Isolation of Circulating Tumor Cells from Seminal Fluid of Patients with Prostate Cancer Using Inertial Microfluidics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfluidic Device and Its Fabrication
2.2. Cell Culture
2.3. Proof-Of-Concept Experiments
2.4. Clinical Samples
2.5. Immunofluorescence Staining
2.6. Cell Enumeration and Data Analysis
3. Results
3.1. Recovery of CTCs from Spiked SF
3.2. Isolation of Putative CTCs from the Patients’ SF
3.3. Potential Diagnostic and Prognostic Value of the Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Gorin, M.A. First point-of-care PSA test for prostate cancer detection. Nat. Rev. Urol. 2019, 16, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Röthke, M.; Anastasiadis, A.; Lichy, M.; Werner, M.; Wagner, P.; Kruck, S.; Claussen, C.D.; Stenzl, A.; Schlemmer, H.-P.; Schilling, D. MRI-guided prostate biopsy detects clinically significant cancer: Analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J. Urol. 2012, 30, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Jolly, C.; Leshchiner, I.; Dentro, S.C.; Gonzalez, S.; Rosebrock, D.; Mitchell, T.J.; Rubanova, Y.; Anur, P.; Yu, K.; et al. The evolutionary history of 2,658 cancers. Nature 2020, 578, 122–128. [Google Scholar] [CrossRef]
- Shin, Y.; Qadir, H.A.; Aabakken, L.; Bergsland, J.; Balasingham, I. Automatic colon polyp detection using region based deep cnn and post learning approaches. IEEE Access 2018, 6, 40950–40962. [Google Scholar] [CrossRef]
- Loh, J.; Jovanovic, L.; Lehman, M.; Capp, A.; Pryor, D.; Harris, M.; Nelson, C.; Martin, J. Circulating tumor cell detection in high-risk non-metastatic prostate cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 2157–2162. [Google Scholar] [CrossRef]
- Pal, S.K.; He, M.; Wilson, T.; Liu, X.; Zhang, K.; Carmichael, C.; Torres, A.; Hernandez, S.; Lau, C.; Agarwal, N.J. Detection and phenotyping of circulating tumor cells in high-risk localized prostate cancer. Clin. Genitourin. Cancer 2015, 13, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, M.; Rack, B.; Horn, T.; Heck, M.M.; Eiber, M.; Kuebler, H.; Retz, M.; Gschwend, J.E.; Andergassen, U.; Nawroth, R. Detection of circulating tumor cells in locally advanced high-risk prostate cancer during neoadjuvant chemotherapy and radical prostatectomy. Anticancer Res. 2015, 35, 5679–5685. [Google Scholar]
- Meyer, C.P.; Pantel, K.; Tennstedt, P.; Stroelin, P.; Schlomm, T.; Heinzer, H.; Riethdorf, S.; Steuber, T. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 235.e11–235.e16. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Satoh, T.; Ishiyama, H.; Tabata, K.-I.; Takenaka, K.; Sekiguchi, A.; Nakamura, M.; Kitano, M.; Hayakawa, K.; Iwamura, M. Perioperative search for circulating tumor cells in patients undergoing prostate brachytherapy for clinically nonmetastatic prostate cancer. Int. J. Mol. Sci. 2017, 18, 128. [Google Scholar] [CrossRef]
- Kuske, A.; Gorges, T.M.; Tennstedt, P.; Tiebel, A.-K.; Pompe, R.; Preißer, F.; Prues, S.; Mazel, M.; Markou, A.; Lianidou, E. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 2016, 6, 39736. [Google Scholar] [CrossRef]
- Broncy, L.; Paterlini-Bréchot, P. Clinical impact of circulating tumor cells in patients with localized prostate cancer. Cells 2019, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Nickens, K.P.; Ali, A.; Scoggin, T.; Tan, S.H.; Ravindranath, L.; McLeod, D.G.; Dobi, A.; Tacha, D.; Sesterhenn, I.A.; Srivastava, S. Prostate cancer marker panel with single cell sensitivity in urine. Prostate 2015, 75, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Rhee, H.; Shaw, M.; Nagubadi, S.; Ray, V.; Guinan, P. Evaluation of cytologic techniques for diagnosis of prostate cancer. Urology 1983, 21, 417–420. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Razavi Bazaz, S.; Ding, L.; Kapitannikova, A.; Sayyadi, N.; Campbell, D.; Walsh, B.; Gillatt, D.; Ebrahimi Warkiani, M.; Zvyagin, A.V. Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics. Cancers 2020, 12, 81. [Google Scholar] [CrossRef]
- van Gils, M.P.; Hessels, D.; van Hooij, O.; Jannink, S.A.; Peelen, W.P.; Hanssen, S.L.; Witjes, J.A.; Cornel, E.B.; Karthaus, H.F.; Smits, G.A. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin. Cancer Res. 2007, 13, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, S.; Birker, I.L.; Smit, F.P.; Leyten, G.H.; de Reijke, T.M.; van Oort, I.M.; Mulders, P.F.; Jannink, S.A.; Schalken, J.A. Prostate cancer biomarker profiles in urinary sediments and exosomes. J. Urol. 2014, 191, 1132–1138. [Google Scholar] [CrossRef]
- Gardiner, R.; Samaratunga, M.; Gwynne, R.; Clague, A.; Seymour, G.; Lavin, M. Abnormal prostatic cells in ejaculates from men with prostatic cancer—A preliminary report. Br. J. Urol. 1996, 78, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Barren, R.J., III; Holmes, E.H.; Boynton, A.L.; Gregorakis, A.; Elgamal, A.A.A.; Cobb, O.E.; Wilson, C.L.; Ragde, H.; Murphy, G.P. Method for identifying prostate cells in semen using flow cytometry. Prostate 1998, 36, 181–188. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.; Bhagat, A.A.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef]
- Aya-Bonilla, C.A.; Marsavela, G.; Freeman, J.B.; Lomma, C.; Frank, M.H.; Khattak, M.A.; Meniawy, T.M.; Millward, M.; Warkiani, M.E.; Gray, E.S. Isolation and detection of circulating tumour cells from metastatic melanoma patients using a slanted spiral microfluidic device. Oncotarget 2017, 8, 67355. [Google Scholar] [CrossRef]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.; Bhagat, A.A.; Irwin, D.; Lau, D.P.; Lim, A.S.; Lim, K.H.; Krisna, S.S.; Lim, W.T.; et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer 2017, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Tran, T.H.P.; Blick, T.; O’Byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.; Chaudhuri, P.K.; Tan, D.S.; Lim, W.T.; Lee, S.C.; Chen, P.C.; et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.-W.; Bhagat, A.A.S.; Lim, W.-T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, Z.; Smith, K.; Kuczler, M.D.; Reyes, D.; Amend, S.R.; Cho, Y.K.; Xue, W.; Pienta, K.J. The combination of size-based separation and selection-free technology provides higher circulating tumour cells detection sensitivity than either method alone in patients with metastatic prostate cancer. BJU Int. 2020, 126, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Panteleakou, Z.; Lembessis, P.; Sourla, A.; Pissimissis, N.; Polyzos, A.; Deliveliotis, C.; Koutsilieris, M. Detection of circulating tumor cells in prostate cancer patients: Methodological pitfalls and clinical relevance. Mol. Med. 2009, 15, 101–114. [Google Scholar] [CrossRef]
- Santana, S.M.; Liu, H.; Bander, N.H.; Gleghorn, J.P.; Kirby, B.J. Immunocapture of prostate cancer cells by use of anti-PSMA antibodies in microdevices. Biomed. Microdevices 2012, 14, 401–407. [Google Scholar] [CrossRef]
- Truong, Q.; Justiniano, I.O.; Nocon, A.L.; Soon, J.T.; Wissmueller, S.; Campbell, D.H.; Walsh, B.J. Glypican-1 as a biomarker for prostate cancer: Isolation and characterization. J. Cancer 2016, 7, 1002. [Google Scholar] [CrossRef][Green Version]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Todenhöfer, T.; Park, E.S.; Duffy, S.; Deng, X.; Jin, C.; Abdi, H.; Ma, H.; Black, P.C. Microfluidic enrichment of circulating tumor cells in patients with clinically localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 483.e9–483.e16. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Lee, R.J.; Kalinich, M.; LiCausi, J.A.; Zheng, Y.; Chen, T.; Milner, J.D.; Emmons, E.; Ho, U.; Broderick, K. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. 2018, 8, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Razavi Bazaz, S.; Mashhadian, A.; Ehsani, A.; Saha, S.C.; Krüger, T.; Ebrahimi Warkiani, M. Computational inertial microfluidics: A review. Lab Chip 2020, 20, 1023–1048. [Google Scholar] [CrossRef] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, F.; An, Q.; Zhang, K.; Wang, X.; Xu, J.; Guo, Y.; Lu, W.; Liang, X.; Gu, Y. Sperm cryopreservation for male cancer patients: More than 10 years of experience, in Beijing China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3256. [Google Scholar] [CrossRef]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.D.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Maas, M.; Hegemann, M.; Rausch, S.; Bedke, J.; Stenzl, A.; Todenhöfer, T.J. Circulating tumor cells and their role in prostate cancer. Asian J. Androl. 2019, 21, 24. [Google Scholar]
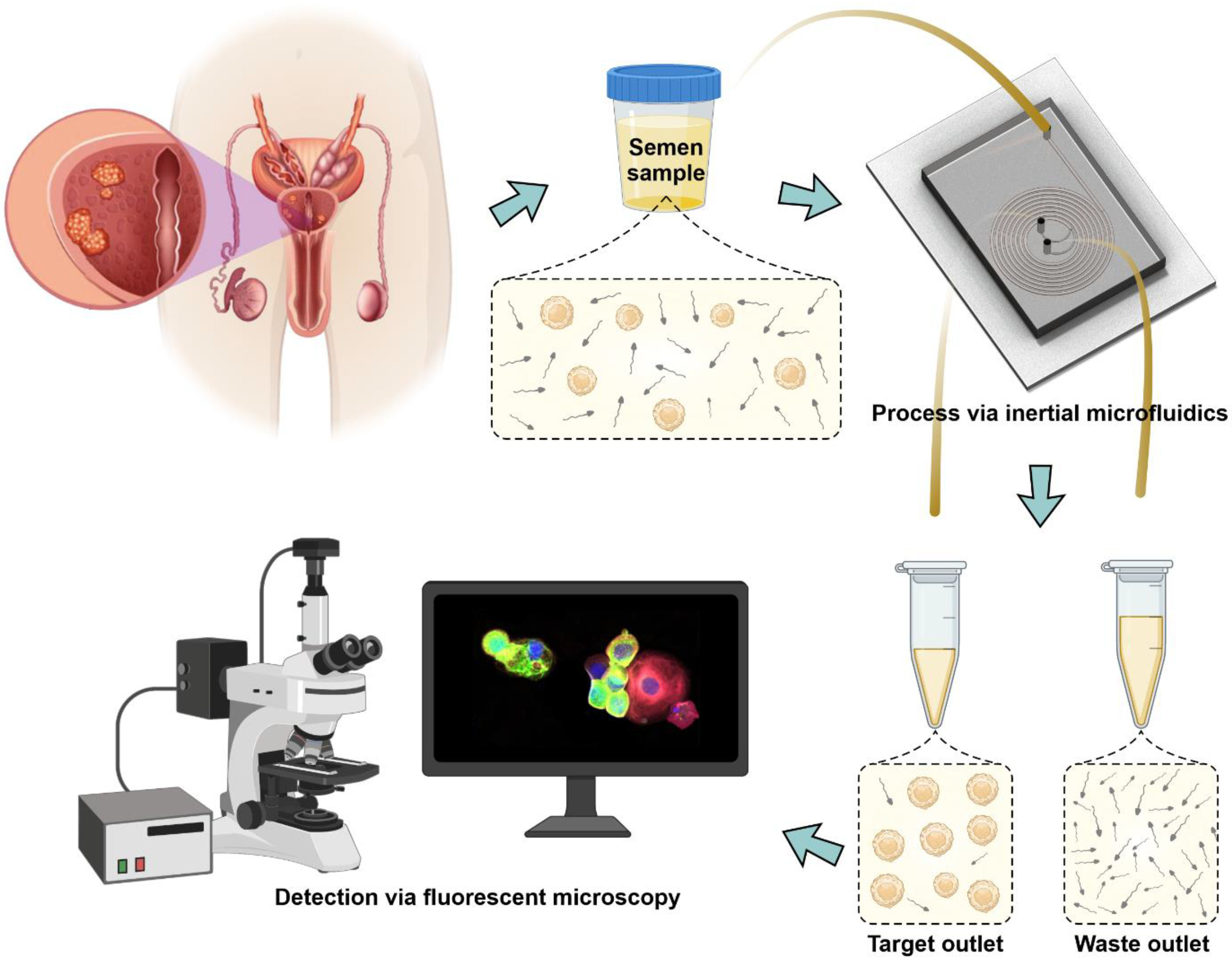
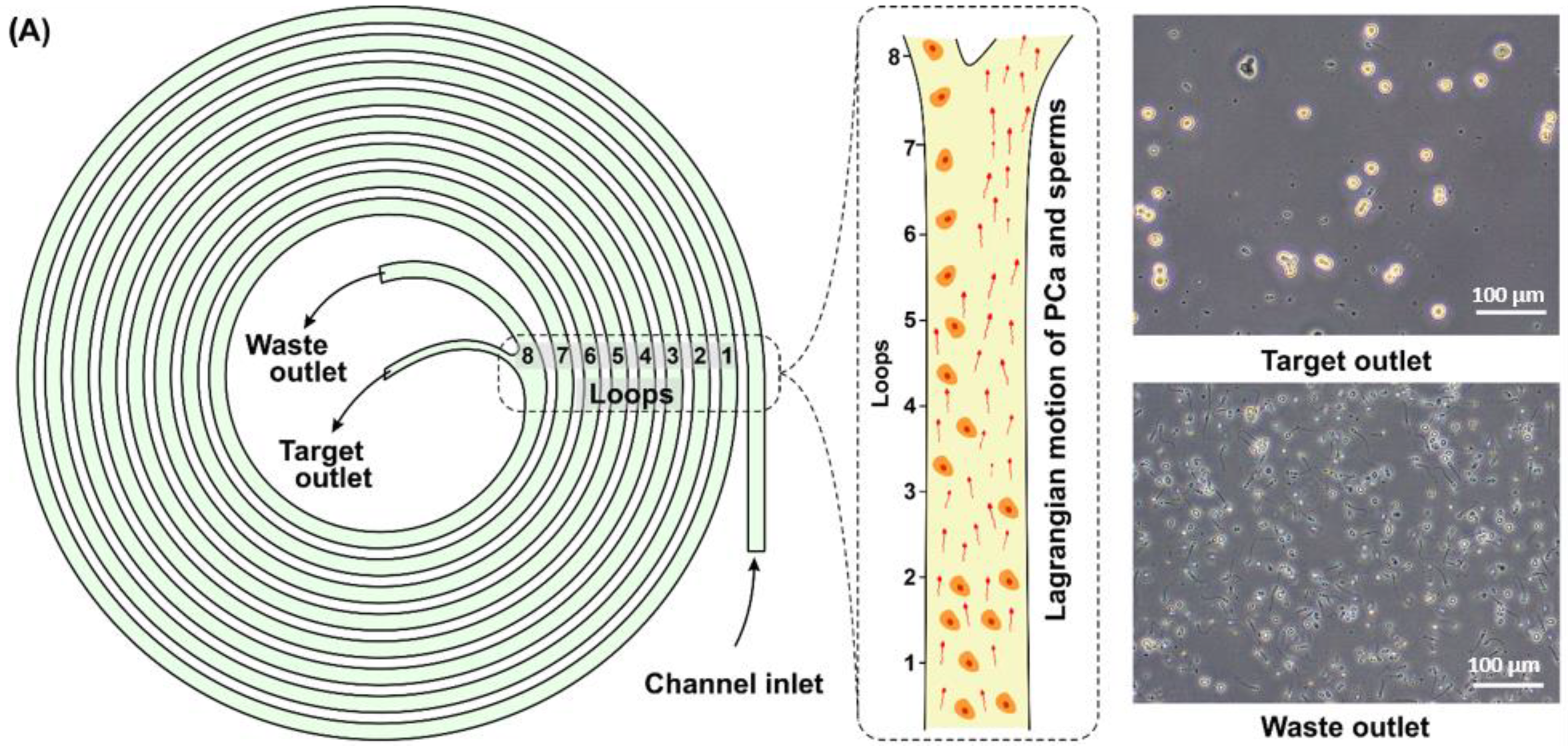





| Patient Number | Age | Tumor Localization (Zone/Lobe) | n * | V ** | n/V | TNM Stage | Gleason Score | PSA Level |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Peripheral /right | 217 | 2.1 | 103.3 | T1cN0M0 | 7 | 10.8 |
| 2 | 69 | Peripheral/right | 321 | 1.6 | 200.6 | T2cN0M0 | 9 | 23 |
| 3 | 56 | Peripheral/left | 238 | 2.3 | 103.5 | T2cN0M0 | 7 | 8.5 |
| 4 | 61 | Peripheral/left and right | 289 | 3.5 | 82.6 | T2cN0M0 | 6 | 6.3 |
| 5 | 51 | Central | 460 | 1.5 | 306.7 | T1bN0M0 | 8 | 19 |
| 6 | 56 | Peripheral/right | 183 | 1.9 | 96.3 | T1cN0M0 | 7 | 6.5 |
| 7 | 51 | Peripheral/right | 174 | 2.6 | 66.9 | T2bN0M0 | 7 | 16 |
| 8 | 59 | Transitory/left | 357 | 3 | 119.0 | T2bN0M0 | 8 | 32.4 |
| 9 | 62 | Peripheral/left | 187 | 2.7 | 69.3 | T1cN0M0 | 7 | 4.1 |
| 10 | 50 | Peripheral/right | 63 | 0.5 | 126 | T1cN0M0 | 7 | 8.9 |
| 11 | 67 | Peripheral/left | 145 | 1.9 | 76.3 | T1cN0M0 | 6 | 2.3 |
| 12 | 70 | Transitory/left | 329 | 1.4 | 235 | T1cN0M0 | 7 | 14.7 |
| 13 | 63 | Peripheral/left | 115 | 1.6 | 71.9 | T1cN0M0 | 7 | 8.2 |
| 14 | 70 | Peripheral/left | 613 | 2.7 | 227 | T2cN0M0 | 10 | 10.2 |
| 15 | 72 | Peripheral/right | 340 | 2.4 | 141.7 | T2cN0M0 | 7 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzhevskiy, A.S.; Kapitannikova, A.Y.; Vasilescu, S.A.; Karashaeva, T.A.; Razavi Bazaz, S.; Taratkin, M.S.; Enikeev, D.V.; Lekarev, V.Y.; Shpot, E.V.; Butnaru, D.V.; et al. Isolation of Circulating Tumor Cells from Seminal Fluid of Patients with Prostate Cancer Using Inertial Microfluidics. Cancers 2022, 14, 3364. https://doi.org/10.3390/cancers14143364
Rzhevskiy AS, Kapitannikova AY, Vasilescu SA, Karashaeva TA, Razavi Bazaz S, Taratkin MS, Enikeev DV, Lekarev VY, Shpot EV, Butnaru DV, et al. Isolation of Circulating Tumor Cells from Seminal Fluid of Patients with Prostate Cancer Using Inertial Microfluidics. Cancers. 2022; 14(14):3364. https://doi.org/10.3390/cancers14143364
Chicago/Turabian StyleRzhevskiy, Alexey S., Alina Y. Kapitannikova, Steven A. Vasilescu, Tamilla A. Karashaeva, Sajad Razavi Bazaz, Mark S. Taratkin, Dmitry V. Enikeev, Vladimir Y. Lekarev, Evgeniy V. Shpot, Denis V. Butnaru, and et al. 2022. "Isolation of Circulating Tumor Cells from Seminal Fluid of Patients with Prostate Cancer Using Inertial Microfluidics" Cancers 14, no. 14: 3364. https://doi.org/10.3390/cancers14143364
APA StyleRzhevskiy, A. S., Kapitannikova, A. Y., Vasilescu, S. A., Karashaeva, T. A., Razavi Bazaz, S., Taratkin, M. S., Enikeev, D. V., Lekarev, V. Y., Shpot, E. V., Butnaru, D. V., Deyev, S. M., Thiery, J. P., Zvyagin, A. V., & Ebrahimi Warkiani, M. (2022). Isolation of Circulating Tumor Cells from Seminal Fluid of Patients with Prostate Cancer Using Inertial Microfluidics. Cancers, 14(14), 3364. https://doi.org/10.3390/cancers14143364








