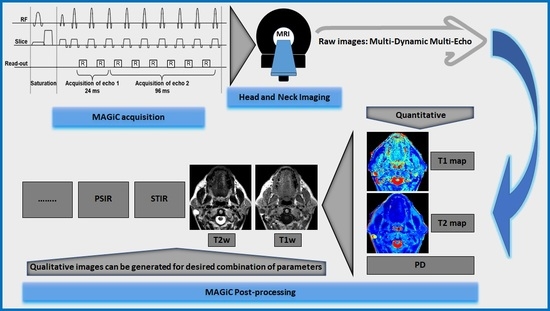
Qualitative and Quantitative Performance of Magnetic Resonance Image Compilation (MAGiC) Method: An Exploratory Analysis for Head and Neck Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. MRI Data Acquisition
2.3. Standard Head and Neck Imaging
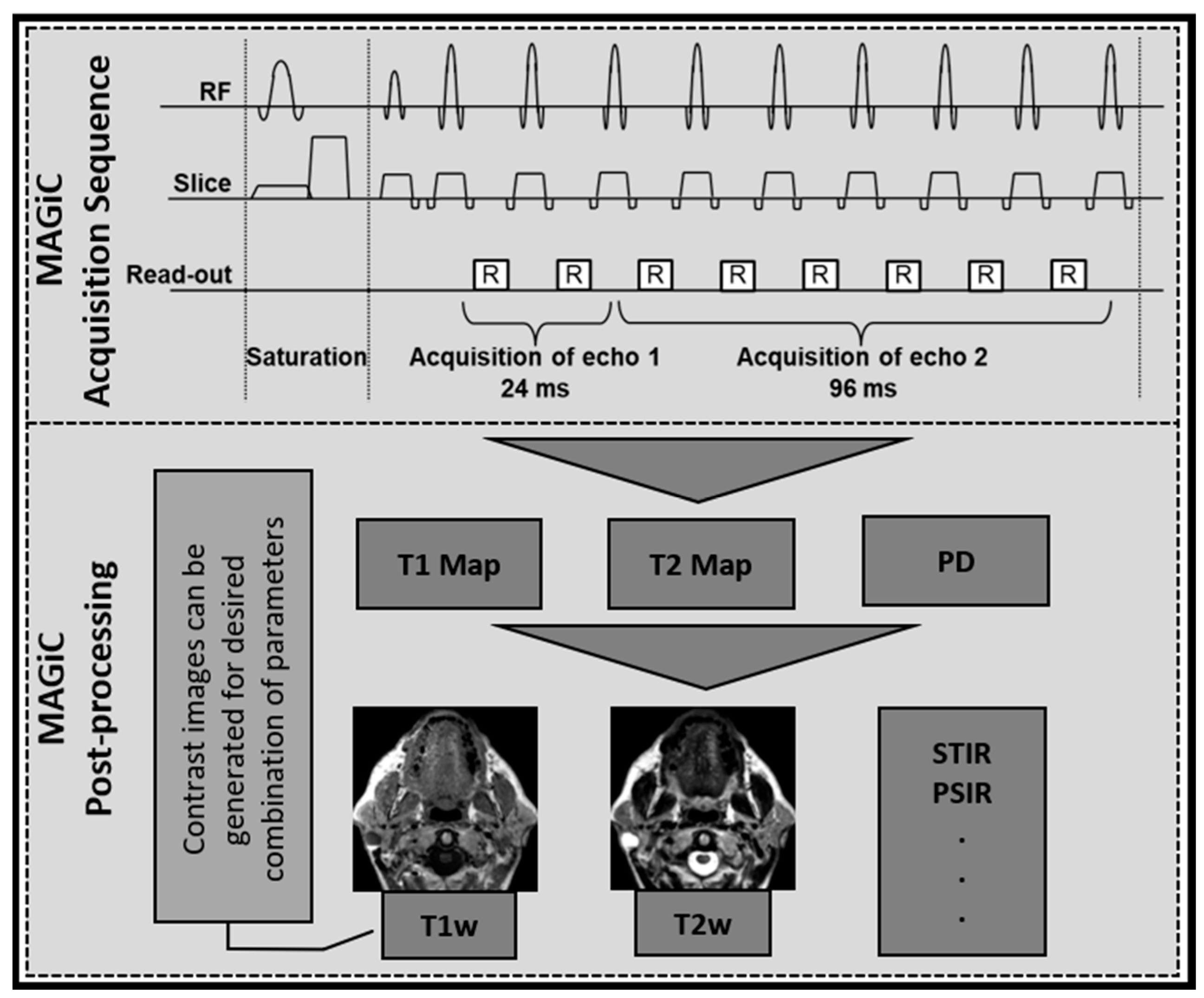
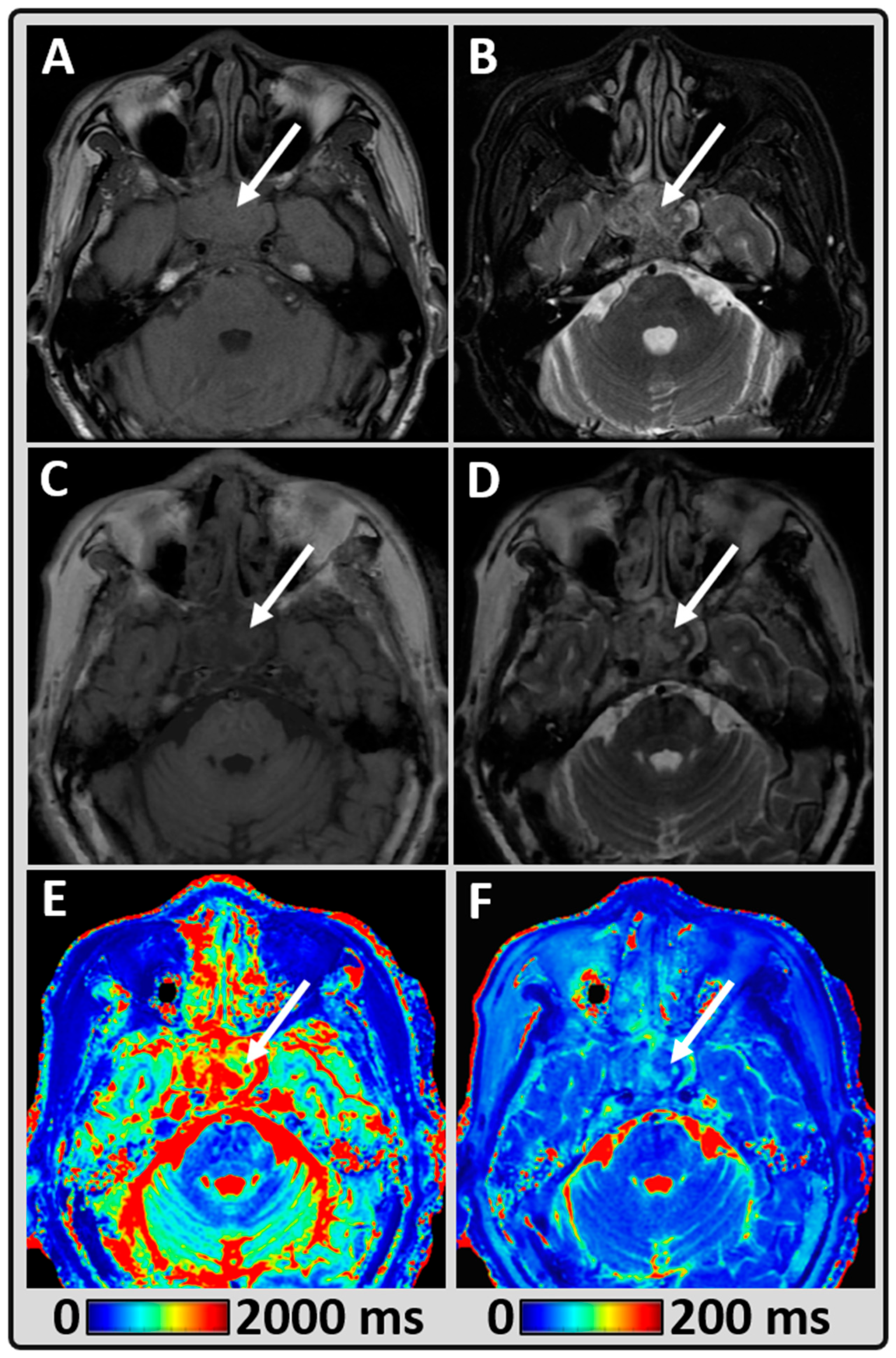
2.4. Synthetic MR Imaging (MAGiC)
2.5. MRI Data Processing
2.6. Regions of Interest Delineation
2.7. Radiological Assessment
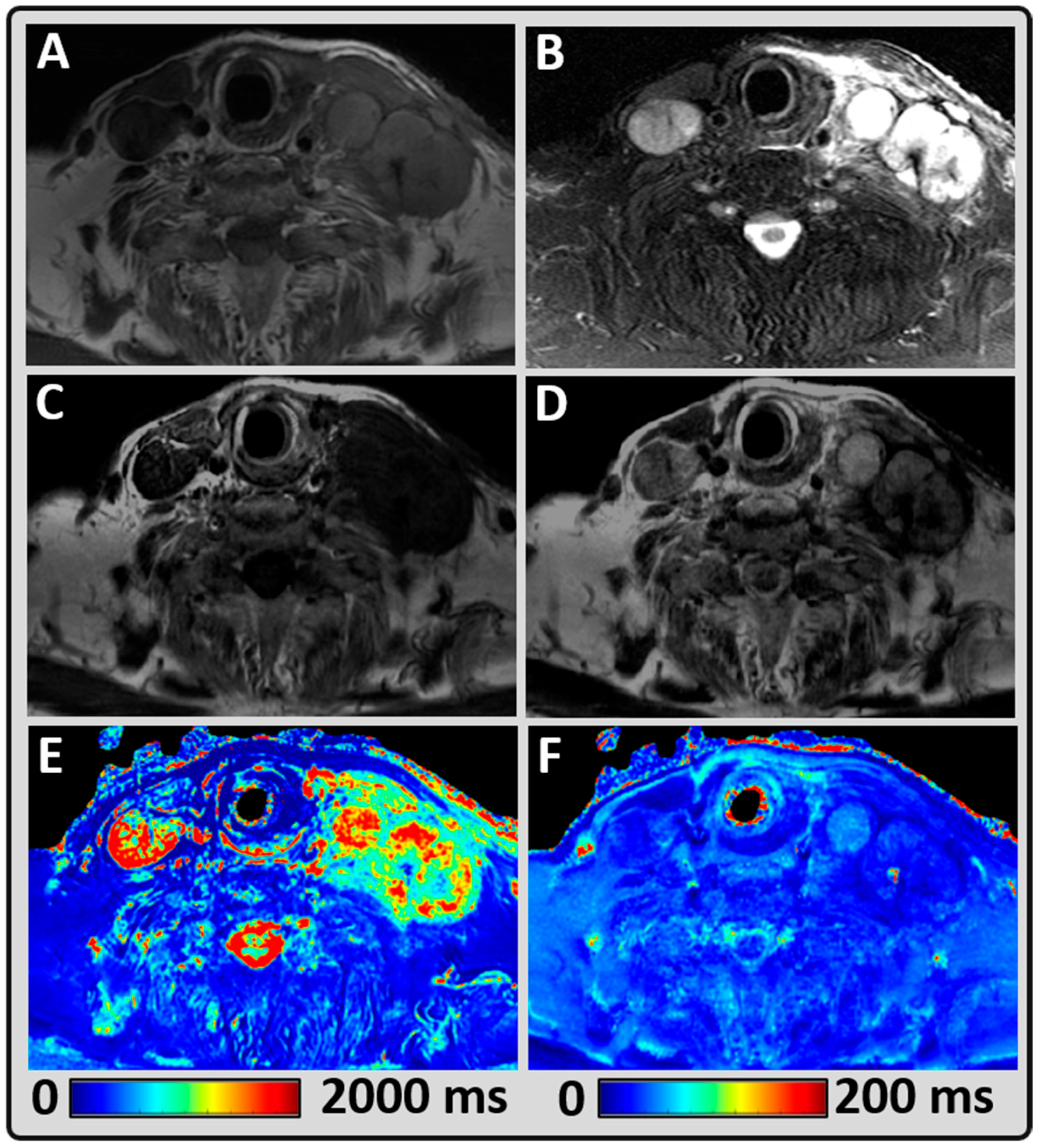
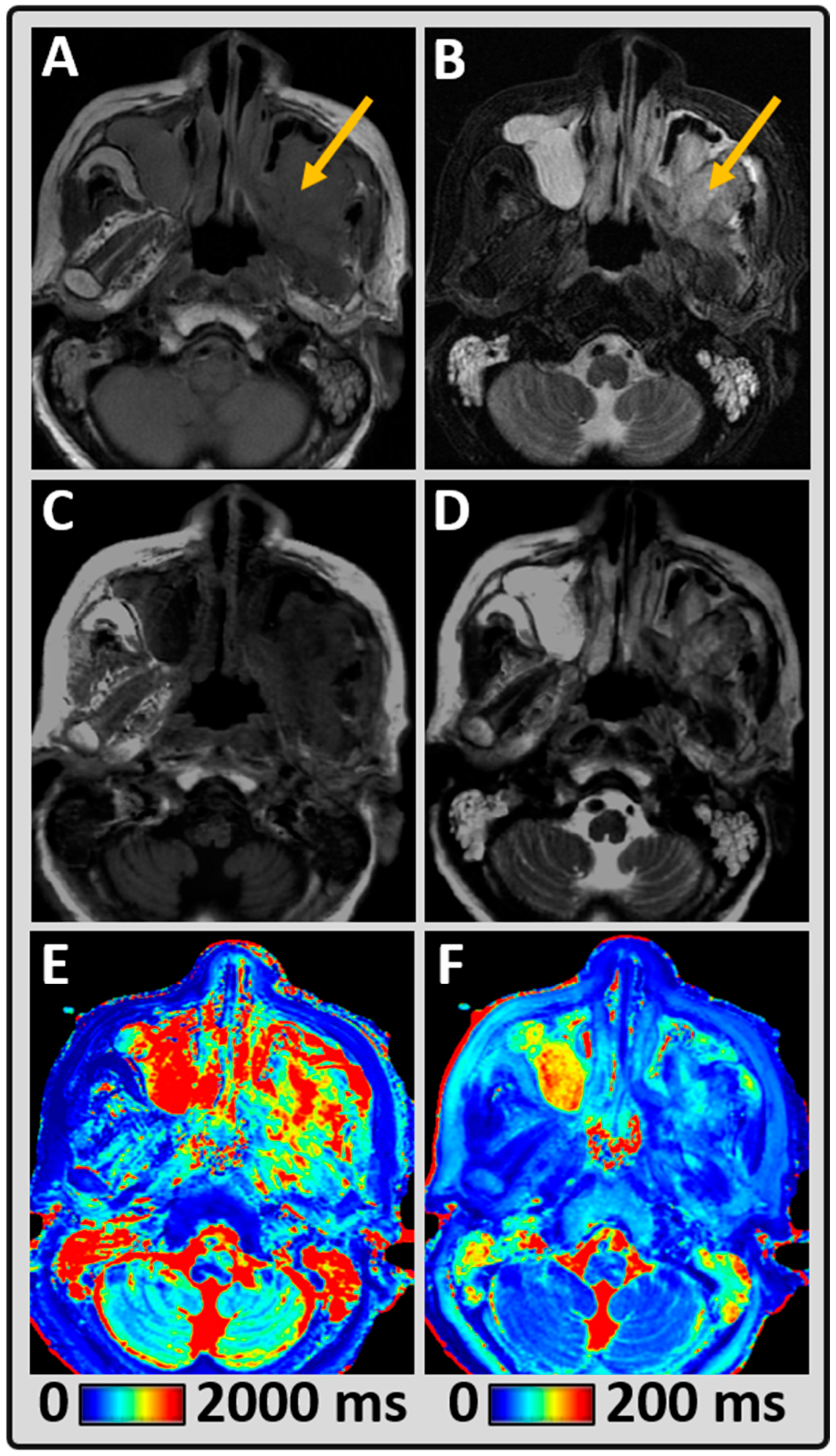
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, M.R.; Schomer, D.F.; Chappell, P.; Enzmann, D.R. MR imaging of head and neck tumors: Comparison of T1-weighted contrast-enhanced fat-suppressed images with conventional T2-weighted and fast spin-echo T2-weighted images. AJR. Am. J. Roentgenol. 1994, 163, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Touska, P.; Connor, S.E.J. Recent advances in MRI of the head and neck, skull base and cranial nerves: New and evolving sequences, analyses and clinical applications. Br. J. Radiol. 2019, 92, 20190513. [Google Scholar] [CrossRef] [PubMed]
- Avey, G. Technical Improvements in Head and Neck MR Imaging: At the Cutting Edge. Neuroimaging Clin. 2020, 30, 295–309. [Google Scholar] [CrossRef]
- Dubin, M.D.; Teresi, L.M.; Bradley, W.G., Jr.; Jordan, J.E.; Pema, P.J.; Goergen, S.K.; Tam, J.K. Conspicuity of tumors of the head and neck on fat-suppressed MR images: T2-weighted fast-spin-echo versus contrast-enhanced T1-weighted conventional spin-echo sequences. AJR. Am. J. Roentgenol. 1995, 164, 1213–1221. [Google Scholar] [CrossRef][Green Version]
- Patel, S.; Bhatt, A.A. Imaging of the sublingual and submandibular spaces. Insights Imaging 2018, 9, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Paudyal, R.; Hatzoglou, V.; Shukla-Dave, A. Quantitative Magnetic Resonance Imaging Biomarkers for Head and Neck and Thyroid Cancers. In Quantitative Imaging in Medicine; AIP Publishing: New York, NY, USA, 2021; pp. 10-11–10-26. [Google Scholar]
- Cheng, H.L.; Stikov, N.; Ghugre, N.R.; Wright, G.A. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012, 36, 805–824. [Google Scholar] [CrossRef]
- Wansapura, J.P.; Holland, S.K.; Dunn, R.S.; Ball, W.S., Jr. NMR relaxation times in the human brain at 3.0 tesla. J. Magn. Reson. Imaging 1999, 9, 531–538. [Google Scholar] [CrossRef]
- Deoni, S.C.L. Quantitative relaxometry of the brain. Top. Magn. Reson. Imaging TMRI 2010, 21, 101–113. [Google Scholar] [CrossRef]
- Granziera, C.; Wuerfel, J.; Barkhof, F.; Calabrese, M.; De Stefano, N.; Enzinger, C.; Evangelou, N.; Filippi, M.; Geurts, J.J.G.; Reich, D.S.; et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021, 144, 1296–1311. [Google Scholar] [CrossRef]
- McSheehy, P.M.; Weidensteiner, C.; Cannet, C.; Ferretti, S.; Laurent, D.; Ruetz, S.; Stumm, M.; Allegrini, P.R. Quantified tumor T1 is a generic early-response imaging biomarker for chemotherapy reflecting cell viability. Clin. Cancer Res. 2010, 16, 212–225. [Google Scholar] [CrossRef]
- Fu, Y.; Tanaka, K.; Nishimura, S. Evaluation of brain edema using magnetic resonance proton relaxation times. Adv. Neurol. 1990, 52, 165–176. [Google Scholar] [PubMed]
- Kim, E.; Lee, E.; Plummer, C.; Gil, S.; Popel, A.S.; Pathak, A.P. Vasculature-specific MRI reveals differential anti-angiogenic effects of a biomimetic peptide in an orthotopic breast cancer model. Angiogenesis 2015, 18, 125–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, M.; Buch, K.; Fujita, A.; Christiansen, C.L.; Jara, H.; Sakai, O. MR relaxometry for the facial ageing assessment: The preliminary study of the age dependency in the MR relaxometry parameters within the facial soft tissue. Dentomaxillofacial Radiol. 2015, 44, 20150047. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Deoni, S.C.; Peters, T.M.; Rutt, B.K. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn. Reson. Med. 2005, 53, 237–241. [Google Scholar] [CrossRef]
- Cheng, H.L.M.; Wright, G.A. Rapid high-resolution T1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2006, 55, 566–574. [Google Scholar] [CrossRef]
- Deoni, S.C.; Peters, T.M.; Rutt, B.K. Determination of optimal angles for variable nutation proton magnetic spin-lattice, T1, and spin-spin, T2, relaxation times measurement. Magn. Reson. Med. 2004, 51, 194–199. [Google Scholar] [CrossRef]
- Riederer, S.J.; Suddarth, S.A.; Bobman, S.A.; Lee, J.N.; Wang, H.Z.; MacFall, J.R. Automated MR image synthesis: Feasibility studies. Radiology 1984, 153, 203–206. [Google Scholar] [CrossRef]
- Warntjes, J.; Dahlqvist, O.; Lundberg, P. Novel method for rapid, simultaneous T1, T* 2, and proton density quantification. Magn. Reson. Med. 2007, 57, 528–537. [Google Scholar] [CrossRef]
- Warntjes, J.B.; Leinhard, O.D.; West, J.; Lundberg, P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn. Reson. Med. 2008, 60, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Yang, D.; Lee, J.; Choi, S.H.; Kim, H.; Kang, K.M. Synthetic MRI: Technologies and Applications in Neuroradiology. J. Magn. Reson. Imaging 2020, 55, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, L.N.; Tsiouris, A.J.; Johnson, A.N.; Naidich, T.P.; DeLano, M.C.; Melhem, E.R.; Quarterman, P.; Parameswaran, S.X.; Shankaranarayanan, A.; Goyen, M.; et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. Am. J. Neuroradiol. 2017, 38, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Di Giuliano, F.; Minosse, S.; Picchi, E.; Marfia, G.A.; Da Ros, V.; Muto, M.; Muto, M.; Pistolese, C.A.; Laghi, A.; Garaci, F.; et al. Comparison between synthetic and conventional magnetic resonance imaging in patients with multiple sclerosis and controls. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 549–557. [Google Scholar] [CrossRef]
- Gouel, P.; Hapdey, S.; Dumouchel, A.; Gardin, I.; Torfeh, E.; Hinault, P.; Vera, P.; Thureau, S.; Gensanne, D. Synthetic MRI for Radiotherapy Planning for Brain and Prostate Cancers: Phantom Validation and Patient Evaluation. Front. Oncol. 2022, 12, 841761. [Google Scholar] [CrossRef]
- Cui, Y.; Han, S.; Liu, M.; Wu, P.Y.; Zhang, W.; Zhang, J.; Li, C.; Chen, M. Diagnosis and Grading of Prostate Cancer by Relaxation Maps From Synthetic MRI. J. Magn. Reson. Imaging 2020, 52, 552–564. [Google Scholar] [CrossRef]
- Moran, C.J. Editorial for “Investigation of synthetic relaxometry and diffusion measures in the differentiation of benign and malignant breast lesions as compared to BI-RADS”. J. Magn. Reson. Imaging 2021, 53, 1128–1129. [Google Scholar] [CrossRef]
- Jung, Y.; Gho, S.M.; Back, S.N.; Ha, T.; Kang, D.K.; Kim, T.H. The feasibility of synthetic MRI in breast cancer patients: Comparison of T(2) relaxation time with multiecho spin echo T(2) mapping method. Br. J. Radiol. 2018, 92, 20180479. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, M.; Wu, P.-y.; Yang, Y.; Zhang, H.; Zhao, X. A preliminary study of synthetic magnetic resonance imaging in rectal cancer: Imaging quality and preoperative assessment. Insights Imaging 2021, 12, 120. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Luo, X.; Lin, Y.; He, B.; Wu, B.; Qu, J.; Wu, T.; Pu-Yeh, W.; Zhang, C.; et al. Quantitative synthetic MRI for evaluation of the lumbar intervertebral disk degeneration in patients with chronic low back pain. Eur. J. Radiol. 2020, 124, 108858. [Google Scholar] [CrossRef]
- Drake-Pérez, M.; Delattre, B.M.A.; Boto, J.; Fitsiori, A.; Lovblad, K.O.; Boudabbous, S.; Vargas, M.I. Normal Values of Magnetic Relaxation Parameters of Spine Components with the Synthetic MRI Sequence. Am. J. Neuroradiol. 2018, 39, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liang, M.; Xie, L.; Yang, Y.; Zhang, H.; Zhao, X. Prediction of pathological prognostic factors of rectal cancer by relaxation maps from synthetic magnetic resonance imaging. Eur. J. Radiol. 2021, 138, 109658. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.L.; Ord, V.A.; Fullerton, G.D. Characterization of proton NMR relaxation times in normal and pathological tissues by correlation with other tissue parameters. Magn. Reson. Imaging 1984, 2, 97–106. [Google Scholar] [CrossRef]
- Komiyama, M.; Yagura, H.; Baba, M.; Yasui, T.; Hakuba, A.; Nishimura, S.; Inoue, Y. MR imaging: Possibility of tissue characterization of brain tumors using T1 and T2 values. Am. J. Neuroradiol. 1987, 8, 65–70. [Google Scholar] [PubMed]
- Maurer, G.D.; Tichy, J.; Harter, P.N.; Nöth, U.; Weise, L.; Quick-Weller, J.; Deichmann, R.; Steinbach, J.P.; Bähr, O.; Hattingen, E. Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma. Cancers 2021, 13, 4060. [Google Scholar] [CrossRef] [PubMed]
- Blystad, I.; Warntjes, J.B.; Smedby, O.; Landtblom, A.M.; Lundberg, P.; Larsson, E.M. Synthetic MRI of the brain in a clinical setting. Acta Radiol. 2012, 53, 1158–1163. [Google Scholar] [CrossRef]
- Du, S.; Gao, S.; Zhao, R.; Liu, H.; Wang, Y.; Qi, X.; Li, S.; Cao, J.; Zhang, L. Contrast-free MRI quantitative parameters for early prediction of pathological response to neoadjuvant chemotherapy in breast cancer. Eur. Radiol. 2022, 32, 5759–5772. [Google Scholar] [CrossRef]
- Konar, A.S.; Shah, A.D.; Paudyal, R.; Fung, M.; Banerjee, S.; Dave, A.; Hatzoglou, V.; Shukla-Dave, A. Quantitative Synthetic Magnetic Resonance Imaging for Brain Metastases: A Feasibility Study. Cancers 2022, 14, 2651. [Google Scholar] [CrossRef]
- Kang, K.M.; Choi, S.H.; Hwang, M.; Yoo, R.-E.; Yun, T.J.; Kim, J.-H.; Sohn, C.-H. Application of Synthetic MRI for Direct Measurement of Magnetic Resonance Relaxation Time and Tumor Volume at Multiple Time Points after Contrast Administration: Preliminary Results in Patients with Brain Metastasis. Korean J. Radiol. 2018, 19, 783–791. [Google Scholar] [CrossRef]
- Blystad, I.; Warntjes, J.B.M.; Smedby, Ö.; Lundberg, P.; Larsson, E.M.; Tisell, A. Quantitative MRI using relaxometry in malignant gliomas detects contrast enhancement in peritumoral oedema. Sci. Rep. 2020, 10, 17986. [Google Scholar] [CrossRef]
- West, H.; Leach, J.L.; Jones, B.V.; Care, M.; Radhakrishnan, R.; Merrow, A.C.; Alvarado, E.; Serai, S.D. Clinical validation of synthetic brain MRI in children: Initial experience. Neuroradiology 2017, 59, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Baek, H.J.; Moon, J.I.; Choi, B.H.; Park, S.E.; Ha, J.Y.; Jeon, K.N.; Bae, K.; Choi, D.S.; Cho, S.B.; et al. Initial clinical experience of synthetic MRI as a routine neuroimaging protocol in daily practice: A single-center study. J. Neuroradiol. 2020, 47, 151–160. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Mäkitie, A.; de Bree, R.; Franchi, A.; de Graaf, P.; Hernández-Prera, J.C.; Strojan, P.; Zidar, N.; Fležar, M.S.; Rodrigo, J.P.; et al. Qualitative and Quantitative Diagnosis in Head and Neck Cancer. Diagnostics 2021, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Total Patients | 14 |
| Demographics | |
| Median age (y) | 61 |
| Age range (y) | 29–83 |
| Male/Female | 8/6 |
| Location of tumor in HN region | |
| Major Salivary Gland | 5 |
| Sinonasal | 3 |
| Larynx | 1 |
| Cheek | 1 |
| Cutaneous | 1 |
| Thyroid | 1 |
| Skull Base | 1 |
| Perineural (pterygopalatine fossa) | 1 |
| Treatment timepoint | |
| Pre-treatment | 3 |
| Post-treatment | 11 |
| Therapy types | |
| CRT | 2 |
| RT and Surgery | 3 |
| CRT and Surgery | 2 |
| RT alone | 1 |
| Proton therapy alone | 1 |
| Surgery alone | 2 |
| Tissue | T1 (in ms) | T2 (in ms) | |||||
|---|---|---|---|---|---|---|---|
| Range [min, max] | Mean ± SD | Median | Range [min, max] | Mean ± SD | Median | ||
| Normal appearing Masseter Muscle (MM) (n = 14) | [729, 950] | 880 ± 52 | 876 | [42, 52] | 46 ± 3 | 45 | |
| Tumor | Untreated (n = 2) | [1455, 2035] | 1745 ± 410 | 1745 | [64, 150] | 107 ± 61 | 107 |
| Treated (n = 8) | [1227, 2751] | 1930 ± 422 | 1905 | [57, 92] | 77 ± 13 | 75 | |
| Diagnostic Quality of the Image (n = 14) | Synthetic (MAGiC) | Conventional | ||
|---|---|---|---|---|
| T1w | T2w | T1w | T2w | |
| Acceptable (rated 5, 4, and 3) | 9 (64%) | 13 (93%) | 14 (100%) | 13 (93%) |
| Excellent (rated 5) | 0 (0%) | 1 (7%) | 2 (14%) | 0 (0%) |
| Good (rated 4) | 1 (7%) | 6 (43%) | 8 (57%) | 5 (36%) |
| Acceptable (rated 3) | 8 (57%) | 6 (43%) | 4 (29%) | 8 (57%) |
| Unacceptable (rated 2 and 1) | 5 (36%) | 1 (7%) | 0 (0%) | 1 (7%) |
| Poor (rated 2) | 4 (29%) | 1 (7%) | 0 (0%) | 1 (7%) |
| Unacceptable (rated 1) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Legibility (n = 14) | SG n (%) | NP n (%) | BoT n (%) | SCM n (%) | Mandible n (%) | Glottis n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1w | T2w | T1w | T2w | T1w | T2w | T1w | T2w | T1w | T2w | T1w | T2w | ||
| Legible | Conventional | 10 (72) | 8 (57) | 13 (93) | 12 (86) | 5 (36) | 3 (21) | 13 (93) | 13 (93) | 11 (79) | 10 (71) | 3 (21) | 3 (21) |
| MAGiC | 3 (21) | 7 (50) | 10 (72) | 11 (79) | 2 (14) | 8 (59) | 10 (72) | 13 (93) | 6 (44) | 9 (64) | 2 (14) | 2 (14) | |
| Illegible | Conventional | 0 (0) | 2 (14) | 0 (0) | 1 (7) | 6 (44) | 8 (59) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (7) | 1 (7) |
| MAGiC | 7 (50) | 3 (21) | 3 (21) | 2 (14) | 9 (66) | 3 (21) | 3 (21) | 0 (0) | 5 (36) | 2 (14) | 2 (13) | 2 (13) | |
| N/A | Conventional | 4 (28) | 1 (7) | 3 (20) | 1 (7) | 3 (20) | 10 (72) | ||||||
| MAGiC | 4 (28) | 1 (7) | 3 (20) | 1 (7) | 3 (20) | 10 (72) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konar, A.S.; Paudyal, R.; Shah, A.D.; Fung, M.; Banerjee, S.; Dave, A.; Lee, N.; Hatzoglou, V.; Shukla-Dave, A. Qualitative and Quantitative Performance of Magnetic Resonance Image Compilation (MAGiC) Method: An Exploratory Analysis for Head and Neck Imaging. Cancers 2022, 14, 3624. https://doi.org/10.3390/cancers14153624
Konar AS, Paudyal R, Shah AD, Fung M, Banerjee S, Dave A, Lee N, Hatzoglou V, Shukla-Dave A. Qualitative and Quantitative Performance of Magnetic Resonance Image Compilation (MAGiC) Method: An Exploratory Analysis for Head and Neck Imaging. Cancers. 2022; 14(15):3624. https://doi.org/10.3390/cancers14153624
Chicago/Turabian StyleKonar, Amaresha Shridhar, Ramesh Paudyal, Akash Deelip Shah, Maggie Fung, Suchandrima Banerjee, Abhay Dave, Nancy Lee, Vaios Hatzoglou, and Amita Shukla-Dave. 2022. "Qualitative and Quantitative Performance of Magnetic Resonance Image Compilation (MAGiC) Method: An Exploratory Analysis for Head and Neck Imaging" Cancers 14, no. 15: 3624. https://doi.org/10.3390/cancers14153624
APA StyleKonar, A. S., Paudyal, R., Shah, A. D., Fung, M., Banerjee, S., Dave, A., Lee, N., Hatzoglou, V., & Shukla-Dave, A. (2022). Qualitative and Quantitative Performance of Magnetic Resonance Image Compilation (MAGiC) Method: An Exploratory Analysis for Head and Neck Imaging. Cancers, 14(15), 3624. https://doi.org/10.3390/cancers14153624