MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice Irradiation and Dosimetry
2.2. Tissue Collection and Processing
2.3. Raman Spectroscopy
2.4. miRNome Analysis by Next-Generation Sequencing (NGS) and Bioinformatics Data Analyses
2.5. Real-Time qPCR
2.6. Cell Lines
3. Results
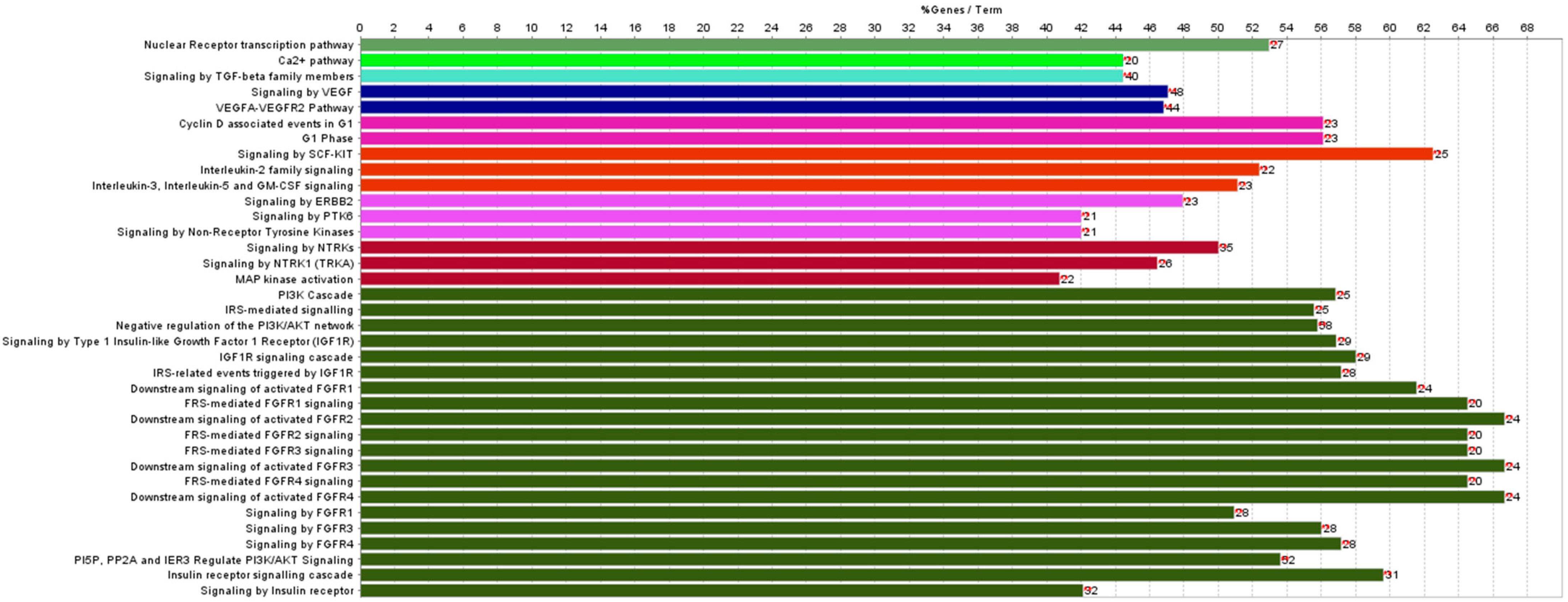
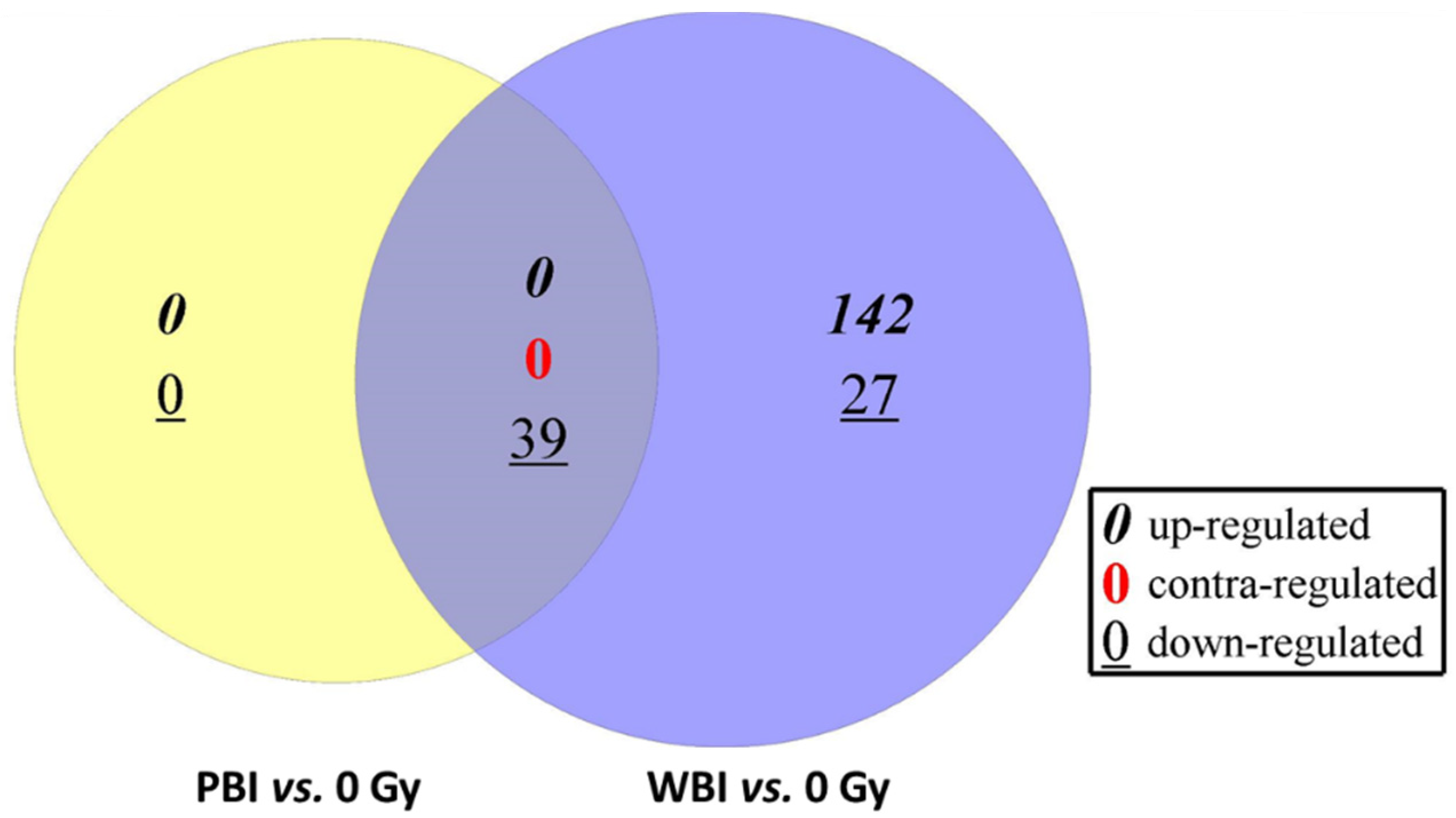
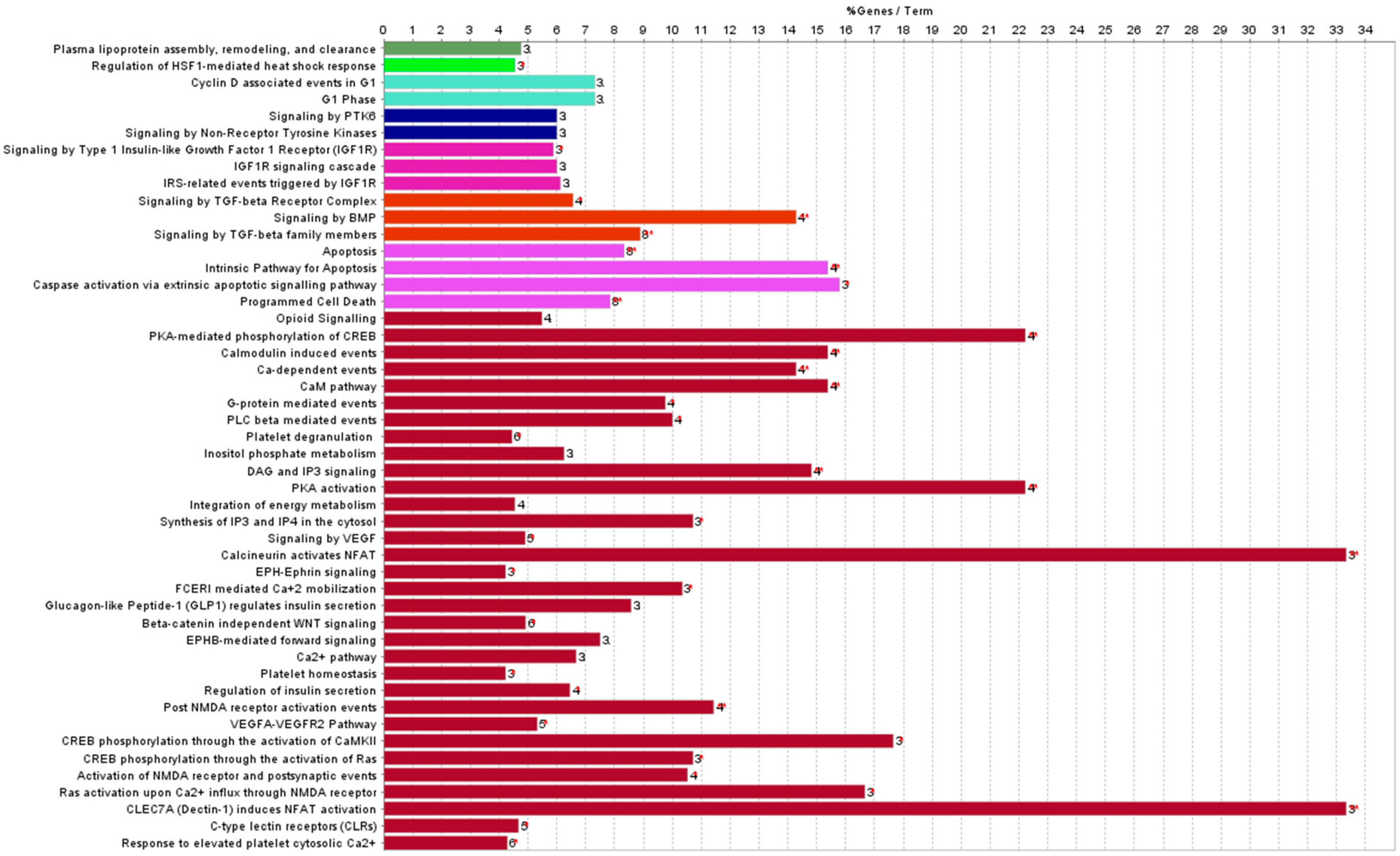
3.1. Differentially Expressed miRNAs in the Hearts of Whole- or Partial-Body-Irradiated Mice and Pathway Analysis
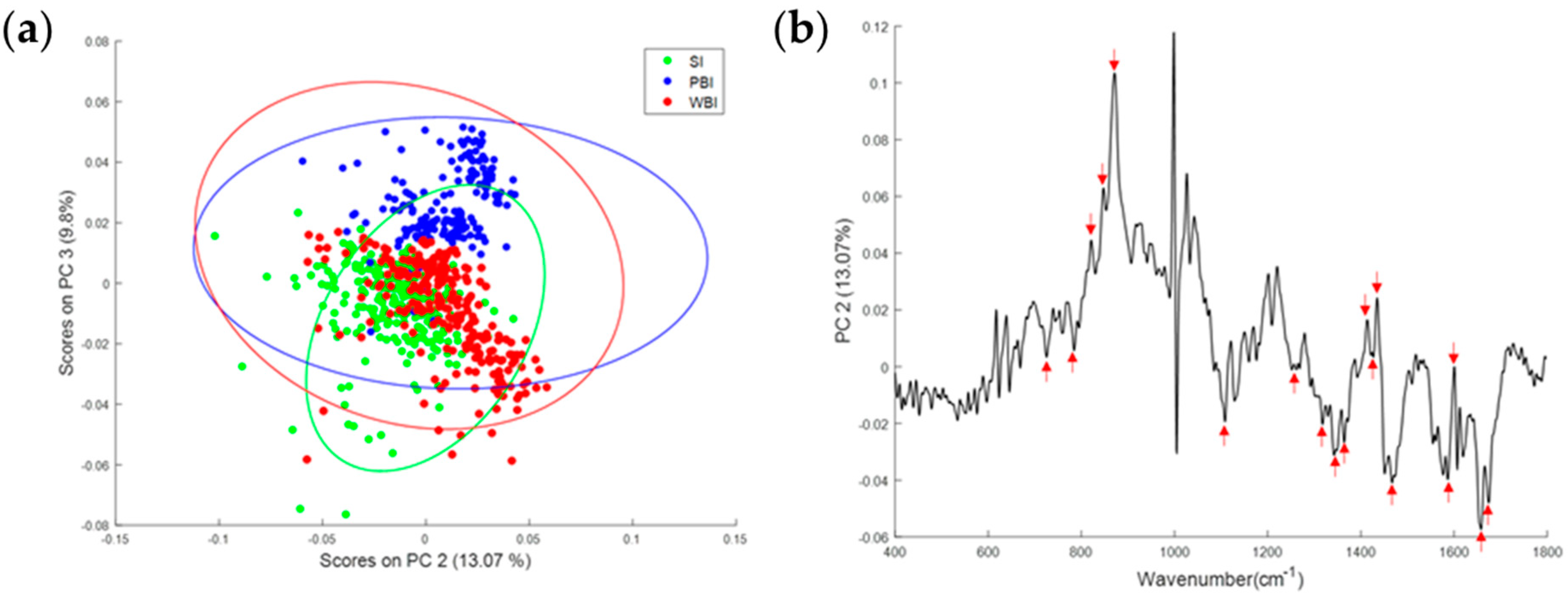
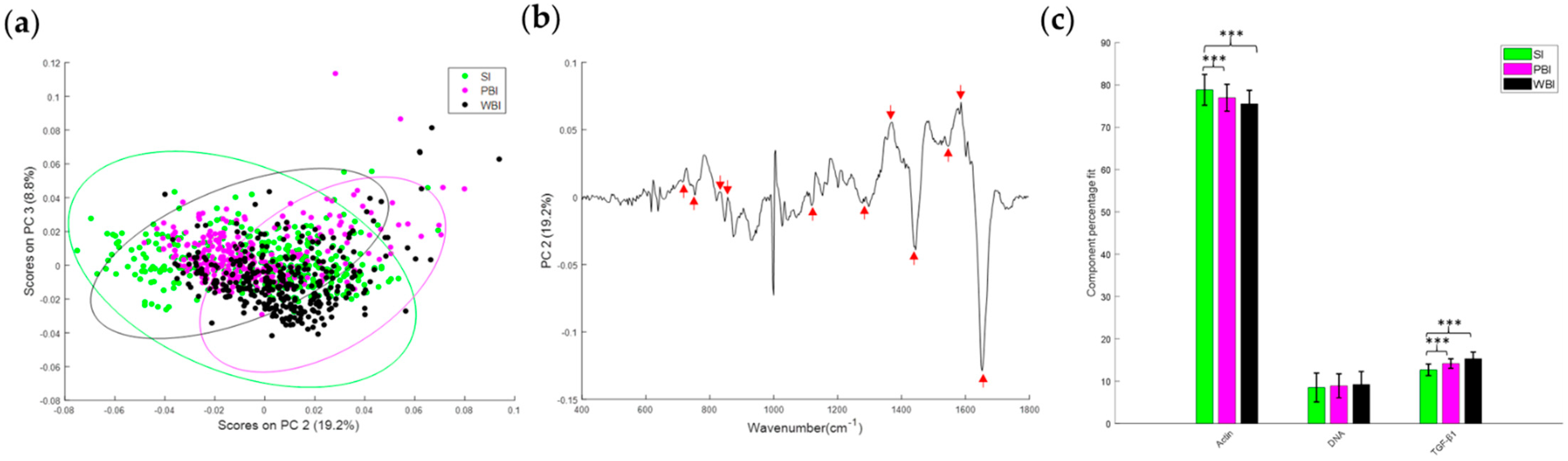
3.2. Effect of In-Field or Out-of-Field Irradiation on the Biochemical Profile of Heart Tissue
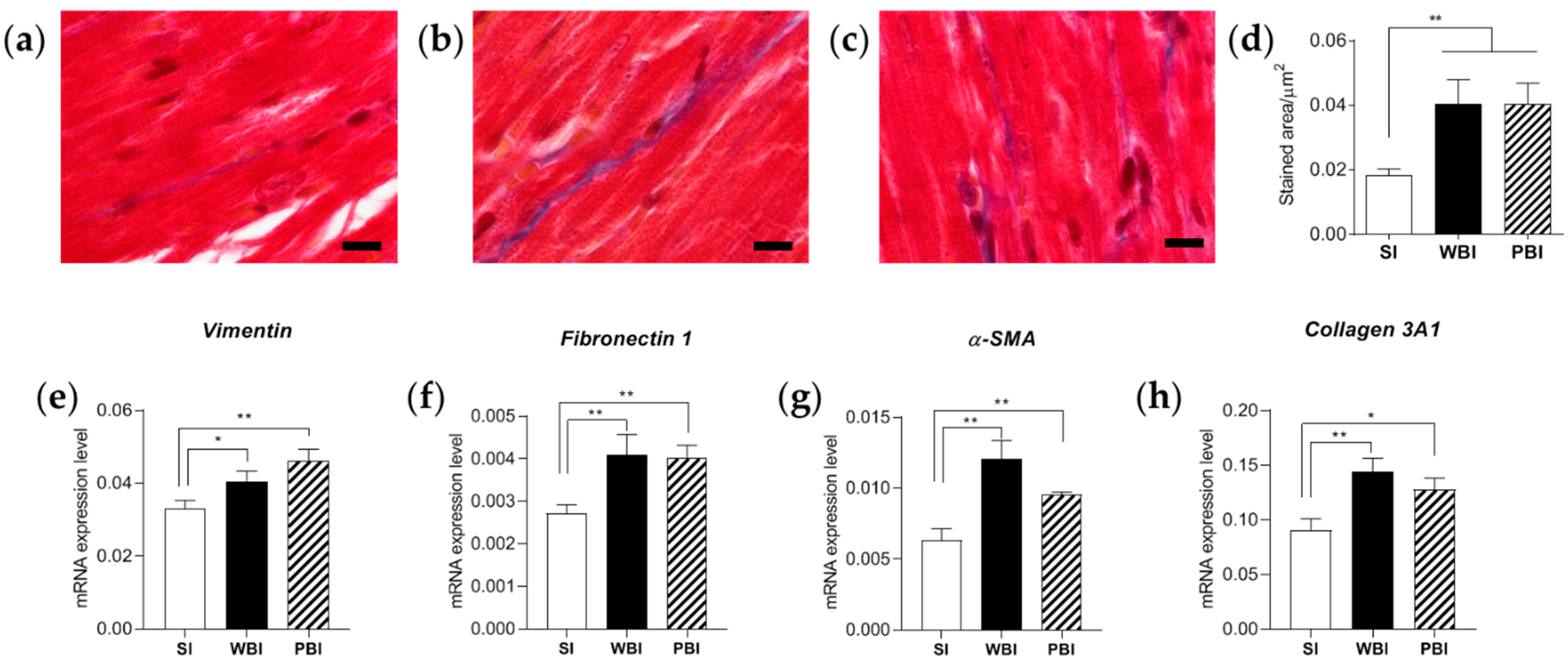
3.3. Impact of Whole- and Partial-Body Irradiation on the Cardiac Tissue 6 Months after Exposure
3.4. Mechanistic Study to Investigate the Propagation of Signals between Irradiated and Non-Irradiated Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, M.P. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat. Environ. Biophys. 2013, 52, 435–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapio, S.; Little, M.P.; Kaiser, J.C.; Impens, N.; Hamada, N.; Georgakilas, A.G.; Simar, D.; Salomaa, S. Ionizing radiation-induced circulatory and metabolic diseases. Environ. Int. 2021, 146, 106235. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Schuster, J.P.; Niu, K.; Huang, Q.; Alexander Rühle, A.; Huber, P.E. Radiotherapy-induced heart disease: A review of the literature. Precis. Clin. Med. 2019, 2, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnellan, E.; Phelan, D.; McCarthy, C.; Collier, P.; Desai, M.; Griffin, B. Radiation induced heart disease: A practical guide to diagnosis and management. Cleve Clin. J. Med. 2016, 83, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Milo, M.L.H.; Thorsen, L.B.J.; Johnsen, S.P.; Nielsen, K.M.; Valentin, J.B.; Alsner, J.; Offersen, B.V. Risk of coronary artery disease after adjuvant radiotherapy in 29,662 early breast cancer patients: A population-based Danish Breast Cancer Group study. Radiother. Oncol. 2021, 157, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Shimizu, Y.; Pierce, D.A.; Suyama, A.; Mabuchi, K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat. Res. 2003, 160, 381–407. [Google Scholar] [CrossRef]
- Howe, G.R.; Zablotska, L.B.; Fix, J.J.; Egel, J.; Buchanan, J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat. Res. 2004, 162, 517–526. [Google Scholar] [CrossRef]
- Puukila, S.; Lemon, J.A.; Lees, S.J.; Tai, T.C.; Boreham, D.R.; Khaper, N. Impact of Ionizing Radiation on the Cardiovascular System: A Review. Radiat. Res. 2017, 188, 539–546. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef]
- Silva, D.C.P.D.; Carneiro, F.D.; Almeida, K.C.; Fernandes-Santos, C. Role of miRNAs on the Pathophysiology of Cardiovascular Diseases. Arq. Bras. Cardiol. 2018, 111, 738–746. [Google Scholar] [CrossRef]
- Rogers, C.J.; Lukaszewicz, A.I.; Yamada-Hanff, J.; Micewicz, E.D.; Ratikan, J.A.; Starbird, M.A.; Miller, T.A.; Nguyen, C.; Lee, J.T.; Olafsen, T.; et al. Identification of miRNA signatures associated with radiation-induced late lung injury in mice. PLoS ONE 2020, 15, e0232411. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; Di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Rebessi, S.; Tanori, M.; Giardullo, P.; Borra, F.; Pazzaglia, S.; Naus, C.C.; Di Majo, V.; et al. Role of connexin43 and ATP in long-range bystander radiation damage and oncogenesis in vivo. Oncogene 2011, 30, 4601–4608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, M.; Giardullo, P.; Leonardi, S.; Pasquali, E.; Casciati, A.; De Stefano, I.; Tanori, M.; Pazzaglia, S.; Saran, A. Dose and spatial effects in long-distance radiation signaling in vivo: Implications for abscopal tumorigenesis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 813–819. [Google Scholar] [CrossRef]
- Chai, Y.; Calaf, G.M.; Zhou, H.; Ghandhi, S.A.; Elliston, C.D.; Wen, G.; Nohmi, T.; Amundson, S.A.; Hei, T.K. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br. J. Cancer 2013, 108, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Pazzaglia, S.; Tanno, B.; Antonelli, F.; Giardullo, P.; Babini, G.; Subedi, P.; Azimzadeh, O.; Khan, Z.N.; Oleksenko, K.; Metzger, F.; et al. Out-of-Field Hippocampus from Partial-Body Irradiated Mice Displays Changes in Multi-Omics Profile and Defects in Neurogenesis. Int. J. Mol. Sci. 2021, 22, 4290. [Google Scholar] [CrossRef]
- Medipally, D.K.R.; Cullen, D.; Untereiner, V.; Bryant, J.; Sockalingum, G.D.; Nguyen, T.N.Q.; Noone, E.; Bradshaw, S.; Finn, M.; Dunne, M.; et al. Effect of haemolysis on FTIR and Raman spectra of blood plasma. J. Biophotonics 2020, 13, e201960173. [Google Scholar] [CrossRef]
- Medipally, D.K.R.; Cullen, D.; Untereiner, V.; Sockalingum, G.D.; Maguire, A.; Nguyen, T.N.Q.; Bryant, J.; Noone, E.; Bradshaw, S.; Finn, M.; et al. Vibrational spectroscopy of liquid biopsies for prostate cancer diagnosis. Ther. Adv. Med. Oncol. 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nguyen, T.N.Q.; Cullen, D.; Meade, A.D.; Wynne, C. Discrimination of immune cell activation using Raman micro-spectroscopy in an in-vitro & ex-vivo model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119118. [Google Scholar] [CrossRef]
- Stanimirovic, O.; Boelens, H.F.; Mank, A.J.; Hoefsloot, H.C.; Smilde, A.K. Profiling of liquid crystal displays with Raman spectroscopy: Preprocessing of spectra. Appl. Spectrosc. 2005, 59, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, B.; Babini, G.; Leonardi, S.; Giardullo, P.; De Stefano, I.; Pasquali, E.; Ottolenghi, A.; Atkinson, M.J.; Saran, A.; Mancuso, M. Ex vivo miRNome analysis in Ptch1+/− cerebellum granule cells reveals a subset of miRNAs involved in radiation-induced medulloblastoma. Oncotarget 2016, 7, 68253–68269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, B.; Babini, G.; Leonardi, S.; De Stefano, I.; Merla, C.; Novelli, F.; Antonelli, F.; Casciati, A.; Tanori, M.; Pasquali, E.; et al. miRNA-Signature of Irradiated Ptch1+/− Mouse Lens is Dependent on Genetic Background. Radiat. Res. 2022, 197, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.E.; Zhang, X.; Li, Y.; Chen, B.; Liu, C.; Ai, X.; Zhang, X.; Tian, Y.; Zhang, C.; et al. Cardiomyocyte PKA Ablation Enhances Basal Contractility While Eliminates Cardiac β-Adrenergic Response Without Adverse Effects on the Heart. Circ. Res. 2019, 124, 1760–1777. [Google Scholar] [CrossRef]
- Saad, N.S.; Elnakish, M.T.; Ahmed, A.A.E.; Janssen, P.M.L. Protein Kinase A as a Promising Target for Heart Failure Drug Development. Arch. Med. Res. 2018, 49, 530–537. [Google Scholar] [CrossRef]
- Elzenaar, I.; Pinto, Y.M.; van Oort, R.J. MicroRNAs in heart failure: New targets in disease management. Clin. Pharmacol. Ther. 2013, 94, 480–489. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Banzet, S. Circulating myomiRs: A new class of biomarkers to monitor skeletal muscle in physiology and medicine. J. Cachexia Sarcopenia Muscle 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Zhang, M.; Ji, R.; Wei, J.; Xin, Y.; Jiang, X. Radiation-induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies. J. Cell Mol. Med. 2020, 24, 7717–7729. [Google Scholar] [CrossRef]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef] [PubMed]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef] [Green Version]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kodama, K.; Nishi, N.; Kasagi, F.; Suyama, A.; Soda, M.; Grant, E.J.; Sugiyama, H.; Sakata, R.; Moriwaki, H.; et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 2010, 340, b5349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, M.P.; Lipshultz, S.E. Low dose radiation and circulatory diseases: A brief narrative review. Cardiooncology 2015, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Monceau, V.; Meziani, L.; Strup-Perrot, C.; Morel, E.; Schmidt, M.; Haagen, J.; Escoubet, B.; Dörr, W.; Vozenin, M.C. Enhanced sensitivity to low dose irradiation of ApoE−/− mice mediated by early pro-inflammatory profile and delayed activation of the TGFβ1 cascade involved in fibrogenesis. PLoS ONE 2013, 8, e57052. [Google Scholar] [CrossRef] [Green Version]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, M.; Pasquali, E.; Giardullo, P.; Leonardi, S.; Tanori, M.; Di Majo, V.; Pazzaglia, S.; Saran, A. The radiation bystander effect and its potential implications for human health. Curr. Mol. Med. 2012, 12, 613–624. [Google Scholar] [CrossRef]
- Ghigo, A.; Laffargue, M.; Li, M.; Hirsch, E. PI3K and Calcium Signaling in Cardiovascular Disease. Circ. Res. 2017, 121, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Cao, L.; Massey, I.Y. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell Biochem. 2021, 476, 4045–4059. [Google Scholar] [CrossRef]
- Moertl, S.; Mutschelknaus, L.; Heider, T.; Atkinson, M.J. MicroRNAs as novel elements in personalized radiotherapy. Transl. Cancer Res. 2016, 5, S1262–S1269. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, J.; Gong, Y.; Wei, S.; Wei, Y.; Yi, L. MicroRNA: A novel implication for damage and protection against ionizing radiation. Environ. Sci. Pollut. Res. 2021, 28, 15584–15596. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. miR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903. [Google Scholar] [CrossRef]
- Sang, H.Q.; Jiang, Z.M.; Zhao, Q.P.; Xin, F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed. Pharmacother. 2015, 71, 185–189. [Google Scholar] [CrossRef]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kho, C. MicroRNAs and Calcium Signaling in Heart Disease. Int. J. Mol. Sci. 2021, 22, 10582. [Google Scholar] [CrossRef]
- Valkov, N.; King, M.E.; Moeller, J.; Liu, H.; Li, X.; Zhang, P. MicroRNA-1-Mediated Inhibition of Cardiac Fibroblast Proliferation Through Targeting Cyclin D2 and CDK6. Front. Cardiovasc. Med. 2019, 6, 65. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Tanno, B.; De Stefano, I.; Giardullo, P.; Leonardi, S.; Merla, C.; Babini, G.; Tuncay Cagatay, S.; Mayah, A.; Kadhim, M.; et al. Micro-RNA and Proteomic Profiles of Plasma-Derived Exosomes from Irradiated Mice Reveal Molecular Changes Preventing Apoptosis in Neonatal Cerebellum. Int. J. Mol. Sci. 2022, 23, 2169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, S.; Natarajan, M.; Ramraj, S.K.; Pandian, V.; Khan, F.H.; Herman, T.S.; Aravindan, N. Abscopal effect of low-LET γ-radiation mediated through Rel protein signal transduction in a mouse model of nontargeted radiation response. Cancer Gene Ther. 2014, 21, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar-Pereira, S.; Fullard, N.; Townsend, P.A.; Banks, P.S.; Ellis, E.L.; Fox, C.; Maxwell, A.G.; Murphy, L.B.; Kirk, A.; Bauer, R.; et al. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 2012, 180, 929–939. [Google Scholar] [CrossRef] [PubMed]
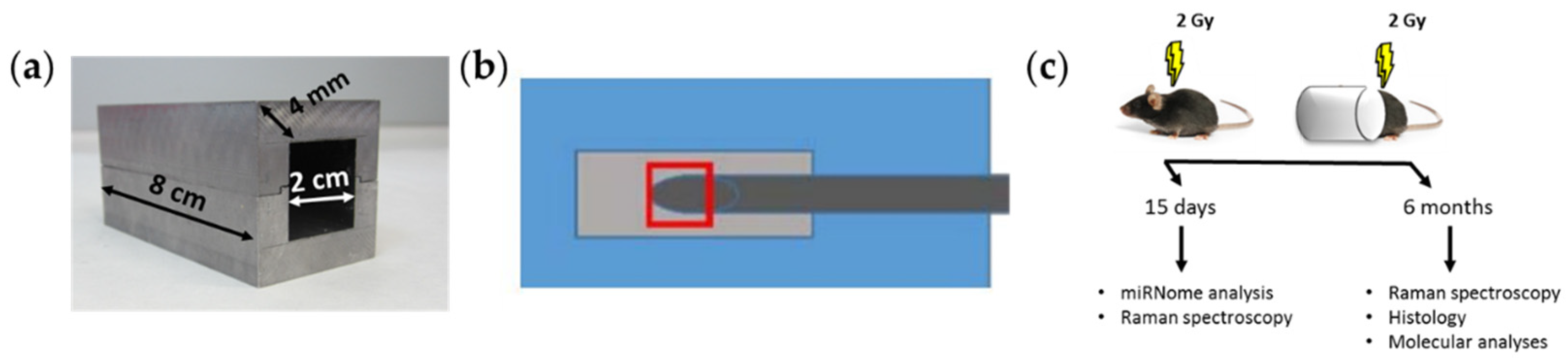

| X-ray Quality Code | HLV/mm Cu | E/KeV | HV/V | I/mA | Dose (Gy) | Scatter Dose (mGy) |
|---|---|---|---|---|---|---|
| H-60 | 0.082 | 38 | 64.9 | 45 | 2 | 2.913 |
| H-100 | 0.29 | 57.3 | 102.7 | 40 | 2 | 6.476 |
| H-200 | 1.61 | 99.3 | 201.5 | 20 | 2 | 9.364 |
| H-250 | 2.44 | 121.5 | 250 | 15 | 2 | 7.614 |
| miRNA | LogFC a | miRNA | LogFC |
|---|---|---|---|
| mmu-miR-208a-3p | −15,223 | mmu-miR-214-3p | −5140 |
| mmu-miR-208a-5p | −12,008 | mmu-miR-378b | −4834 |
| mmu-miR-133a-5p | −10,531 | mmu-miR-378a-5p | −4785 |
| mmu-miR-133a-3p | −10,067 | mmu-miR-199a-5p | −4757 |
| mmu-miR-1a-3p | −9184 | mmu-miR-155-5p | −4727 |
| mmu-miR-1a-1-5p | −10,678 | mmu-miR-199a-3p | −4441 |
| mmu-miR-133b-3p | −9139 | mmu-miR-199b-3p | −4357 |
| mmu-miR-499-5p | −8787 | mmu-miR-486-3p | −4422 |
| mmu-miR-499-3p | −10,697 | mmu-miR-224-5p | −4046 |
| mmu-miR-1a-2-5p | −8451 | mmu-miR-122-5p | −3708 |
| mmu-miR-208b-3p | −6388 | mmu-miR-223-3p | −3185 |
| mmu-miR-10a-5p | −6035 | mmu-miR-143-5p | −3091 |
| mmu-miR-378d | −5986 | mmu-miR-322-5p | −2994 |
| mmu-miR-10a-3p | −6466 | mmu-miR-450b-3p | −2982 |
| mmu-miR-199b-5p | −5793 | mmu-miR-145a-3p | −2846 |
| mmu-miR-10b-5p | −5396 | mmu-miR-490-3p | −2716 |
| mmu-miR-486-5p | −5366 | mmu-miR-126a-5p | −2577 |
| mmu-miR-3107-5p | −5354 | mmu-miR-126a-3p | −2577 |
| mmu-miR-378c | −5239 | mmu-miR-27a-3p | −2564 |
| mmu-miR-378a-3p | −4946 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanno, B.; Novelli, F.; Leonardi, S.; Merla, C.; Babini, G.; Giardullo, P.; Kadhim, M.; Traynor, D.; Medipally, D.K.R.; Meade, A.D.; et al. MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation. Cancers 2022, 14, 3463. https://doi.org/10.3390/cancers14143463
Tanno B, Novelli F, Leonardi S, Merla C, Babini G, Giardullo P, Kadhim M, Traynor D, Medipally DKR, Meade AD, et al. MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation. Cancers. 2022; 14(14):3463. https://doi.org/10.3390/cancers14143463
Chicago/Turabian StyleTanno, Barbara, Flavia Novelli, Simona Leonardi, Caterina Merla, Gabriele Babini, Paola Giardullo, Munira Kadhim, Damien Traynor, Dinesh K. R. Medipally, Aidan D. Meade, and et al. 2022. "MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation" Cancers 14, no. 14: 3463. https://doi.org/10.3390/cancers14143463
APA StyleTanno, B., Novelli, F., Leonardi, S., Merla, C., Babini, G., Giardullo, P., Kadhim, M., Traynor, D., Medipally, D. K. R., Meade, A. D., Lyng, F. M., Tapio, S., Marchetti, L., Saran, A., Pazzaglia, S., & Mancuso, M. (2022). MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation. Cancers, 14(14), 3463. https://doi.org/10.3390/cancers14143463









