Crosstalk of Redox-Related Subtypes, Establishment of a Prognostic Model and Immune Responses in Endometrial Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Consensus Clustering Analysis of RRGs
2.3. Functional Annotation and Enrichment Analysis
2.4. Establishment of the Prognostic Model in Light of RRGs
2.5. Stratification Analyses
2.6. Correlation between the RBS and Other Biological Processes
2.7. Exploration of Immune Status between Different Subgroups
2.8. Prediction of Immunotherapy Response
2.9. Phenotypes of DNAss and RNAss Differentiation
2.10. Assessment of Drug Sensitivity
2.11. Construction of a Nomograph System
2.12. Statistical Analysis
3. Results
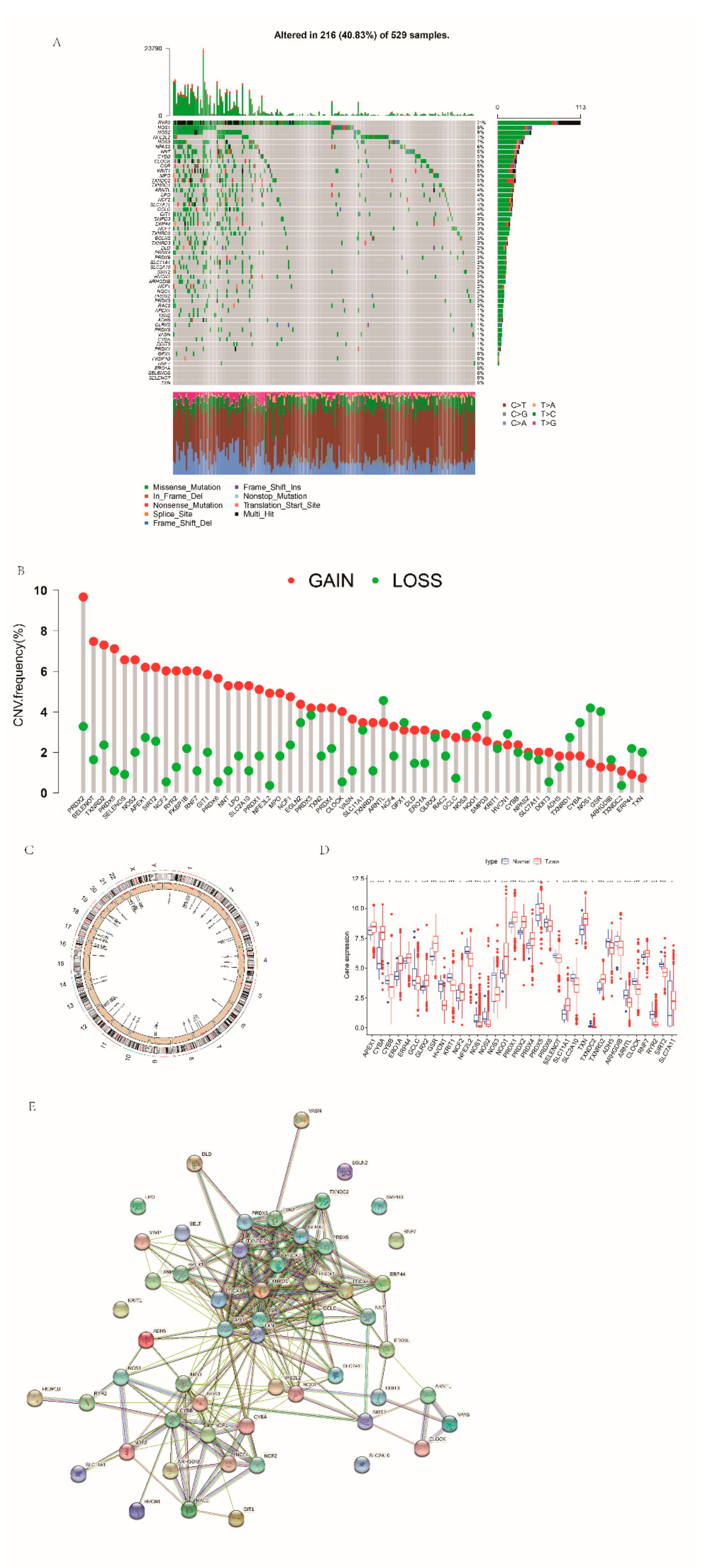
3.1. Genetic Features of RRGs in EC
3.2. Identification of Redox-Associated Molecular Subtype in EC
3.3. Characteristics of the TME in Distinct RRG Clusters
3.4. Identification of Gene Clusters Based on DEGs
3.5. Development and Validation of the RBS
3.6. Comparison of the Risk Score of Different Clinical Characteristics and Stratified Analysis
3.7. Estimation of TME on the Basis of the RRG Score
3.8. Relationships between RRG Score and Tumor Stem Cells as well as TMB
3.9. Analysis of Drug Sensitivity
3.10. Development of Nomograms for Survival Prediction
3.11. RRG Score Is a Novel Predictor for EC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| CNVs | copy number variations |
| DCA | decision curve analysis |
| DEGs | differentially expressed genes |
| EC | endometrial carcinoma |
| GEO | Gene Expression Omnibus |
| HLA | human leukocyte antigen |
| IC50 | half maximum inhibitor concentration |
| IPS | immunophenoscore |
| LASSO | least absolute shrinkage and selection operator |
| MSI | microsatellite instability |
| OS | overall survival |
| PCA | principal component analysis |
| RMS | restricted mean survival |
| ROC | receiver operating characteristic |
| RRGs | redox-related genes |
| TCGA | The Cancer Genome Atlas |
| TIIC | tumor-infiltrating immune cells |
| TMB | tumor mutation burden |
| TME | tumor microenvironment |
| GSVA | gene set variation analysis |
| GSEA | gene set enrichment analysis |
References
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased incidence of endometrial cancer following the women’s health initiative: An assessment of risk factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.L.; Ruterbusch, J.J.; Olson, S.H.; Lu, K.; Ali-Fehmi, R. The growing burden of endometrial cancer: A major racial disparity affecting black women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Wan, X.P. Prognostic significance of immune landscape in tumour microenvironment of endometrial cancer. J. Cell Mol. Med. 2020, 24, 7767–7777. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- McAlpine, J.; Leon-Castillo, A.; Bosse, T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018, 244, 538–549. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Pradip; Jennifer, A.; Nandini, D. Cancer-associated fibroblasts in conversation with tumor cells in endometrial cancers: A partner in crime. Int. J. Mol. Sci. 2021, 22, 9121. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2017, 168, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Liu, D.; Liu, F.; Wu, Y.; Peng, X.; Song, F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J. Control. Release 2021, 332, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, J.; Lei, Y.; Zhou, S.; Wei, Y.; Huang, C. Targeting metabolic-redox circuits for cancer therapy. Trends Biochem. Sci. 2019, 44, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Modulating intracellular oxidative stress via engineered nanotherapeutics. J. Control. Release 2020, 319, 333–343. [Google Scholar] [CrossRef]
- Liao, S.; Yang, Y.; Chen, S.; Bi, Y.; Huang, Q.; Wei, Z.; Qin, A.; Liu, B. IL-24 inhibits endometrial cancer cell proliferation by promoting apoptosis through the mitochondrial intrinsic signaling pathway. Biomed. Pharm. 2020, 124, 109831. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the thioredoxin system for cancer therapy. Trends Pharm. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szczesniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Lin, C.J.; Hatcher, A.; Lozzi, B.; Kong, K.; Huang-Hobbs, E.; Cheng, Y.T.; Beechar, V.B.; Zhu, W.; Zhang, Y.; et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 2020, 578, 166–171. [Google Scholar] [CrossRef]
- Li, L.; Cai, S.; Liu, S.; Feng, H.; Zhang, J. Bioinformatics analysis to screen the key prognostic genes in ovarian cancer. J. Ovarian Res. 2017, 10, 27. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Ma, B.; Jiang, P.; Yang, H.M. Identification of methylation-regulated differentially expressed genes and related pathways in hepatocellular carcinoma: A study based on TCGA database and bioinformatics analysis. Front. Oncol. 2021, 11, 636093. [Google Scholar] [CrossRef]
- Bloniarz, A.; Liu, H.; Zhang, C.H.; Sekhon, J.S.; Yu, B. Lasso adjustments of treatment effect estimates in randomized experiments. Proc. Natl. Acad. Sci. USA 2016, 113, 7383–7390. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, K.; Shahmoradgoli, M.; Martinez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Trevino, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Ling, J.; Zhu, X.; Jiang, P.; Tang, X.; Zhou, H.; Li, R. Construction of a glycolysis-related long noncoding RNA signature for predicting survival in endometrial cancer. J. Cancer 2021, 12, 1431–1444. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Charoentong, P.; Finotello, F.; Angelova, M.; Mayer, C.; Efremova, M.; Rieder, D.; Hackl, H.; Trajanoski, Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017, 18, 248–262. [Google Scholar] [CrossRef] [Green Version]
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. TMB or not TMB as a biomarker: That is the question. Crit. Rev. Oncol. Hematol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, J.; Liu, S. Cancer stem cells and neovascularization. Cells 2021, 10, 1070. [Google Scholar] [CrossRef]
- Reinhold, W.C.; Sunshine, M.; Liu, H.; Varma, S.; Kohn, K.W.; Morris, J.; Doroshow, J.; Pommier, Y. CellMiner: A web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the NCI-60 cell line set. Cancer Res. 2012, 72, 3499–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, N.; Hida, K.; Sakai, Y.; Osada, S.; Idani, H.; Sato, T.; Takii, Y.; Bando, H.; Shiomi, A.; Saito, N. Nomogram for predicting anastomotic leakage after low anterior resection for rectal cancer. Int. J. Colorectal. Dis. 2018, 33, 411–418. [Google Scholar] [CrossRef]
- Nie, K.; Zheng, Z.; Wen, Y.; Shi, L.; Xu, S.; Wang, X.; Zhou, Y.; Fu, B.; Li, X.; Deng, Z.; et al. Construction and validation of a TP53-associated immune prognostic model for gastric cancer. Genomics 2020, 112, 4788–4795. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Wang, K.; Ming, H.; Zuo, J.; Tian, H.L.; Huang, C.H. A review of the redox regulation of tumor metabolism. Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 57–63. [Google Scholar] [CrossRef]
- Pan, S.; Zhan, Y.; Chen, X.; Wu, B.; Liu, B. Bladder cancer exhibiting high immune infiltration shows the lowest response rate to immune checkpoint inhibitors. Front. Oncol. 2019, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, L.; Cai, K.; Zhao, J.; He, S.; Shen, J.; Wei, Q.; Wang, Z.; Sooranna, S.; Li, H.; et al. A Tumor-infiltration CD8+ T cell-based gene signature for facilitating the prognosis and estimation of immunization responses in HPV+ head and neck squamous cell cancer. Front. Oncol. 2021, 11, 749398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Zhang, J.; Wang, Q. Expression of acap1 is associated with tumor immune infiltration and clinical outcome of ovarian cancer. DNA Cell Biol. 2020, 39, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mei, J.; Li, S.; Wu, Z.; Zhang, Y. Establishment of a novel cell cycle-related prognostic signature predicting prognosis in patients with endometrial cancer. Cancer Cell Int. 2020, 20, 329. [Google Scholar] [CrossRef] [PubMed]
- Nabeel-Shah, S.; Garg, J.; Saettone, A.; Ashraf, K.; Lee, H.; Wahab, S.; Ahmed, N.; Fine, J.; Derynck, J.; Pu, S.; et al. Functional characterization of RebL1 highlights the evolutionary conservation of oncogenic activities of the RBBP4/7 orthologue in Tetrahymena thermophila. Nucleic Acids Res. 2021, 49, 6196–6212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, S.; Situ, B.; Zhong, J.; Hu, Y.; Li, S.; Huang, J.; Xu, J.; Wu, S.; Lin, J.; et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J. Exp. Clin. Cancer Res. 2018, 37, 127. [Google Scholar] [CrossRef]
- Shah, F.H.; Kim, S.J. Identification of medicinal compounds as potential inhibitors for mutated isocitrate dehydrogenases against chondrosarcoma. Saudi J. Biol. Sci. 2022, 29, 161–167. [Google Scholar] [CrossRef]
- Smith, D.; Stewart, C.J.R.; Clarke, E.M.; Lose, F.; Davies, C.; Armes, J.; Obermair, A.; Brennan, D.; Webb, P.M.; Nagle, C.M.; et al. ER and PR expression and survival after endometrial cancer. Gynecol. Oncol. 2018, 148, 258–266. [Google Scholar] [CrossRef]
- Luo, L.; Xu, L.; Tang, L. The expression of ER, PR in endometrial cancer and analysis of their correlation with ERK signaling pathway. Cancer Biomark 2017, 21, 145–149. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, B.; Ye, Z.; Chen, S.; Yu, H.; Chen, C.; Zhang, X.; Zhou, K.; Zeng, J. Triple-high expression of phosphatase and tensin homolog (PTEN), estrogen receptor (ER) and progesterone receptor (PR) may predict favorable prognosis for patients with Type I endometrial carcinoma. J. Cancer 2020, 11, 1436–1445. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Tuominen, I.; Sousa, S.; Gerbens, F.; van Dijk-Bos, K.; Osinga, J.; Kooi, K.A.; Sanjabi, B.; Esendam, C.; Oliveira, C.; et al. New target genes in endometrial tumors show a role for the estrogen-receptor pathway in microsatellite-unstable cancers. Hum. Mutat. 2014, 35, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.J.; Hoivik, E.A.; Halle, M.K.; Taylor-Weiner, A.; Cherniack, A.D.; Berg, A.; Holst, F.; Zack, T.I.; Werner, H.M.; Staby, K.M.; et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat. Genet 2016, 48, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Flindris, S.; Katsoulas, N.; Goussia, A.; Lazaris, A.C.; Navrozoglou, I.; Paschopoulos, M.; Thymara, I. The expression of NRIP1 and LCOR in endometrioid endometrial cancer. In Vivo 2021, 35, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Guo, Y.; Yu, H.; Guo, T. RNA editing affects cis-regulatory elements and predicts adverse cancer survival. Cancer Med. 2021, 10, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Z.; Shang, X.; Tian, D.; Wang, D.; Wu, K.; Fan, D.; Xia, L. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology 2015, 61, 1920–1933. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.M.; Oliemuller, E.; Howard, B.A. Regulatory roles for SOX11 in development, stem cells and cancer. Semin. Cancer Biol. 2020, 67, 3–11. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A Magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic Therapy. Adv. Mater. 2018, 30, e1706090. [Google Scholar] [CrossRef]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An ultrasound activated vesicle of janus Au-MnO nanoparticles for promoted tumor penetration and sono-chemodynamic therapy of orthotopic liver cancer. Angew Chem. Int. Ed. Engl. 2020, 59, 1682–1688. [Google Scholar] [CrossRef]
- Chen, P.; Yang, Y.; Zhang, Y.; Jiang, S.; Li, X.; Wan, J. Identification of prognostic immune-related genes in the tumor microenvironment of endometrial cancer. Aging 2020, 12, 3371–3387. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Kiyotani, K.; Yew, P.Y.; Sato, S.; Imai, Y.; Yamaguchi, R.; Miyano, S.; Fujiwara, K.; Hasegawa, K.; Nakamura, Y. Clinical significance of T cell clonality and expression levels of immune-related genes in endometrial cancer. Oncol. Rep. 2017, 37, 2603–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.V.; Shen, Z.; Rodriguez-Garcia, M.; Usherwood, E.J.; Tafe, L.J.; Wira, C.R. Endometrial cancer suppresses CD8+ T Cell-mediated cytotoxicity in postmenopausal women. Front. Immunol. 2021, 12, 657326. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, G.; Biswas, S.; Anadon, C.M.; Yu, X.; Gatenbee, C.D.; Prabhakaran, S.; Payne, K.K.; Chaurio, R.A.; Martin, A.; Innamarato, P.; et al. IgA-dominated humoral immune responses govern patients’ outcome in endometrial cancer. Cancer Res. 2022, 82, 859–871. [Google Scholar] [CrossRef]
- Yu, K.; Ravoor, A.; Malats, N.; Pineda, S.; Sirota, M. A pan-cancer analysis of tumor-infiltrating B cell repertoires. Front. Immunol. 2021, 12, 790119. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Su, H.; Xu, L.; Wang, Y.; Su, R.; Zhang, Z.; Guan, G.; Li, Z. Different co-culture models reveal the pivotal role of TBBPA-promoted M2 macrophage polarization in the deterioration of endometrial cancer. J. Hazard Mater. 2021, 413, 125337. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Chang, W.C.; Li, C.H.; Huang, S.C.; Chang, D.Y.; Chou, L.Y.; Sheu, B.C. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer 2010, 116, 5777–5788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.S.; Weil, R.; Dovey, Z.; Davis, A.; Tewari, A.K. The tumor microenvironment and immunotherapy in prostate and bladder cancer. Urol. Clin. North Am. 2020, 47, e17–e54. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, R.; Ni, S.; Cai, L.; Yang, S.; Shao, F.; Bai, J. Pyroptosis-related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with UCEC. Mol. Nucleic. Acids 2022, 27, 1036–1055. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Marchetti, C.; Tombolini, V.; Panici, P.B. Immune check-point in endometrial cancer. Int. J. Clin. Oncol. 2019, 24, 910–916. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Hu, Y.; Huang, W. Landscape of immune microenvironment under immune cell infiltration pattern in breast cancer. Front. Immunol. 2021, 12, 711433. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, S.; Wu, Z.; Jiang, Y.; Wan, Y.; Li, S.; Meng, H.; Zhou, S.; Cheng, W. Exploration of a novel prognostic risk signatures and immune checkpoint molecules in endometrial carcinoma microenvironment. Genomics 2020, 112, 3117–3134. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Lu, H.; Ju, D.D.; Yang, G.D.; Zhu, L.Y.; Yang, X.M.; Li, J.; Song, W.W.; Wang, J.H.; Zhang, C.C.; Zhang, Z.G.; et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019, 40, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Brandmaier, A.; Hou, S.Q.; Demaria, S.; Formenti, S.C.; Shen, W.H. PTEN at the interface of immune tolerance and tumor suppression. Front. Biol. 2017, 12, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Liang, B. PTEN mutation: A potential prognostic factor associated with immune infiltration in endometrial carcinoma. Pathol. Res. Pract. 2020, 216, 152943. [Google Scholar] [CrossRef]
- Pan, Y.; Jia, L.P.; Liu, Y.; Han, Y.; Deng, Q. Alteration of tumor associated neutrophils by PIK3CA expression in endometrial carcinoma from TCGA data. J. Ovarian Res. 2019, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.W.; Ellenson, L.H. Molecular genetics of endometrial carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, J.T.; Brady, M.F.; Homesley, H.D.; Malfetano, J.; DuBeshter, B.; Burger, R.A.; Liao, S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A gynecologic oncology group study. J. Clin. Oncol. 2004, 22, 3902–3908. [Google Scholar] [CrossRef]
- Long, H.J., 3rd; Nelimark, R.A.; Podratz, K.C.; Suman, V.; Keeney, G.L.; Nikcevich, D.A.; Kugler, J.W.; Rowland, K.M., Jr.; Kardinal, C.G.; Wos, E.J. Phase III comparison of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) vs. doxorubicin and cisplatin (AC) in women with advanced primary or recurrent metastatic carcinoma of the uterine endometrium. Gynecol. Oncol. 2006, 100, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Sun, W.; Shen, N.; Huang, X.; Fu, S. Identification of a metabolism-related gene expression prognostic model in endometrial carcinoma patients. BMC Cancer 2020, 20, 864. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Liang, Y.; Qin, S.; Zhu, Y.; Liu, J.; Yao, S. A Cholesterol homeostasis-related gene signature predicts prognosis of endometrial cancer and correlates with immune infiltration. Front. Genet. 2021, 12, 763537. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, X.; Li, B. Expression profile of epithelial-mesenchymal transition-related genes as a prognostic biomarker for endometrial cancer. J. Cancer 2021, 12, 6484–6496. [Google Scholar] [CrossRef]
- Weijiao, Y.; Fuchun, L.; Mengjie, C.; Xiaoqing, Q.; Hao, L.; Yuan, L.; Desheng, Y. Immune infiltration and a ferroptosis-associated gene signature for predicting the prognosis of patients with endometrial cancer. Aging 2021, 13, 16713–16732. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, R.; Song, J.; Zhong, Z.; Ni, S.; Liu, W.; He, Z.; Gan, S.; Huang, Q.; Yu, H.; Bai, J.; et al. Crosstalk of Redox-Related Subtypes, Establishment of a Prognostic Model and Immune Responses in Endometrial Carcinoma. Cancers 2022, 14, 3383. https://doi.org/10.3390/cancers14143383
Geng R, Song J, Zhong Z, Ni S, Liu W, He Z, Gan S, Huang Q, Yu H, Bai J, et al. Crosstalk of Redox-Related Subtypes, Establishment of a Prognostic Model and Immune Responses in Endometrial Carcinoma. Cancers. 2022; 14(14):3383. https://doi.org/10.3390/cancers14143383
Chicago/Turabian StyleGeng, Rui, Jiahang Song, Zihang Zhong, Senmiao Ni, Wen Liu, Zhiqiang He, Shilin Gan, Qinghao Huang, Hao Yu, Jianling Bai, and et al. 2022. "Crosstalk of Redox-Related Subtypes, Establishment of a Prognostic Model and Immune Responses in Endometrial Carcinoma" Cancers 14, no. 14: 3383. https://doi.org/10.3390/cancers14143383
APA StyleGeng, R., Song, J., Zhong, Z., Ni, S., Liu, W., He, Z., Gan, S., Huang, Q., Yu, H., Bai, J., & Liu, J. (2022). Crosstalk of Redox-Related Subtypes, Establishment of a Prognostic Model and Immune Responses in Endometrial Carcinoma. Cancers, 14(14), 3383. https://doi.org/10.3390/cancers14143383





