Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Performance Requirements
2.2. Gel Formulation and Studies
2.3. Gel and Imaging Gel Application
2.4. Cell Culture Preparation for Orthotopic Implants
2.5. Animal and Tumor Model
2.6. Histology of Tissue Samples
2.7. Imaging of Animal BCa Models
2.8. Imaging of the Human BCa Tissue
2.9. Statistics (Mouse Studies)
3. Results
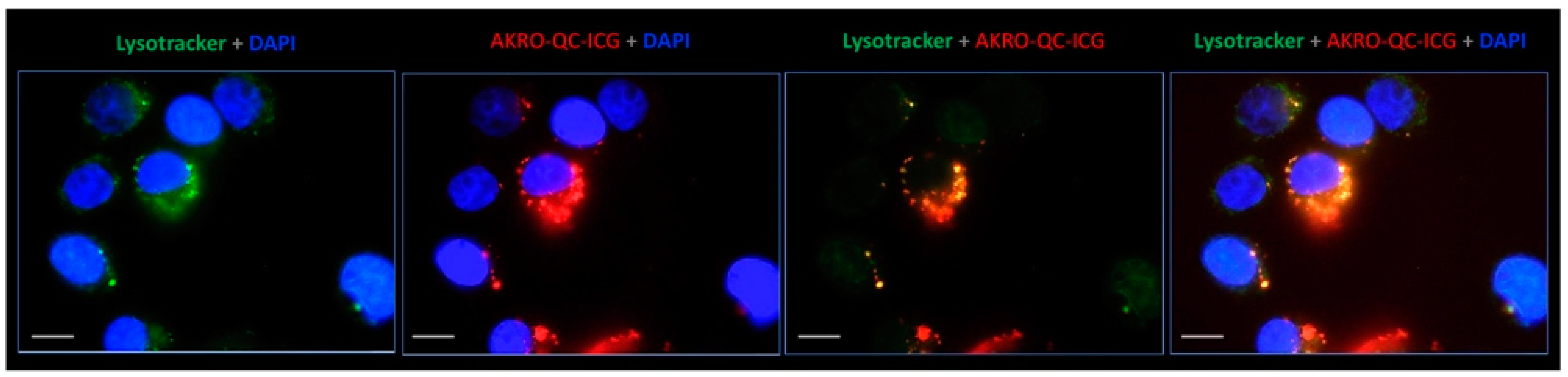
3.1. AKRO-QC-ICG Specifically Accumulates and Is Activated in Human BCa Cells
3.2. Human Breast Cancer Cell Lysate Initiates Specific Fluorescence of the AKRO-QC-ICG Probe In Vitro
3.3. Thermo-Sensitive Gel Formulation of AKRO-QC-ICG Enables Uniform Topical Distribution of Probe along the Surfaces of the Surgical Cavity
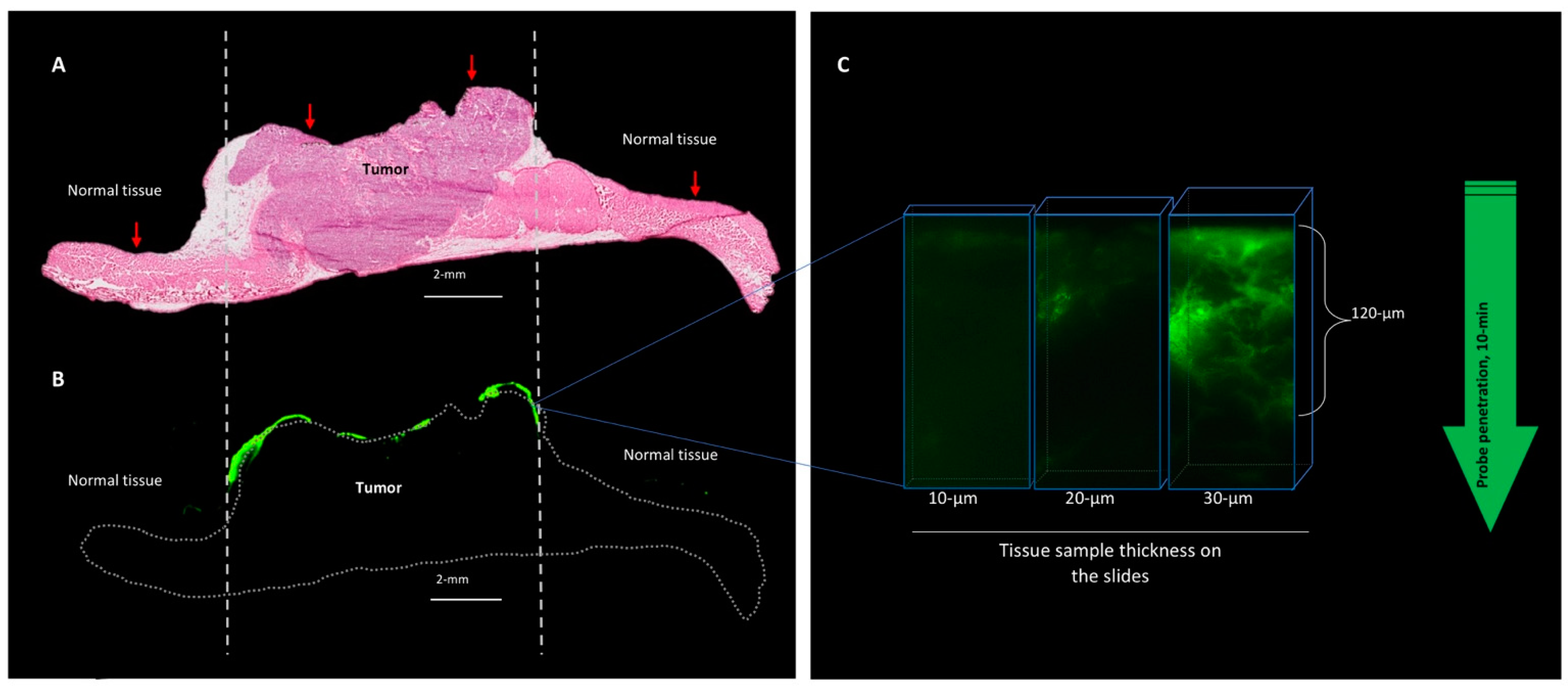
3.3.1. The Gel Formulation of AKRO-QC-ICG Enables the Visualization and Discrimination of Human BCa from Normal Mouse Tissue In Vivo
3.3.2. The Behavior of the AKRO-QC-ICG Gel Formulation on the Surface of the Human BCa Tissue In Vivo
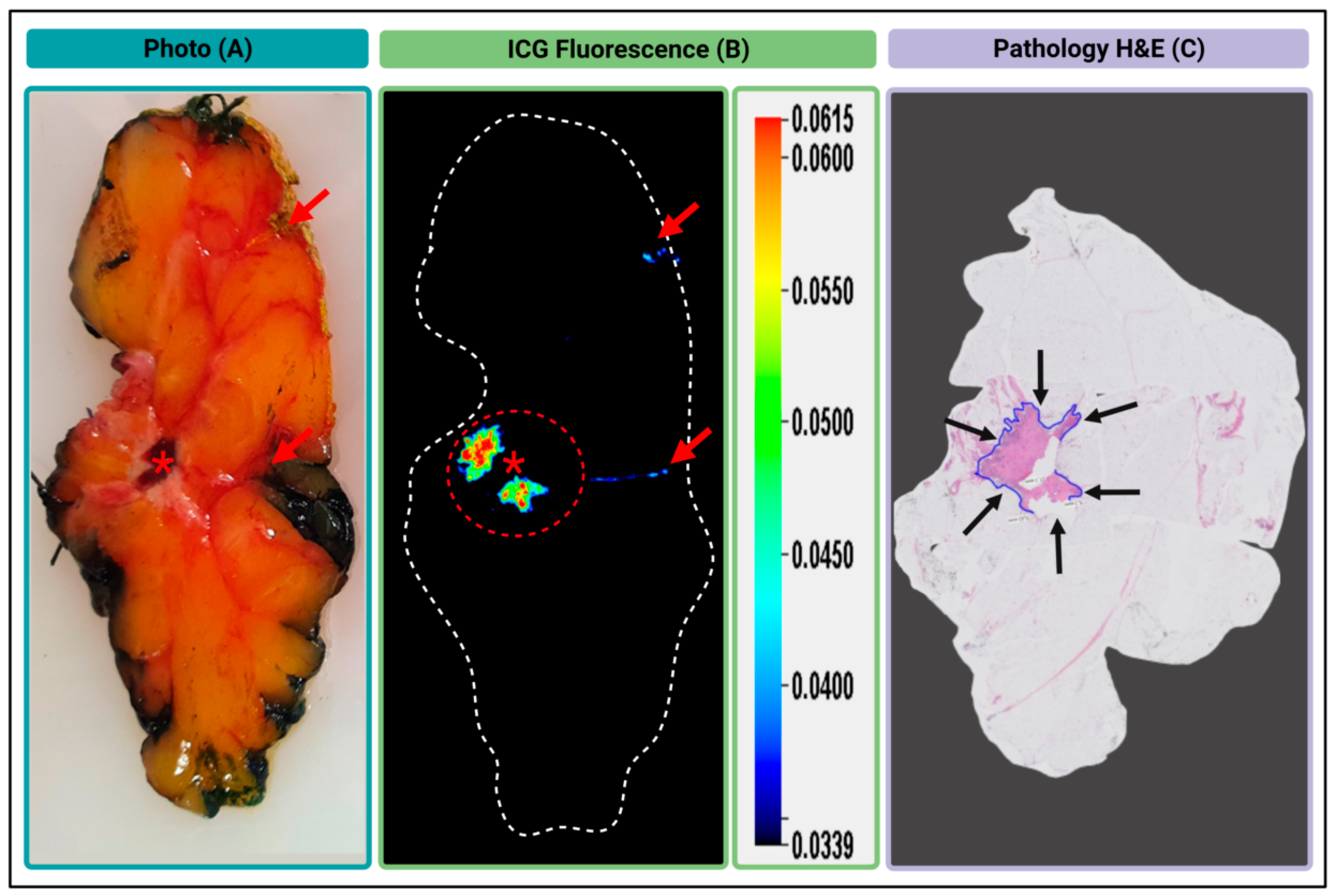
3.3.3. Gel Formulated AKRO-QC-ICG can Detect BCa in an Excised Human Lumpectomy Sample
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Breast Cancer Facts & Figures 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 11 July 2022).
- American Cancer Society. Breast Cancer Facts and Figures 2019–2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html (accessed on 11 July 2022).
- Gomes, D.C.; Farshid, G. Change of blue ink margin colour to black in immunohistochemistry stained slides: A potential pitfall for pathology cancer margin assessment. Pathology 2021, 53, 558–559. [Google Scholar] [CrossRef]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann. Surg. Oncol. 2014, 21, 704–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevan, R.; Cromwell, D.A.; Trivella, M.; Lawrence, G.; Kearins, O.; Pereira, J.; Sheppard, C.; Caddy, C.M.; van der Meulen, J.H. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ 2012, 345, e4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wazer, D.E.; Schmidt-Ullrich, R.K.; Ruthazer, R.; Schmid, C.H.; Graham, R.; Safaii, H.; Rothschild, J.; McGrath, J.; Erban, J.K. Factors determining outcome for breast-conserving irradiation with margin-directed dose escalation to the tumor bed. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 851–858. [Google Scholar] [CrossRef]
- Blair, S.L.; Thompson, K.; Rococco, J.; Malcarne, V.; Beitsch, P.D.; Ollila, D.W. Attaining negative margins in breast-conservation operations: Is there a consensus among breast surgeons? J. Am. Coll. Surg. 2009, 209, 608–613. [Google Scholar] [CrossRef]
- Pleijhuis, R.G.; Graafland, M.; de Vries, J.; Bart, J.; de Jong, J.S.; van Dam, G.M. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: Current modalities and future directions. Ann. Surg. Oncol. 2009, 16, 2717–2730. [Google Scholar] [CrossRef] [Green Version]
- Menes, T.S.; Tartter, P.I.; Bleiweiss, I.; Godbold, J.H.; Estabrook, A.; Smith, S.R. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann. Surg. Oncol. 2005, 12, 881–885. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Lagios, M.D.; Martino, S.; Lewinsky, B.S.; Craig, P.H.; Beron, P.J.; Gamagami, P.; Waisman, J.R. Outcome after invasive local recurrence in patients with ductal carcinoma in situ of the breast. J. Clin. Oncol. 1998, 16, 1367–1373. [Google Scholar] [CrossRef]
- Solin, L.J.; Recht, A.; Fourquet, A.; Kurtz, J.; Kuske, R.; McNeese, M.; McCormick, B.; Cross, M.A.; Schultz, D.J.; Bornstein, B.A. Ten-year results of breast-conserving surgery and definitive irradiation for intraductal carcinoma (ductal carcinoma in situ) of the breast. Cancer 1991, 68, 2337–2344. [Google Scholar] [CrossRef]
- McCahill, L.E.; Single, R.M.; Aiello Bowles, E.J.; Feigelson, H.S.; James, T.A.; Barney, T.; Engel, J.M.; Onitilo, A.A. Variability in reexcision following breast conservation surgery. JAMA 2012, 307, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.M.; Houssami, N. Bigger margins are not better in breast conserving surgery. BMJ. 2012, 345, e5855. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, S.J.; Moran, M.S.; Houssami, N.; Morrow, M. The Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer: Perspectives for Pathologists. Arch. Pathol. Lab. Med. 2015, 139, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Azu, M.; Abrahamse, P.; Katz, S.J.; Jagsi, R.; Morrow, M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann. Surg. Oncol. 2010, 17, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Erguvan-Dogan, B.; Whitman, G.J.; Nguyen, V.A.; Dryden, M.J.; Stafford, R.J.; Hazle, J.; McAlee, K.R.; Phelps, M.J.; Ice, M.F.; Kuerer, H.M.; et al. Specimen radiography in confirmation of MRI-guided needle localization and surgical excision of breast lesions. AJR Am. J. Roentgenol. 2006, 187, 339–344. [Google Scholar] [CrossRef]
- Goldfeder, S.; Davis, D.; Cullinan, J. Breast specimen radiography: Can it predict margin status of excised breast carcinoma? Acad. Radiol. 2006, 13, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Karni, T.; Pappo, I.; Sandbank, J.; Lavon, O.; Kent, V.; Spector, R.; Morgenstern, S.; Lelcuk, S. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am. J. Surg. 2007, 194, 467–473. [Google Scholar] [CrossRef]
- Karni, T.; Pappo, I.; Sandbank, J.; Lavon, O.; Kent, V.; Spector, R.; Morgenstern, S.; Lelcuk, S. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem. Biol. 2012, 19, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Krekel, N.M.; Haloua, M.H.; Cardozo, A.M.L.; de Wit, R.H.; Bosch, A.M.; de Widt-Levert, L.M.; Muller, S.; van der Veen, H.; Bergers, E.; Klerk, E.S.D.L.D.; et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): A multicentre, randomised controlled trial. Lancet Oncol. 2013, 14, 48–54. [Google Scholar] [CrossRef]
- Ramos, M.; Díaz, J.C.; Ramos, T.; Ruano, R.; Aparicio, M.; Sancho, M.; González-Orús, J.M. Ultrasound-guided excision combined with intraoperative assessment of gross macroscopic margins decreases the rate of reoperations for non-palpable invasive breast cancer. Breast 2013, 22, 520–524. [Google Scholar] [CrossRef]
- James, T.A.; Harlow, S.; Sheehey-Jones, J.; Hart, M.; Gaspari, C.; Stanley, M.; Krag, D.; Ashikaga, T.; McCahill, L.E. Intraoperative Ultrasound Versus Mammographic Needle Localization for Ductal Carcinoma In Situ. Ann. Surg. Oncol. 2009, 16, 1164–1169. [Google Scholar] [CrossRef]
- Olsha, O.; Shemesh, D.; Carmon, M.; Sibirsky, O.; Abu Dalo, R.; Rivkin, L.; Ashkenazi, I. Resection Margins in Ultrasound-Guided Breast-Conserving Surgery. Ann. Surg. Oncol. 2010, 18, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, X. Activatable Molecular Probes for Cancer Imaging. Curr. Top. Med. Chem. 2010, 10, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Hori, S.S.; Tummers, W.S.; Gambhir, S.S. Cancer diagnostics: On-target probes for early detection. Nat. Biomed. Eng. 2017, 1, 62. [Google Scholar] [CrossRef]
- Liu, H.-W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.-B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef]
- Garland, M.; Yim, J.J.; Bogyo, M. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef] [Green Version]
- Cutter, J.L.; Cohen, N.T.; Wang, J.; Sloan, A.E.; Cohen, A.R.; Panneerselvam, A.; Schluchter, M.; Blum, G.; Bogyo, M.; Basilion, J.P. Topical Application of Activity-based Probes for Visualization of Brain Tumor Tissue. PLoS ONE 2012, 7, e33060. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.; Gopalakrishnan, R.; Bogyo, M.; Basilion, J.P. Microscopic detection of quenched activity-based optical imaging probes using an antibody detection system: Localizing protease activity. Mol. Imaging Biol. 2014, 16, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.; Mann, M.; Honda, K.; Vidimos, A.; Schluchter, M.D.; Straight, B.; Bogyo, M.; Popkin, D.; Basilion, J.P. Rapid visualization of nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2016, 76, 209–216.e9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Walker, E.; Iyer, S.R.; Biro, M.; Kim, I.; Zhou, B.; Straight, B.; Bogyo, M.; Basilion, J.P.; Popkin, D.L.; et al. Molecular imaging and validation of margins in surgically excised nonmelanoma skin cancer specimens. J. Med. Imaging 2019, 6, 016001. [Google Scholar] [CrossRef]
- A Foekens, J.; Kos, J.; A Peters, H.; Krasovec, M.; Look, M.P.; Cimerman, N.; Gelder, M.E.M.-V.; Henzen-Logmans, S.C.; Van Putten, W.L.; Klijn, J.G. Prognostic significance of cathepsins B and L in primary human breast cancer. J. Clin. Oncol. 1998, 16, 1013–1021. [Google Scholar] [CrossRef]
- Lah, T.T.; Kalman, E.; Najjar, D.; Gorodetsky, E.; Brennan, P.; Somers, R.; Daskal, I. Cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum. Pathol. 2000, 31, 149–160. [Google Scholar] [CrossRef]
- Levičar, N.; Kos, J.; Blejec, A.; Golouh, R.; Vrhovec, I.; Frkovič-Grazio, S.; Lah, T.T. Comparison of potential biological markers cathepsin B, cathepsin L, stefin A and stefin B with urokinase and plasminogen activator inhibitor-1 and clinicopathological data of breast carcinoma patients. Cancer Detect. Prev. 2002, 26, 42–49. [Google Scholar] [CrossRef]
- Withana, N.P.; Blum, G.; Sameni, M.; Slaney, C.; Anbalagan, A.; Olive, M.B.; Bidwell, B.N.; Edgington, L.; Wang, L.; Moin, K.; et al. Cathepsin B Inhibition Limits Bone Metastasis in Breast Cancer. Cancer Res. 2012, 72, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clin. Exp. Metastasis 2013, 30, 891–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengsch, F.; Buck, A.K.; Gunther, S.; Seiz, J.R.; Tacke, M.; Pfeifer, D.; von Elverfeldt, D.; Sevenich, L.; Hillebrand, L.E.; Kern, U.; et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene 2014, 33, 4474–4484. [Google Scholar] [CrossRef] [Green Version]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, J.O.; Matrisian, L.M. Molecular imaging of proteolytic activity in cancer. J. Cell. Biochem. 2003, 90, 1087–1097. [Google Scholar] [CrossRef]
- Fonovic, M. Activity Based Probes for Proteases: Applications to Biomarker Discovery, Molecular Imaging and Drug Screening. Curr. Pharm. Des. 2007, 13, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Gocheva, V.; Wang, H.-W.; Gadea, B.B.; Shree, T.; Hunter, K.E.; Garfall, A.L.; Berman, T.; Joyce, J.A. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010, 24, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, U.; Weissleder, R. Near-infrared optical imaging of proteases in cancer. Mol. Cancer Ther. 2003, 2, 489–496. [Google Scholar]
- Blum, G.; Von Degenfeld, G.; Merchant, M.J.; Blau, H.M.; Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 2007, 3, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Blum, G.; Mullins, S.R.; Keren, K.; Fonovic, M.; Jedeszko, C.; Rice, M.J.; Sloane, B.F.; Bogyo, M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat. Chem. Biol. 2005, 1, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.B.; Witte, M.D.; Blum, G.; Bogyo, M. Design of cell-permeable, fluorescent activity-based probes for the lysosomal cysteine protease asparaginyl endopeptidase (AEP)/legumain. Bioorg. Med. Chem. Lett. 2007, 17, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofori, L.O.; Withana, N.P.; Prestwood, T.R.; Verdoes, M.; Brady, J.J.; Winslow, M.M.; Sorger, J.; Bogyo, M. Design of Protease Activated Optical Contrast Agents That Exploit a Latent Lysosomotropic Effect for Use in Fluorescence-Guided Surgery. ACS Chem. Biol. 2015, 10, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Dixon, J.M.; Irwig, L.; Brennan, M.E.; Solin, L.J. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer 2010, 46, 3219–3232. [Google Scholar] [CrossRef]
- Withana, N.P.; Garland, M.; Verdoes, M.; O Ofori, L.; Segal, E.; Bogyo, M. Labeling of active proteases in fresh-frozen tissues by topical application of quenched activity-based probes. Nat. Protoc. 2015, 11, 184–191. [Google Scholar] [CrossRef]
- Walker, E.; Liu, Y.; Kim, I.; Biro, M.; Iyer, S.R.; Ezaldein, H.; Scott, J.; Merati, M.; Mistur, R.; Zhou, B.; et al. A Protease-Activated Fluorescent Probe Allows Rapid Visualization of Keratinocyte Carcinoma during Excision. Cancer Res. 2020, 80, 2045–2055. [Google Scholar] [CrossRef] [Green Version]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2008, 68, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release Off. J. Control. Release Soc. 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Cohn, D.; Sosnik, A.; Levy, A. Improved reverse thermo-responsive polymeric systems. Biomaterials 2003, 24, 3707–3714. [Google Scholar] [CrossRef]
- Sosnik, A.; Cohn, D. Ethoxysilane-capped PEO–PPO–PEO triblocks: A new family of reverse thermo-responsive polymers. Biomaterials 2004, 25, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Tholen, M.; Klaassen, A.; Sorger, J.; Bogyo, M. Optimization of a Protease Activated Probe for Optical Surgical Navigation. Mol. Pharm. 2017, 15, 750–758. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Tjalma, J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2019, 61, 655–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, A.; Predina, J.; Mison, M.; Runge, J.; Bradley, C.; Stefanovski, D.; Singhal, S.; Holt, D. Intraoperative near-infrared imaging can identify canine mammary tumors, a spontaneously occurring, large animal model of human breast cancer. PLoS ONE 2020, 15, e0234791. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef]
- Liu, J.-N.; Bu, W.; Shi, J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef]
- Bu, L.; Shen, B.; Cheng, Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv. Drug Deliv. Rev. 2014, 76, 21–38. [Google Scholar] [CrossRef] [Green Version]




| Measure | Group Examined | n | Estimate | 95% CI 2-Sided |
|---|---|---|---|---|
| Sensitivity | All samples | 12 | 0.923 (12/12) | (0.621–0.996) |
| Specificity | All samples | 12 | 1.000 (11/12) | (0.679–1.000) |
| Measure | Group Examined | n | Estimate | 95% CI 2-Sided |
|---|---|---|---|---|
| Sensitivity | All samples | 16 | 0.941 (16/16) | (0.692–0.997) |
| Specificity | All samples | 20 | 1.000 (19/20) | (0.790–1.000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, E.; Linders, D.G.J.; Abenojar, E.; Wang, X.; Hazelbag, H.M.; Straver, M.E.; Bijlstra, O.D.; March, T.L.; Vahrmeijer, A.L.; Exner, A.; et al. Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity. Cancers 2022, 14, 3459. https://doi.org/10.3390/cancers14143459
Walker E, Linders DGJ, Abenojar E, Wang X, Hazelbag HM, Straver ME, Bijlstra OD, March TL, Vahrmeijer AL, Exner A, et al. Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity. Cancers. 2022; 14(14):3459. https://doi.org/10.3390/cancers14143459
Chicago/Turabian StyleWalker, Ethan, Daan G. J. Linders, Eric Abenojar, Xinning Wang, Hans Marten Hazelbag, Marieke E. Straver, Okker D. Bijlstra, Taryn L. March, Alexander L. Vahrmeijer, Agata Exner, and et al. 2022. "Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity" Cancers 14, no. 14: 3459. https://doi.org/10.3390/cancers14143459
APA StyleWalker, E., Linders, D. G. J., Abenojar, E., Wang, X., Hazelbag, H. M., Straver, M. E., Bijlstra, O. D., March, T. L., Vahrmeijer, A. L., Exner, A., Bogyo, M., Basilion, J. P., & Straight, B. (2022). Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity. Cancers, 14(14), 3459. https://doi.org/10.3390/cancers14143459






