The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
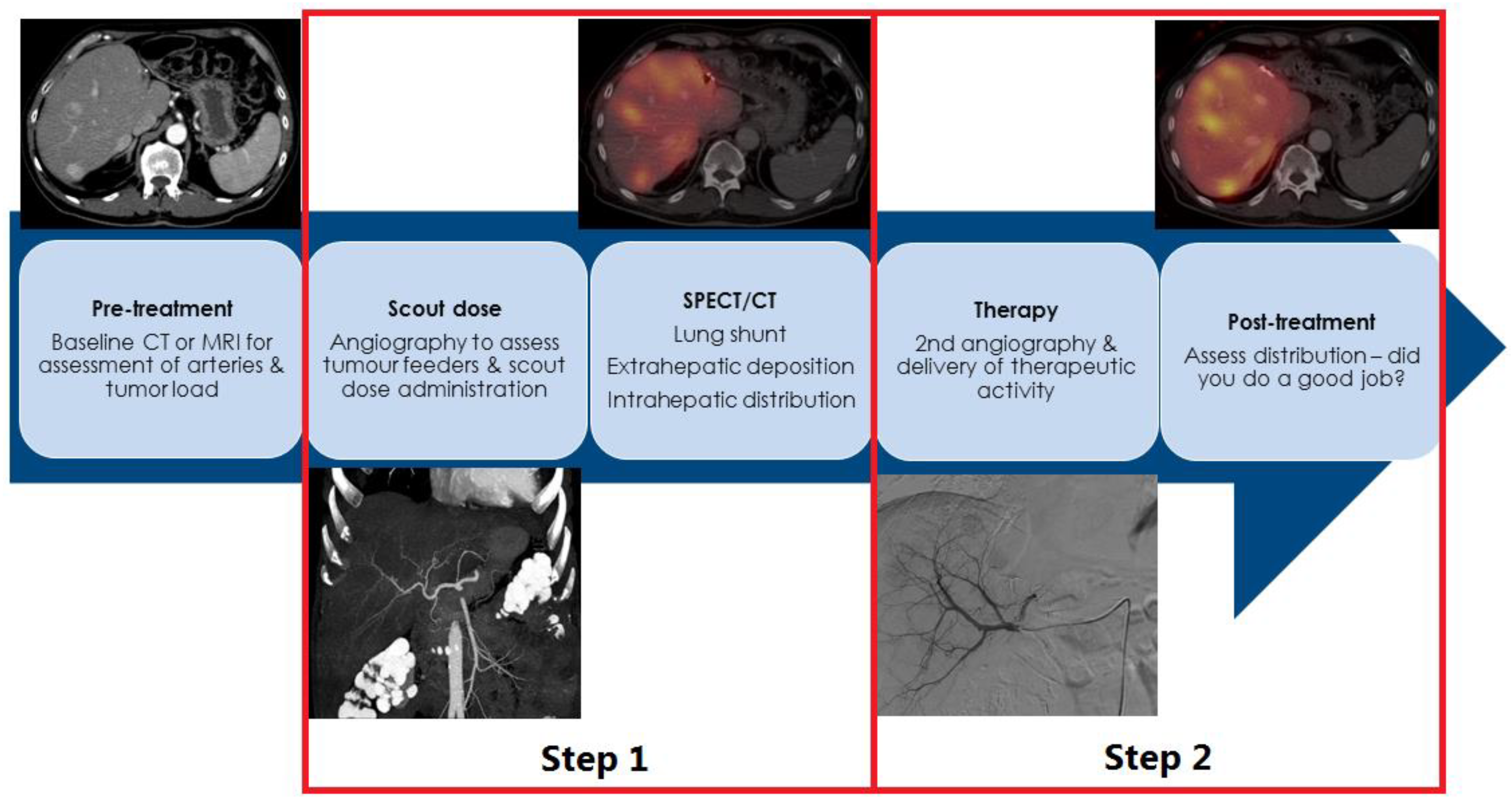
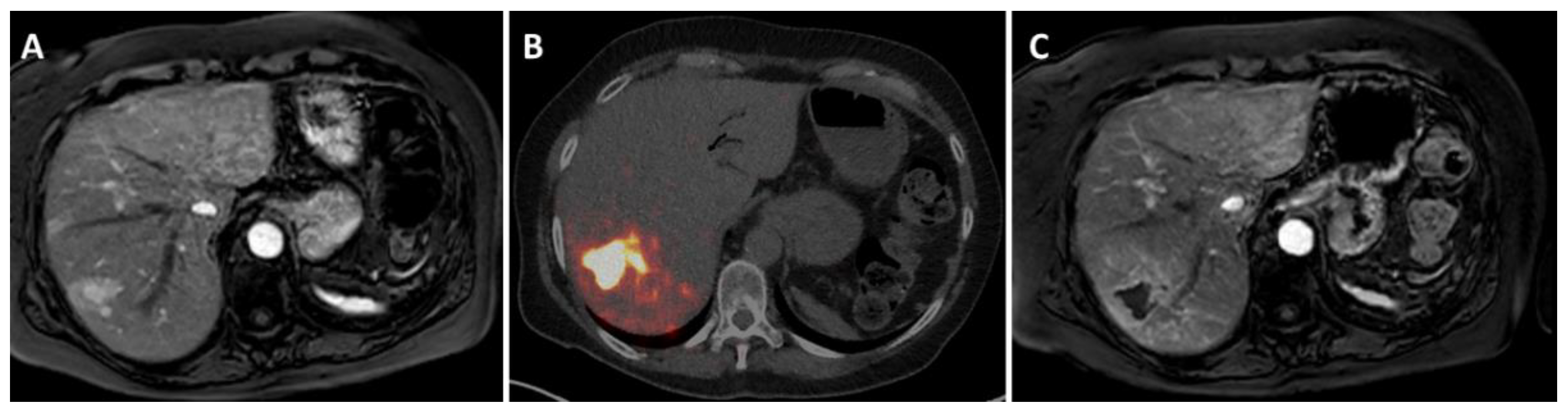
2. How Is Radioembolization Performed?
3. SIRT in NEN: Salvage Setting
4. Radioembolization in Earlier Lines or Combinations Treatments
5. Concerns, Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bosman, F.T.; Carneiro, F. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System, 4th ed.; IARC: Lyon, France, 2010. [Google Scholar]
- Capelli, P.; Fassan, M.; Scarpa, A. Pathology-grading and staging of gep-nets. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with g3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. Enets consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (nen) and nen of unknown primary site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after carcinoid: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the united states. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R.; Barcelona Consensus Conference participants. Enets consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Braat, A.J.A.T.; Smits, M.L.J.; Braat, M.N.G.J.A.; van den Hoven, A.F.; Prince, J.F.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. 90y hepatic radioembolization: An update on current practice and recent developments. J. Nucl. Med. 2015, 56, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Braat, A.J.A.T.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)y resin microspheres of neuroendocrine liver metastases: International multicenter study on efficacy and toxicity. Cardiovasc. Intervent. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef]
- Devcic, Z.; Rosenberg, J.; Braat, A.J.A.T.; Techasith, T.; Banerjee, A.; Sze, D.Y.; Lam, M.G.E.H. The efficacy of hepatic 90y resin radioembolization for metastatic neuroendocrine tumors: A meta-analysis. J. Nucl. Med. 2014, 55, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Uliel, L.; Royal, H.D.; Darcy, M.D.; Zuckerman, D.A.; Sharma, A.; Saad, N.E. From the angio suite to the gamma-camera: Vascular mapping and 99mtc-maa hepatic perfusion imaging before liver radioembolization—A comprehensive pictorial review. J. Nucl. Med. 2012, 53, 1736–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Kim, D.J.; Kim, K.A.; Yoon, S.W.; Lee, J.T. Feasibility of mdct angiography for determination of tumor-feeding vessels in chemoembolization of hepatocellular carcinoma. J. Comput. Assist. Tomogr. 2014, 38, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, M.H.; Hwang, G.L.; Louie, J.D.; Kothary, N.; Hofmann, L.V.; Kuo, W.T.; Hovsepian, D.M.; Sze, D.Y. Development of new hepaticoenteric collateral pathways after hepatic arterial skeletonization in preparation for yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2010, 21, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Stella, M.; van Rooij, R.; Lam, M.G.E.H.; de Jong, H.W.A.M.; Braat, A.J.A.T. Lung dose measured on post-radioembolization (90)y-pet/ct and incidence of radiation pneumonitis. J. Nucl. Med. 2021, 63, 1075–1080. [Google Scholar] [CrossRef]
- Cholapranee, A.; van Houten, D.; Deitrick, G.; Dagli, M.; Sudheendra, D.; Mondschein, J.I.; Soulen, M.C. Risk of liver abscess formation in patients with prior biliary intervention following yttrium-90 radioembolization. Cardiovasc. Intervent. Radiol. 2015, 38, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.T.M.; Mees, E.; Powerski, M.J.; Bruijnen, R.C.G.; van den Bosch, M.A.A.J.; Lam, M.G.E.H.; Smits, M.L.J. Radioembolisation in Europe: A Survey Amongst CIRSE Members. Cardiovasc. Intervent. Radiol. 2018, 41, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Peker, A.; Cicek, O.; Soydal, C.; Kucuk, N.O.; Bilgic, S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn. Interv. Radiol. 2015, 21, 54–59. [Google Scholar] [CrossRef]
- Barbier, C.E.; Garske-Roman, U.; Sandstrom, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1425–1431. [Google Scholar] [CrossRef]
- Schaarschmidt, B.M.; Wildgruber, M.; Kloeckner, R.; Nie, J.; Steinle, V.; Braat, A.J.A.T.; Lohoefer, F.; Kim, H.S.; Lahner, H.; Weber, M.; et al. 90y radioembolization in the treatment of neuroendocrine neoplasms: Results of an international multicenter retrospective study. J. Nucl. Med. 2022, 63, 679–685. [Google Scholar] [CrossRef]
- Wong, T.Y.; Zhang, K.S.; Gandhi, R.T.; Collins, Z.S.; O’Hara, R.; Wang, E.A.; Vaheesan, K.; Matsuoka, L.; Sze, D.Y.; Kennedy, A.S.; et al. Long-term outcomes following 90y radioembolization of neuroendocrine liver metastases: Evaluation of the radiation-emitting sir-spheres in non-resectable liver tumor (resin) registry. BMC Cancer 2022, 22, 224. [Google Scholar] [CrossRef]
- Braat, A.J.A.T.; Kwekkeboom, D.J.; Kam, B.L.R.; Teunissen, J.J.M.; de Herder, W.W.; Dreijerink, K.M.A.; van Rooij, R.; Krijger, G.C.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; et al. Additional hepatic 166ho-radioembolization in patients with neuroendocrine tumours treated with 177lu-dotatate; a single center, interventional, non-randomized, non-comparative, open label, phase ii study (hepar plus trial). BMC Gastroenterol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)lu-dotatate: An analysis of the netter-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepage, C.; Dahan, L.; Bouarioua, N.; Toumpanakis, C.; Legoux, J.L.; Le Malicot, K.; Guimbaud, R.; Smith, D.; Tougeron, D.; Lievre, A.; et al. Evaluating lanreotide as maintenance therapy after first-line treatment in patients with non-resectable duodeno-pancreatic neuroendocrine tumours. Dig. Liver Dis. 2017, 49, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Phelip, J.M.; Lièvre, A.; Le Malicot, K.; Tougeron, D.; Dahan, L.; Toumpanakis, C.; Di Fiore, F.; Bohas, C.L.; Borbath, I.; et al. 1163p lanreotide as maintenance therapy after first-line treatment in patients with non-resectable duodeno-pancreatic neuroendocrine tumours (nets): An international double-blind, placebo-controlled randomized phase ii trial. Ann. Oncol. 2020, 31, S774. [Google Scholar] [CrossRef]
- Soulen, M.C.; van Houten, D.; Teitelbaum, U.R.; Damjanov, N.; Cengel, K.A.; Metz, D.C. Safety and feasibility of integrating yttrium-90 radioembolization with capecitabine-temozolomide for grade 2 liver-dominant metastatic neuroendocrine tumors. Pancreas 2018, 47, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Shaib, W.L.; Zhang, C.; Nagaraju, G.P.; Wu, C.; Alese, O.B.; Chen, Z.; Brutcher, E.; Renfroe, M.; El-Rayes, B.F. Phase 1b study of pasireotide, everolimus, and selective internal radioembolization therapy for unresectable neuroendocrine tumors with hepatic metastases. Cancer 2018, 124, 1992–2000. [Google Scholar] [CrossRef] [Green Version]
- Braat, A.J.A.T.; Bruijnen, R.C.G.; van Rooij, R.; Braat, M.N.G.J.A.; Wessels, F.J.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; de Herder, W.W.; Hofland, J.; Tesselaar, M.E.T.; et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (hepar plus): A single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020, 21, 561–570. [Google Scholar] [CrossRef]
- Ebbers, S.C.; Brabander, T.; Tesselaar, M.E.T.; Hofland, J.; Braat, M.N.G.J.A.; Wessels, F.J.; Barentsz, M.W.; Lam, M.G.E.H.; Braat, A.J.A.T. Inflammatory markers and long term hematotoxicity of holmium-166-radioembolization in liver-dominant metastatic neuroendocrine tumors after initial peptide receptor radionuclide therapy. EJNMMI Res. 2022, 12, 7. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Braat, A.J.A.T.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef]
- Braat, M.N.G.J.A.; van Erpecum, K.J.; Zonnenberg, B.A.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. Radioembolization-induced liver disease: A systematic review. Eur. J. Gastroenterol. Hepatol. 2017, 29, 144–152. [Google Scholar] [CrossRef]
- Currie, B.M.; Nadolski, G.; Mondschein, J.; Dagli, M.; Sudheendra, D.; Stavropoulos, S.W.; Soulen, M.C. Chronic hepatotoxicity in patients with metastatic neuroendocrine tumor: Transarterial chemoembolization versus transarterial radioembolization. J. Vasc. Interv. Radiol. 2020, 31, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.A. Radioembolization versus chemoembolization for neuroendocrine metastases. J. Vasc. Interv. Radiol. 2021, 32, 482–483. [Google Scholar] [CrossRef]
- Currie, B.M.; Nadolski, G.; Soulen, M.C. Response letter to correspondence regarding chronic hepatotoxicity in patients with metastatic neuroendocrine tumor: Transarterial chemoembolization versus transarterial radioembolization. J. Vasc. Interv. Radiol. 2021, 32, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Riff, B.P.; Yang, Y.X.; Soulen, M.C.; Pryma, D.A.; Bennett, B.; Wild, D.; Nicolas, G.; Teitelbaum, U.R.; Metz, D.C. Peptide receptor radionuclide therapy-induced hepatotoxicity in patients with metastatic neuroendocrine tumors. Clin. Nucl. Med. 2015, 40, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Mackey, R.V.; Riaz, A.; Gates, V.L.; Benson, A.B., 3rd; Miller, F.H.; Yaghmai, V.; Gabr, A.; Salem, R.; Lewandowski, R.J. Long-term hepatotoxicity of yttrium-90 radioembolization as treatment of metastatic neuroendocrine tumor to the liver. J. Vasc. Interv. Radiol. 2017, 28, 1520–1526. [Google Scholar] [CrossRef]
- Tomozawa, Y.; Jahangiri, Y.; Pathak, P.; Kolbeck, K.J.; Schenning, R.C.; Kaufman, J.A.; Farsad, K. Long-term toxicity after transarterial radioembolization with yttrium-90 using resin microspheres for neuroendocrine tumor liver metastases. J. Vasc. Interv. Radiol. 2018, 29, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (dosisphere-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Hermann, A.L.; Dieudonne, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V.; et al. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with (90)y in the sarah study. Radiology 2020, 296, 673–684. [Google Scholar] [CrossRef]
- Lam, M.G.E.H.; Garin, E.; Maccauro, M.; Kappadath, S.C.; Sze, D.Y.; Turkmen, C.; Cantasdemir, M.; Haste, P.; Herrmann, K.; Alsuhaibani, H.S.; et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with yttrium-90: The target study. Eur. J. Nucl. Med. Mol. Imaging 2022, 1–13. [Google Scholar] [CrossRef]
- Ebbers, S.C.; van Roekel, C.; Braat, M.N.G.J.A.; Barentsz, M.W.; Lam, M.G.E.H.; Braat, A.J.A.T. Dose-response relationship after yttrium-90-radioembolization with glass microspheres in patients with neuroendocrine tumor liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1700–1710. [Google Scholar] [CrossRef]
- Sommer, W.H.; Ceelen, F.; Garcia-Albeniz, X.; Paprottka, P.M.; Auernhammer, C.J.; Armbruster, M.; Nikolaou, K.; Haug, A.R.; Reiser, M.F.; Theisen, D. Defining predictors for long progression-free survival after radioembolisation of hepatic metastases of neuroendocrine origin. Eur. Radiol. 2013, 23, 3094–3103. [Google Scholar] [CrossRef] [PubMed]


| Clinical Assessment | Laboratory Testing | Imaging Work-Up |
|---|---|---|
| Minimal | ||
| ECOG performance score | Bilirubin, ALP, AST, ALT, albumin | gdMRI/CECT for intrahepatic tumor load 1 |
| Signs of hepatic dysfunction (Child–Pugh score) | Creatinine, eGFR | Early-phase CECT for arterial vasculature |
| NET hormone-related symptoms | Tumor markers (e.g., CgA, gastrin) | |
| Additional | ||
| In selected cases, Fibroscan or gastroscopy to assess esophageal varices | Hb, hematocrit, WBC, platelets | SSTR-PET/CT for total body tumor load 1 |
| Coagulation (e.g., Prothrombin time or INR) | FDG-PET/CT for tumor grade distinction, excluding aggressive disease. |
| Year | N | ORR * | DCR * | PFS | OS | REILD | |
|---|---|---|---|---|---|---|---|
| % | % | Months | Months | n (%) | |||
| Devcic et al. † [10] | 2014 | 435 | 50 | 86 | NR | 28.5 | NR |
| Peker et al. [18] | 2015 | 38 | 46 | 83 | NR | 39 | 0 |
| Barbier et al. [19] | 2016 | 54 | 54 | 94 | NR | 34.8 | 1 (1.8) |
| Braat et al. [9] | 2019 | 244 | 16 | 91 | NR | 31 | 2 (0.8) |
| 43 | 91 | ||||||
| Schaarschmidt et al. [20] | 2022 | 297 | 41.3 | 83.5 | 15.9 | 30.6 | 2 (0.8) |
| Wong et al. [21] | 2022 | 170 | 36 | 69 | 25 | 33 | 1 (0.6) |
| Author | Year | n | Population | Procedures | ORR * | PFS † | OS |
|---|---|---|---|---|---|---|---|
| Soulen et al. [26] | 2018 | 21 | Grade 2 NELM | capecitabin 600 mg/m2 twice daily for 14 days temozolomide 150 to 200 mg/m2 in divided on days 10 to 14. 90Y resin radioembolization 7th day of cycle 2 | 74% | NR | NR |
| Kim et al. [27] | 2018 | 13 | Grade 1 + 2 NELM | 3 + 3 dose escalation of everolimus 2,5–5–10 mg Pasitreotide 600 µg twice daily 90Y resin radioembolization day 9 and 37 | 46% | 18.6 | 46.3 |
| Braat et al. [28] | 2020 | 31 | Grade 1 + 2 NELM | Standard 4 cycles of 7.4 GBq 177Lu-PRRT 166Ho-radioembolization <20 weeks after 4th PRRT | 43% | 30.1 | 40.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramdhani, K.; Braat, A.J.A.T. The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases. Cancers 2022, 14, 3415. https://doi.org/10.3390/cancers14143415
Ramdhani K, Braat AJAT. The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases. Cancers. 2022; 14(14):3415. https://doi.org/10.3390/cancers14143415
Chicago/Turabian StyleRamdhani, Khalil, and Arthur J. A. T. Braat. 2022. "The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases" Cancers 14, no. 14: 3415. https://doi.org/10.3390/cancers14143415
APA StyleRamdhani, K., & Braat, A. J. A. T. (2022). The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases. Cancers, 14(14), 3415. https://doi.org/10.3390/cancers14143415






