Non-Canonical Programmed Cell Death in Colon Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Mitotic Catastrophe and Colon Cancer
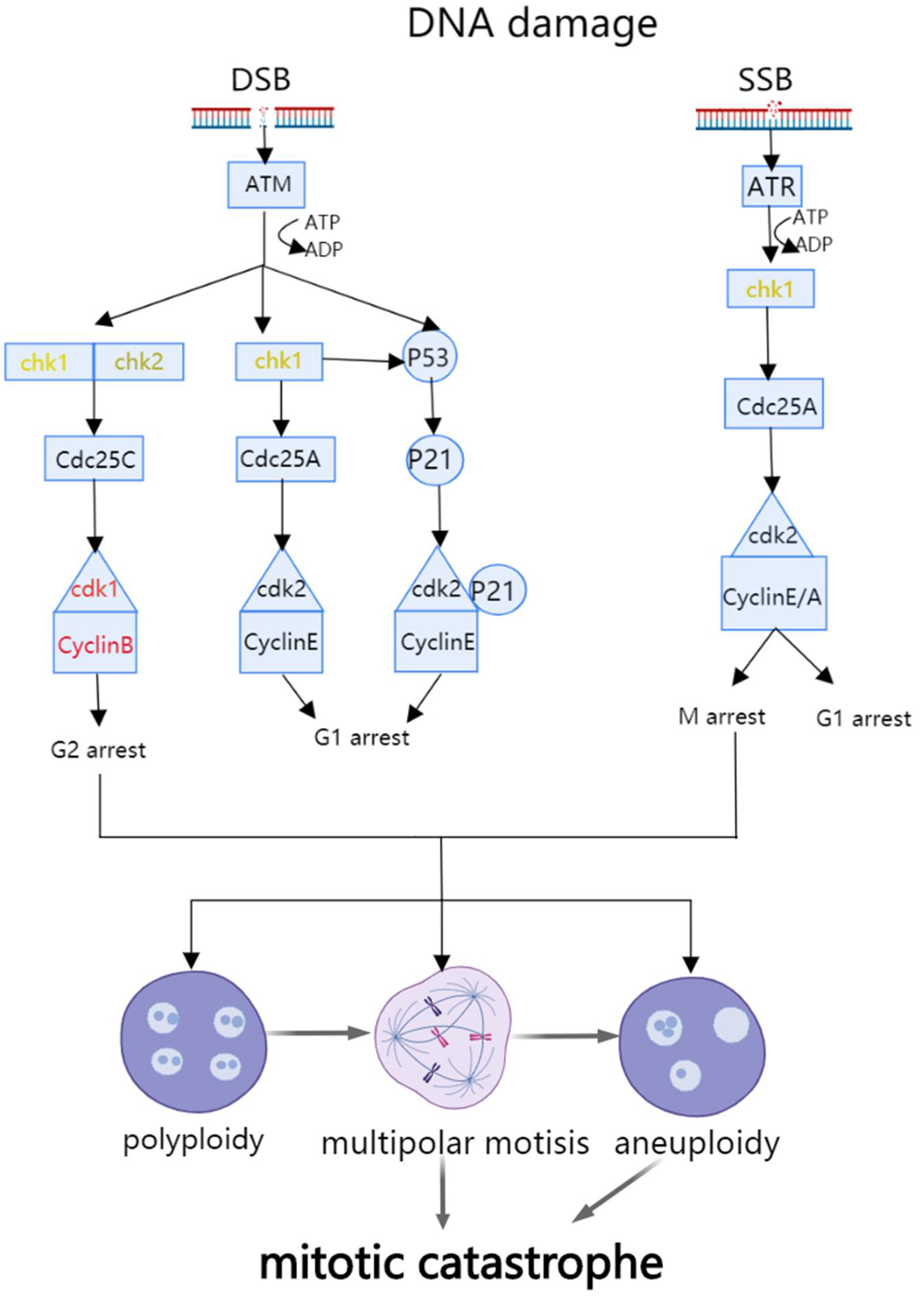
2.1. The Mechanism of Mitotic Catastrophe
2.2. The Relationship of Mitotic Catastrophe with Initiation and Progression of Colon Cancer
2.3. Anti-Tumor Treatments Inducing Mitotic Catastrophe
2.4. Prevention of Colon Cancer by Mitotic Catastrophe
3. Ferroptosis and Colon Cancer
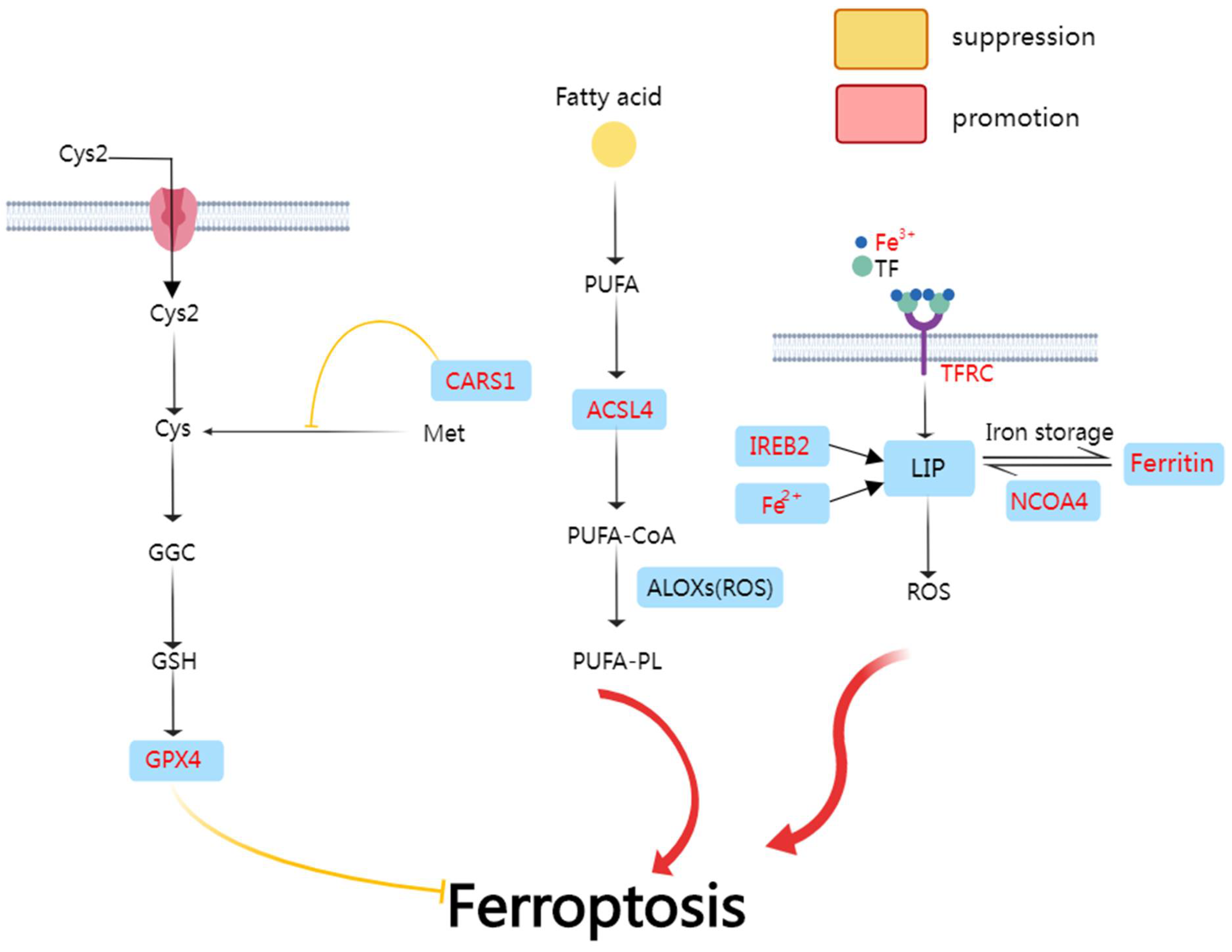
3.1. The Mechanism of Ferroptosis
3.2. Ferroptosis-Related Genes and RNAs in Colon Cancer
3.3. Anti-Tumor Treatments Inducing Ferroptosis in Colon Cancer
3.3.1. Anti-Tumor Treatments Inducing Ferroptosis
3.3.2. Ferroptosis Activators
3.3.3. The Role of Ferroptosis in Tumor Vaccines
3.4. Contradiction of NCOA4 Effect
4. Pyroptosis and Colon Cancer
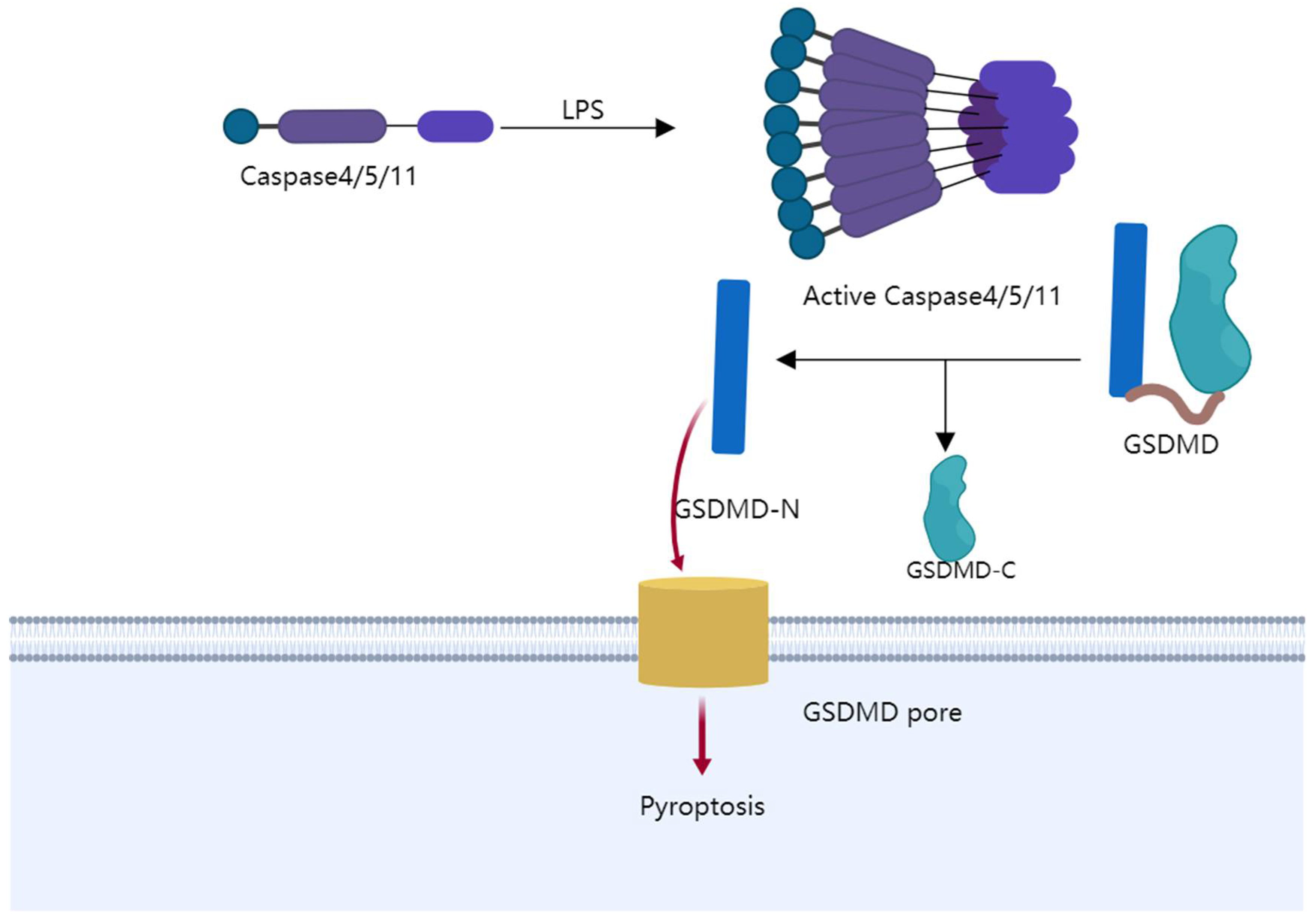
4.1. The Mechanism of Pyroptosis
4.2. Pyroptosis-Related Genes in Colon Cancer
4.3. Anti-Tumor Treatments Inducing Pyroptosis
4.4. Prevention of Colon Cancer by Inhibiting Pyroptosis
4.5. The Role of Caspase-2 in Pyroptosis of Colon Cancer
5. Necroptosis and Colon Cancer
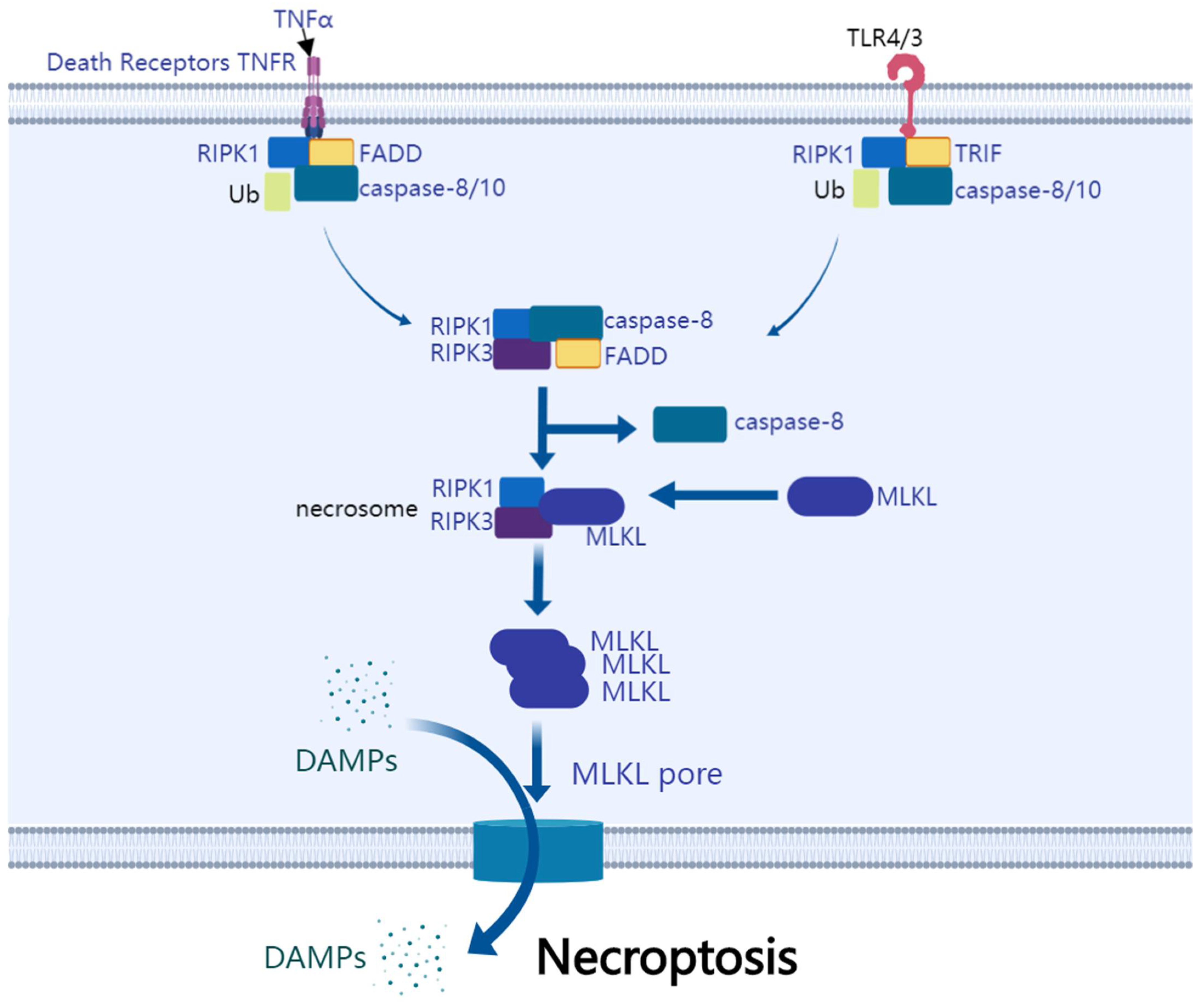
5.1. The Mechanism of Necroptosis
5.2. Necroptosis-Related DNA and RNA of Colon Cancer
5.3. Anti-Tumor Treatments Inducing Necroptosis
5.4. Prevention of Colon Cancer by Inhibiting Necroptosis
5.5. Relationship of Necroptosis with Colon Cancer Prognosis
6. Parthanatos and Colon Cancer
6.1. The Mechanism of Parthanatos
6.2. Anti-Tumor Treatments Inducing Parthanatos
7. Oxeiptosis and Colon Cancer
8. NETosis and Colon Cancer
9. PANoptosis Inhibits Colon Cancer
10. Entosis and Colon Cancer
11. Summary and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gibellini, L.; Moro, L. Programmed Cell Death in Health and Disease. Cells 2021, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, P.; Deng, G.; Han, Y.; Chen, Y.; Cai, C.; Shen, H.; Deng, G.; Zeng, S. Honokiol induces ferroptosis in colon cancer cells by regulating GPX4 activity. Am. J. Cancer Res. 2021, 11, 3039–3054. [Google Scholar] [PubMed]
- Moriwaki, K.; Bertin, J.; Gough, P.J.; Orlowski, G.M.; Chan, F.K. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015, 6, e1636. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, J.; Guo, J. Development and validation of a five-immune gene prognostic risk model in colon cancer. BMC Cancer 2020, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Shen, B.; Sun, X.F. Chromogranin-A Expression as a Novel Biomarker for Early Diagnosis of Colon Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2919. [Google Scholar] [CrossRef] [PubMed]
- Nfonsam, V.N.; Jecius, H.; Chen, D.; Omesiete, P.N.; Ewongwo, A.N.; Elquza, E.; Scott, A.J.; Jandova, J. Increasing Incidence of Colon Cancer in the Young: Assessing the Tumor Biology. J. Am. Coll. Surg. 2019, 229, 79–90. [Google Scholar] [CrossRef]
- Thaker, A.I.; Shaker, A.; Rao, M.S.; Ciorba, M.A. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J. Vis. Exp. 2012, e4100. [Google Scholar] [CrossRef]
- Sureban, S.M.; Ramalingam, S.; Natarajan, G.; May, R.; Subramaniam, D.; Bishnupuri, K.S.; Morrison, A.R.; Dieckgraefe, B.K.; Brackett, D.J.; Postier, R.G.; et al. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene 2008, 27, 4544–4556. [Google Scholar] [CrossRef]
- Jemaà, M.; Vitale, I.; Kepp, O.; Berardinelli, F.; Galluzzi, L.; Senovilla, L.; Mariño, G.; Malik, S.A.; Rello-Varona, S.; Lissa, D.; et al. Selective killing of p53-deficient cancer cells by SP600125. EMBO Mol. Med. 2012, 4, 500–514. [Google Scholar] [CrossRef]
- Andreassen, P.R.; Lacroix, F.B.; Lohez, O.D.; Margolis, R.L. Neither p21WAF1 nor 14-3-3sigma prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001, 61, 7660–7668. [Google Scholar]
- Sureban, S.M.; May, R.; George, R.J.; Dieckgraefe, B.K.; McLeod, H.L.; Ramalingam, S.; Bishnupuri, K.S.; Natarajan, G.; Anant, S.; Houchen, C.W. Knockdown of RNA binding protein musashi-1 leads to tumor regression in vivo. Gastroenterology 2008, 134, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Natarajan, G.; Schafer, C.; Subramaniam, D.; May, R.; Ramachandran, I.; Queimado, L.; Houchen, C.W.; Anant, S. Novel intestinal splice variants of RNA-binding protein CUGBP2: Isoform-specific effects on mitotic catastrophe. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G971–G981. [Google Scholar] [CrossRef] [PubMed]
- Bravo-San Pedro, J.M.; Kepp, O.; Sauvat, A.; Rello-Varona, S.; Kroemer, G.; Senovilla, L. Clonogenic Assays to Detect Cell Fate in Mitotic Catastrophe. Methods Mol. Biol. 2021, 2267, 227–239. [Google Scholar] [CrossRef]
- Castedo, M.; Perfettini, J.L.; Roumier, T.; Valent, A.; Raslova, H.; Yakushijin, K.; Horne, D.; Feunteun, J.; Lenoir, G.; Medema, R.; et al. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 2004, 23, 4362–4370. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Sorokina, I.V.; Gogvadze, V.; Zhivotovsky, B. Mitotic catastrophe and cancer drug resistance: A link that must to be broken. Drug Resist. Updat. 2016, 24, 1–12. [Google Scholar] [CrossRef]
- Ramalingam, S.; Ramamoorthy, P.; Subramaniam, D.; Anant, S. Reduced Expression of RNA Binding Protein CELF2, a Putative Tumor Suppressor Gene in Colon Cancer. Immuno-Gastroenterol. 2012, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Sun, L.; Shan, P.; Zhang, X.; Huan, J.; Zhang, X.; Li, D.; Wang, T.; Wei, T.; Zhang, X.; et al. Loss of KLF14 triggers centrosome amplification and tumorigenesis. Nat. Commun. 2015, 6, 8450. [Google Scholar] [CrossRef]
- Tu, S.P.; Cui, J.T.; Liston, P.; Huajiang, X.; Xu, R.; Lin, M.C.; Zhu, Y.B.; Zou, B.; Ng, S.S.; Jiang, S.H.; et al. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology 2005, 128, 361–375. [Google Scholar] [CrossRef]
- Sharma, D.; Malik, A.; Guy, C.S.; Karki, R.; Vogel, P.; Kanneganti, T.D. Pyrin Inflammasome Regulates Tight Junction Integrity to Restrict Colitis and Tumorigenesis. Gastroenterology 2018, 154, 948–964.e948. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Izdebska, M.; Gagat, M.; Grzanka, A. Overexpression of lamin B1 induces mitotic catastrophe in colon cancer LoVo cells and is associated with worse clinical outcomes. Int. J. Oncol. 2018, 52, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Karthikeyan, C.; Pathak, R.; Hussein, N.; Christman, R.; Robey, R.; Ashby, C.R., Jr.; Trivedi, P.; Malhotra, A.; Tiwari, A.K. Thienopyrimidine derivatives exert their anticancer efficacy via apoptosis induction, oxidative stress and mitotic catastrophe. Eur. J. Med. Chem. 2017, 138, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Králová, V.; Hanušová, V.; Rudolf, E.; Čáňová, K.; Skálová, L. Flubendazole induces mitotic catastrophe and senescence in colon cancer cells in vitro. J. Pharm. Pharmacol. 2016, 68, 208–218. [Google Scholar] [CrossRef]
- Alotaibi, M.R.; Asnake, B.; Di, X.; Beckman, M.J.; Durrant, D.; Simoni, D.; Baruchello, R.; Lee, R.M.; Schwartz, E.L.; Gewirtz, D.A. Stilbene 5c, a microtubule poison with vascular disrupting properties that induces multiple modes of growth arrest and cell death. Biochem. Pharmacol. 2013, 86, 1688–1698. [Google Scholar] [CrossRef]
- Hyzy, M.; Bozko, P.; Konopa, J.; Skladanowski, A. Antitumour imidazoacridone C-1311 induces cell death by mitotic catastrophe in human colon carcinoma cells. Biochem. Pharmacol. 2005, 69, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Sedic, M.; Poznic, M.; Gehrig, P.; Scott, M.; Schlapbach, R.; Hranjec, M.; Karminski-Zamola, G.; Pavelic, K.; Kraljevic Pavelic, S. Differential antiproliferative mechanisms of novel derivative of benzimidazo[1,2-alpha]quinoline in colon cancer cells depending on their p53 status. Mol. Cancer Ther. 2008, 7, 2121–2132. [Google Scholar] [CrossRef]
- Puig, P.E.; Guilly, M.N.; Bouchot, A.; Droin, N.; Cathelin, D.; Bouyer, F.; Favier, L.; Ghiringhelli, F.; Kroemer, G.; Solary, E.; et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008, 32, 1031–1043. [Google Scholar] [CrossRef]
- Jain, C.K.; Roychoudhury, S.; Majumder, H.K. Selective killing of G2 decatenation checkpoint defective colon cancer cells by catalytic topoisomerase II inhibitor. Biochim. Biophys. Actaiochim. Biophys. Acta 2015, 1853, 1195–1204. [Google Scholar] [CrossRef]
- Vié, N.; Copois, V.; Bascoul-Mollevi, C.; Denis, V.; Bec, N.; Robert, B.; Fraslon, C.; Conseiller, E.; Molina, F.; Larroque, C.; et al. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol. Cancer 2008, 7, 14. [Google Scholar] [CrossRef]
- Guo, X.; Dai, X.; Ni, J.; Ma, X.; Xue, J.; Wang, X. Geraniin Differentially Modulates Chromosome Stability of Colon Cancer and Noncancerous Cells by Oppositely Regulating their Spindle Assembly Checkpoint. Environ. Mol. Mutagenesis 2019, 60, 254–268. [Google Scholar] [CrossRef]
- VanderPorten, E.C.; Taverna, P.; Hogan, J.N.; Ballinger, M.D.; Flanagan, W.M.; Fucini, R.V. The Aurora kinase inhibitor SNS-314 shows broad therapeutic potential with chemotherapeutics and synergy with microtubule-targeted agents in a colon carcinoma model. Mol. Cancer Ther. 2009, 8, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Pisanti, S.; Grimaldi, C.; Izzo, A.A.; Borrelli, F.; Proto, M.C.; Malfitano, A.M.; Gazzerro, P.; Laezza, C.; Bifulco, M. Rimonabant inhibits human colon cancer cell growth and reduces the formation of precancerous lesions in the mouse colon. Int. J. Cancer 2009, 125, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Jemaà, M.; Galluzzi, L.; Kepp, O.; Boilève, A.; Lissa, D.; Senovilla, L.; Harper, F.; Pierron, G.; Berardinelli, F.; Antoccia, A.; et al. Preferential killing of p53-deficient cancer cells by reversine. Cell Cycle 2012, 11, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.N.; Schwartz, G.K. Potentiation of cytotoxicity of topoisomerase i poison by concurrent and sequential treatment with the checkpoint inhibitor UCN-01 involves disparate mechanisms resulting in either p53-independent clonogenic suppression or p53-dependent mitotic catastrophe. Cancer Res. 2004, 64, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xue, J.; Gu, W.Z.; Bui, M.; Li, G.; Tao, Z.F.; Lin, N.H.; Sowin, T.J.; Zhang, H. Cyclin B1 is an efficacy-predicting biomarker for Chk1 inhibitors. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2008, 13, 579–596. [Google Scholar] [CrossRef]
- Xiao, Z.; Xue, J.; Semizarov, D.; Sowin, T.J.; Rosenberg, S.H.; Zhang, H. Novel indication for cancer therapy: Chk1 inhibition sensitizes tumor cells to antimitotics. Int. J. Cancer 2005, 115, 528–538. [Google Scholar] [CrossRef]
- Castedo, M.; Perfettini, J.L.; Roumier, T.; Yakushijin, K.; Horne, D.; Medema, R.; Kroemer, G. The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene 2004, 23, 4353–4361. [Google Scholar] [CrossRef]
- Dewi, F.R.P.; Domoto, T.; Hazawa, M.; Kobayashi, A.; Douwaki, T.; Minamoto, T.; Wong, R.W. Colorectal cancer cells require glycogen synthase kinase-3β for sustaining mitosis via translocated promoter region (TPR)-dynein interaction. Oncotarget 2018, 9, 13337–13352. [Google Scholar] [CrossRef]
- Castiel, A.; Visochek, L.; Mittelman, L.; Dantzer, F.; Izraeli, S.; Cohen-Armon, M. A phenanthrene derived PARP inhibitor is an extra-centrosomes de-clustering agent exclusively eradicating human cancer cells. BMC Cancer 2011, 11, 412. [Google Scholar] [CrossRef]
- Lentini, L.; Amato, A.; Schillaci, T.; Insalaco, L.; Di Leonardo, A. Aurora-A transcriptional silencing and vincristine treatment show a synergistic effect in human tumor cells. Oncol. Res. 2008, 17, 115–125. [Google Scholar] [CrossRef]
- Nakahata, K.; Miyakoda, M.; Suzuki, K.; Kodama, S.; Watanabe, M. Heat shock induces centrosomal dysfunction, and causes non-apoptotic mitotic catastrophe in human tumour cells. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2002, 18, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Sonar, V.N.; Muthusamy, V.; Sasi, S.; Laszlo, A.; Sawani, J.; Horikoshi, N.; Higashikubo, R.; Bristow, R.G.; Borrelli, M.J.; et al. Novel chemical enhancers of heat shock increase thermal radiosensitization through a mitotic catastrophe pathway. Cancer Res. 2007, 67, 695–701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perletti, G.; Marras, E.; Dondi, D.; Osti, D.; Congiu, T.; Ferrarese, R.; de Eguileor, M.; Tashjian, A.H., Jr. p21(Waf1/Cip1) and p53 are downstream effectors of protein kinase C delta in tumor suppression and differentiation in human colon cancer cells. Int. J. Cancer 2005, 113, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zuazua-Villar, P.; Rodriguez, R.; Gagou, M.E.; Eyers, P.A.; Meuth, M. DNA replication stress in CHK1-depleted tumour cells triggers premature (S-phase) mitosis through inappropriate activation of Aurora kinase B. Cell Death Dis. 2014, 5, e1253. [Google Scholar] [CrossRef] [PubMed]
- Llovera, L.; Mansilla, S.; Portugal, J. Apoptotic-like death occurs through a caspase-independent route in colon carcinoma cells undergoing mitotic catastrophe. Cancer Lett. 2012, 326, 114–121. [Google Scholar] [CrossRef]
- Bataller, M.; Méndez, C.; Salas, J.A.; Portugal, J. Mithramycin SK modulates polyploidy and cell death in colon carcinoma cells. Mol. Cancer Ther. 2008, 7, 2988–2997. [Google Scholar] [CrossRef]
- Martini, E.; Schneider, E.; Neufert, C.; Neurath, M.F.; Becker, C. Survivin is a guardian of the intestinal stem cell niche and its expression is regulated by TGF-β. Cell Cycle 2016, 15, 2875–2881. [Google Scholar] [CrossRef]
- Xue, Z.; Sun, P.H.; Zhu, L.M.; Jiang, S.H.; Qiao, M.M.; Chi, A.L.; Tu, S.P. Adeno-associated virus-mediated survivin mutant Thr34Ala cooperates with oxaliplatin to inhibit tumor growth and angiogenesis in colon cancer. Oncol. Rep. 2011, 25, 1039–1046. [Google Scholar] [CrossRef][Green Version]
- Fujie, Y.; Yamamoto, H.; Ngan, C.Y.; Takagi, A.; Hayashi, T.; Suzuki, R.; Ezumi, K.; Takemasa, I.; Ikeda, M.; Sekimoto, M.; et al. Oxaliplatin, a potent inhibitor of survivin, enhances paclitaxel-induced apoptosis and mitotic catastrophe in colon cancer cells. Jpn. J. Clin. Oncol. 2005, 35, 453–463. [Google Scholar] [CrossRef]
- Hematulin, A.; Ingkaninan, K.; Limpeanchob, N.; Sagan, D. Ethanolic extract from Derris scandens Benth mediates radiosensitzation via two distinct modes of cell death in human colon cancer HT-29 cells. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 1871–1877. [Google Scholar] [CrossRef][Green Version]
- Riesterer, O.; Matsumoto, F.; Wang, L.; Pickett, J.; Molkentine, D.; Giri, U.; Milas, L.; Raju, U. A novel Chk inhibitor, XL-844, increases human cancer cell radiosensitivity through promotion of mitotic catastrophe. Investig. New Drugs 2011, 29, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Ramalingam, S.; Ramachandran, I.; May, R.; Queimado, L.; Houchen, C.W.; Anant, S. CUGBP2 downregulation by prostaglandin E2 protects colon cancer cells from radiation-induced mitotic catastrophe. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1235–G1244. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wuthrick, E.; Rappaport, J.A.; Kraft, C.; Lin, J.E.; Marszalowicz, G.; Snook, A.E.; Zhan, T.; Hyslop, T.M.; Waldman, S.A. GUCY2C Signaling Opposes the Acute Radiation-Induced GI Syndrome. Cancer Res. 2017, 77, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Boilève, A.; Senovilla, L.; Vitale, I.; Lissa, D.; Martins, I.; Métivier, D.; van den Brink, S.; Clevers, H.; Galluzzi, L.; Castedo, M.; et al. Immunosurveillance against tetraploidization-induced colon tumorigenesis. Cell Cycle 2013, 12, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Zhang, Y.; Wang, Z.; Wang, Z.; Li, Z.; Zhou, T.; Xu, H.; Liu, J.; Jiang, B.; Li, X.; et al. The deacetylation-phosphorylation regulation of SIRT2-SMC1A axis as a mechanism of antimitotic catastrophe in early tumorigenesis. Sci. Adv. 2021, 7, eabe5518. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Ye, S.; Xu, M.; Zhu, T.; Chen, J.; Shi, S.; Jiang, H.; Zheng, Q.; Liao, Q.; Ding, X.; Xi, Y. Cytoglobin promotes sensitivity to ferroptosis by regulating p53-YAP1 axis in colon cancer cells. J. Cell Mol. Med. 2021, 25, 3300–3311. [Google Scholar] [CrossRef]
- Ghoochani, A.; Hsu, E.C.; Aslan, M.; Rice, M.A.; Nguyen, H.M.; Brooks, J.D.; Corey, E.; Paulmurugan, R.; Stoyanova, T. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res. 2021, 81, 1583–1594. [Google Scholar] [CrossRef]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef]
- Angius, A.; Uva, P.; Pira, G.; Muroni, M.R.; Sotgiu, G.; Saderi, L.; Uleri, E.; Caocci, M.; Ibba, G.; Cesaraccio, M.R.; et al. Integrated Analysis of miRNA and mRNA Endorses a Twenty miRNAs Signature for Colorectal Carcinoma. Int. J. Mol. Sci. 2019, 20, 4067. [Google Scholar] [CrossRef]
- Zhu, H.; Klement, J.D.; Lu, C.; Redd, P.S.; Yang, D.; Smith, A.D.; Poschel, D.B.; Zou, J.; Liu, D.; Wang, P.G.; et al. Asah2 Represses the p53-Hmox1 Axis to Protect Myeloid-Derived Suppressor Cells from Ferroptosis. J. Immunol. 2021, 206, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kong, W.; Xie, Z. Expression and Prognostic Characteristics of Ferroptosis-Related Genes in Colon Cancer. Int. J. Mol. Sci. 2021, 22, 5652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, H.B.; Chen, Z.M.; Meng, L.; Xu, A.M. Identification of a ferroptosis-related gene signature predictive model in colon cancer. World J. Surg. Oncol. 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, R.; Lin, Y.; Yan, D.; Zuo, J.; Chen, J.; Shen, B. A Ferroptosis-Related Gene Signature Identified as a Novel Prognostic Biomarker for Colon Cancer. Front. Genet. 2021, 12, 692426. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Huang, T.; Jin, M.; Zheng, Z.; Zhang, H.; Ye, M.; Liu, K. Establishment of a novel ferroptosis-related lncRNA pair prognostic model in colon adenocarcinoma. Aging 2021, 13, 23072. [Google Scholar] [CrossRef]
- Cai, H.J.; Zhuang, Z.C.; Wu, Y.; Zhang, Y.Y.; Liu, X.; Zhuang, J.F.; Yang, Y.F.; Gao, Y.; Chen, B.; Guan, G.X. Development and validation of a ferroptosis-related lncRNAs prognosis signature in colon cancer. Bosn. J. Basic Med. Sci. 2021, 21, 569–576. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.; Kim, M.; Jin, E.J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim. Cells Syst. 2018, 22, 334–340. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Noulala, C.G.T.; Samba, A.R.M.; Tankeo, S.B.; Abdelfatah, S.; Fotso, G.W.; Happi, E.N.; Ngadjui, B.T.; Beng, V.P.; Kuete, V.; et al. The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem. Biol. Interact. 2021, 333, 109334. [Google Scholar] [CrossRef]
- Song, J.; Liu, T.; Yin, Y.; Zhao, W.; Lin, Z.; Yin, Y.; Lu, D.; You, F. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021, 22, e51162. [Google Scholar] [CrossRef]
- Shen, L.D.; Qi, W.H.; Bai, J.J.; Zuo, C.Y.; Bai, D.L.; Gao, W.D.; Zong, X.L.; Hao, T.T.; Ma, Y.; Cao, G.C. Resibufogenin inhibited colorectal cancer cell growth and tumorigenesis through triggering ferroptosis and ROS production mediated by GPX4 inactivation. Anat. Rec. 2021, 304, 313–322. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Qi, Y.; Wang, S.; Li, L.; Li, W.; Xie, T.; Zhu, H.; Tang, Z.; Zhou, M. H(2) S-Scavenged and Activated Iron Oxide-Hydroxide Nanospindles for MRI-Guided Photothermal Therapy and Ferroptosis in Colon Cancer. Small 2020, 16, e2001356. [Google Scholar] [CrossRef]
- Chaudhary, N.; Choudhary, B.S.; Shah, S.G.; Khapare, N.; Dwivedi, N.; Gaikwad, A.; Joshi, N.; Raichanna, J.; Basu, S.; Gurjar, M.; et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int. J. Cancer 2021, 149, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bu, D.; Zhu, J.; Yue, T.; Guo, S.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. Endogenous hydrogen sulfide regulates xCT stability through persulfidation of OTUB1 at cysteine 91 in colon cancer cells. Neoplasia 2021, 23, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.M.; Çelik, İ.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomed. Int. J. Phytother. Phytopharm. 2018, 43, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, D.H.; Jeong, S.Y.; Park, S.H.; Oh, S.C.; Park, Y.S.; Yu, J.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-inducing agents enhance TRAIL-induced apoptosis through upregulation of death receptor 5. J. Cell. Biochem. 2019, 120, 928–939. [Google Scholar] [CrossRef]
- Singhal, R.; Mitta, S.R.; Das, N.K.; Kerk, S.A.; Sajjakulnukit, P.; Solanki, S.; Andren, A.; Kumar, R.; Olive, K.P.; Banerjee, R.; et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J. Clin. Investig. 2021, 131, e143691. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Hou, Z.; Luan, W.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Novel antitumor compound optimized from natural saponin Albiziabioside A induced caspase-dependent apoptosis and ferroptosis as a p53 activator through the mitochondrial pathway. Eur. J. Med. Chem. 2018, 157, 759–772. [Google Scholar] [CrossRef]
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Hasan, M.; Reddy, S.M.; Das, N.K. Ferritinophagy is not required for colon cancer cell growth. Cell Biol. Int. 2020, 44, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeng, L.; Zhu, S.; Xie, Y.; Liu, J.; Wen, Q.; Cao, L.; Xie, M.; Ran, Q.; Kroemer, G.; et al. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe 2018, 24, 97–108.e104. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Erkes, D.A.; Cai, W.; Sanchez, I.M.; Purwin, T.J.; Rogers, C.; Field, C.O.; Berger, A.C.; Hartsough, E.J.; Rodeck, U.; Alnemri, E.S.; et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020, 10, 254–269. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.; Chen, J.; Zhi, F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef]
- Allen, I.C.; TeKippe, E.M.; Woodford, R.M.; Uronis, J.M.; Holl, E.K.; Rogers, A.B.; Herfarth, H.H.; Jobin, C.; Ting, J.P. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010, 207, 1045–1056. [Google Scholar] [CrossRef]
- Moon, S.W.; Son, H.J.; Mo, H.Y.; Yoo, N.J.; Lee, S.H. Somatic Mutation of NLRP Genes in Gastric and Colonic Cancers. Pathol. Oncol. Res. 2021, 27, 607385. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.; Qi, J.; Chen, Z.; Wu, Y.; Guo, J.; Wang, K.; Sun, X.; Zheng, J. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019, 10, 193. [Google Scholar] [CrossRef]
- Zhuang, Z.; Cai, H.; Lin, H.; Guan, B.; Wu, Y.; Zhang, Y.; Liu, X.; Zhuang, J.; Guan, G. Development and Validation of a Robust Pyroptosis-Related Signature for Predicting Prognosis and Immune Status in Patients with Colon Cancer. J. Oncol. 2021, 2021, 5818512. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, S.; Yu, G.; Guan, X.; Liu, H.; Quan, J.; Jiang, Z.; Wang, X. Deciphering the Pyroptosis-Related Prognostic Signature and Immune Cell Infiltration Characteristics of Colon Cancer. Front. Genet. 2021, 12, 755384. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, N.D.; Tuarita, M.Z.; Khatib, A.; Laila, F.; Sukarno, S. GC-MS metabolomics revealed protocatechuic acid as a cytotoxic and apoptosis-inducing compound from black rice brans. Food Sci. Biotechnol. 2020, 29, 825–835. [Google Scholar] [CrossRef]
- Ren, Q.; Yang, B.; Zhu, G.; Wang, S.; Fu, C.; Zhang, H.; Ross, R.P.; Stanton, C.; Chen, H.; Chen, W. Antiproliferation Activity and Mechanism of c9, t11, c15-CLNA and t9, t11, c15-CLNA from Lactobacillus plantarum ZS2058 on Colon Cancer Cells. Molecules 2020, 25, 1225. [Google Scholar] [CrossRef] [PubMed]
- Courtaut, F.; Derangère, V.; Chevriaux, A.; Ladoire, S.; Cotte, A.K.; Arnould, L.; Boidot, R.; Rialland, M.; Ghiringhelli, F.; Rébé, C. Liver X receptor ligand cytotoxicity in colon cancer cells and not in normal colon epithelial cells depends on LXRβ subcellular localization. Oncotarget 2015, 6, 26651–26662. [Google Scholar] [CrossRef]
- Derangère, V.; Chevriaux, A.; Courtaut, F.; Bruchard, M.; Berger, H.; Chalmin, F.; Causse, S.Z.; Limagne, E.; Végran, F.; Ladoire, S.; et al. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014, 21, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Rébé, C.; Derangère, V.; Ghiringhelli, F. Induction of pyroptosis in colon cancer cells by LXRβ. Mol. Cell. Oncol. 2015, 2, e970094. [Google Scholar] [CrossRef]
- Chen, C.; Wang, B.; Sun, J.; Na, H.; Chen, Z.; Zhu, Z.; Yan, L.; Ren, S.; Zuo, Y. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death Dis. 2015, 6, e1602. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, S.; Nakayama, S.; Xu, L.; Pilon, A.L.; Kimura, S. Secretoglobin 3A2 eliminates human cancer cells through pyroptosis. Cell Death Discov. 2021, 7, 12. [Google Scholar] [CrossRef]
- Serna, N.; Álamo, P.; Ramesh, P.; Vinokurova, D.; Sánchez-García, L.; Unzueta, U.; Gallardo, A.; Céspedes, M.V.; Vázquez, E.; Villaverde, A.; et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4(+) colorectal cancer stem cells. J. Control. Release Off. J. Control. Release Soc. 2020, 320, 96–104. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, J.; Mu, M.; Chen, Z.; Xu, Z.; Zhao, C.; Yang, K.; Qin, X.; Sun, X.; Yu, J. GW4064 enhances the chemosensitivity of colorectal cancer to oxaliplatin by inducing pyroptosis. Biochem. Biophys. Res. Commun. 2021, 548, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Liu, P.; Zhao, F.; Liu, J.; Hong, Z.; Ren, H.; Gu, G.; Wang, G.; Wu, X.; Zheng, T.; et al. STING-mediated Syk Signaling Attenuates Tumorigenesis of Colitis-associated Colorectal Cancer Through Enhancing Intestinal Epithelium Pyroptosis. Inflamm. Bowel Dis. 2021, 28, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Lai, L. Yu Shi An Chang Fang Ameliorates TNBS-Induced Colitis in Mice by Reducing Inflammatory Response and Protecting the Intestinal Mucosal Barrier. Evid. Based Complementary Altern. Med. Ecam 2021, 2021, 8870901. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Rut, W.; Groborz, K.; Snipas, S.J.; Salvesen, G.S.; Drag, M. Potent and selective caspase-2 inhibitor prevents MDM-2 cleavage in reversine-treated colon cancer cells. Cell Death Differ. 2019, 26, 2695–2709. [Google Scholar] [CrossRef]
- Hitomi, J.; Christofferson, D.E.; Ng, A.; Yao, J.; Degterev, A.; Xavier, R.J.; Yuan, J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008, 135, 1311–1323. [Google Scholar] [CrossRef]
- Chesnokov, M.; Khan, I.; Chefetz, I. Induction and Detection of Necroptotic Cell Death in Mammalian Cell Culture. Methods Mol. Biol. 2021, 2255, 119–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yu, D.; Zhu, X.; Liu, X.; Liao, J.; Li, S.; Wang, H. The MLKL kinase-like domain dimerization is an indispensable step of mammalian MLKL activation in necroptosis signaling. Cell Death Dis. 2021, 12, 638. [Google Scholar] [CrossRef]
- Najafov, A.; Chen, H.; Yuan, J. Necroptosis and Cancer. Trends Cancer 2017, 3, 294–301. [Google Scholar] [CrossRef]
- Le, D.T.; Jung, S.; Quynh, N.T.N.; Sandag, Z.; Lee, B.S.; Kim, S.; Lee, H.; Lee, H.; Lee, M.S. Inhibitory role of AMP-activated protein kinase in necroptosis of HCT116 colon cancer cells with p53 null mutation under nutrient starvation. Int. J. Oncol. 2019, 54, 702–712. [Google Scholar] [CrossRef]
- Grassilli, E.; Narloch, R.; Federzoni, E.; Ianzano, L.; Pisano, F.; Giovannoni, R.; Romano, G.; Masiero, L.; Leone, B.E.; Bonin, S.; et al. Inhibition of GSK3B bypass drug resistance of p53-null colon carcinomas by enabling necroptosis in response to chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3820–3831. [Google Scholar] [CrossRef]
- Grassilli, E.; Ianzano, L.; Bonomo, S.; Missaglia, C.; Cerrito, M.G.; Giovannoni, R.; Masiero, L.; Lavitrano, M. GSK3A is redundant with GSK3B in modulating drug resistance and chemotherapy-induced necroptosis. PLoS ONE 2014, 9, e100947. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, Y.; Zheng, K.; Zou, C.; Wu, H.; Wang, S.; Ou, L.; Wang, Y.; Huang, B.; Wang, X. Identification of the circRNA-miRNA-mRNA regulatory network of Hsp90 inhibitor-induced cell death in colorectal cancer by integrated analysis. Gene 2020, 727, 144232. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Saki, G.; Bavarsad, N.; Mombeini, M. Silymarin induces a multi-targeted cell death process in the human colon cancer cell line HT-29. Biomed. Pharmacother. 2017, 94, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Liccardi, G.; Ramos Garcia, L.; Tenev, T.; Annibaldi, A.; Legrand, A.J.; Robertson, D.; Feltham, R.; Anderton, H.; Darding, M.; Peltzer, N.; et al. RIPK1 and Caspase-8 Ensure Chromosome Stability Independently of Their Role in Cell Death and Inflammation. Mol. Cell 2019, 73, 413–428.e147. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, X.; Cui, W.; Wen, W.; Lu, F.; Sun, X.; Ma, D.; Yuan, Y.; Li, Z.; Hou, N.; et al. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca2+ Uptake and Promotes Colorectal Oncogenesis. Cancer Res. 2018, 78, 2876–2885. [Google Scholar] [CrossRef]
- Agarwalla, P.; Banerjee, R. N-end rule pathway inhibition assists colon tumor regression via necroptosis. Mol. Ther. Oncolytics 2016, 3, 16020. [Google Scholar] [CrossRef]
- Oliver Metzig, M.; Fuchs, D.; Tagscherer, K.E.; Gröne, H.J.; Schirmacher, P.; Roth, W. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-α-dependent necroptosis driven by RIP1 kinase and NF-κB. Oncogene 2016, 35, 3399–3409. [Google Scholar] [CrossRef]
- Bozec, D.; Iuga, A.C.; Roda, G.; Dahan, S.; Yeretssian, G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget 2016, 7, 46384–46400. [Google Scholar] [CrossRef]
- Mouratidis, P.X.; Rivens, I.; Ter Haar, G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int. J. Hyperth. 2015, 31, 476–488. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Liu, H.; Yang, C.; Jiang, A.; Wei, H.; Sun, D.; Cai, Z.; Zheng, Y. Versatile cationic liposomes for RIP3 overexpression in colon cancer therapy and RIP3 downregulation in acute pancreatitis therapy. J. Drug Target. 2020, 28, 627–642. [Google Scholar] [CrossRef]
- Hou, X.; Yang, C.; Zhang, L.; Hu, T.; Sun, D.; Cao, H.; Yang, F.; Guo, G.; Gong, C.; Zhang, X.; et al. Killing colon cancer cells through PCD pathways by a novel hyaluronic acid-modified shell-core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials 2017, 124, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Aredia, F.; Czaplinski, S.; Fulda, S.; Scovassi, A.I. Molecular features of the cytotoxicity of an NHE inhibitor: Evidence of mitochondrial alterations, ROS overproduction and DNA damage. BMC Cancer 2016, 16, 851. [Google Scholar] [CrossRef] [PubMed]
- Tortola, L.; Nitsch, R.; Bertrand, M.J.M.; Kogler, M.; Redouane, Y.; Kozieradzki, I.; Uribesalgo, I.; Fennell, L.M.; Daugaard, M.; Klug, H.; et al. The Tumor Suppressor Hace1 Is a Critical Regulator of TNFR1-Mediated Cell Fate. Cell Rep. 2016, 15, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- He, G.W.; Günther, C.; Thonn, V.; Yu, Y.Q.; Martini, E.; Buchen, B.; Neurath, M.F.; Stürzl, M.; Becker, C. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J. Exp. Med. 2017, 214, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Takemura, R.; Takaki, H.; Okada, S.; Shime, H.; Akazawa, T.; Oshiumi, H.; Matsumoto, M.; Teshima, T.; Seya, T. PolyI:C-Induced, TLR3/RIP3-Dependent Necroptosis Backs Up Immune Effector-Mediated Tumor Elimination In Vivo. Cancer Immunol. Res. 2015, 3, 902–914. [Google Scholar] [CrossRef]
- Kang, J.I.; Hong, J.Y.; Choi, J.S.; Lee, S.K. Columbianadin Inhibits Cell Proliferation by Inducing Apoptosis and Necroptosis in HCT116 Colon Cancer Cells. Biomol. Ther. 2016, 24, 320–327. [Google Scholar] [CrossRef]
- Cui, X.; Wang, R.; Wang, Z. Cationic peroxidase from proso millet induces human colon cancer cell necroptosis by regulating autocrine TNF-α and RIPK3 demethylation. Food Funct. 2018, 9, 1878–1888. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Li, M.; Liu, Y.; Han, Y.; Zhang, X.; Li, X.M.; Wu, X.; Qin, J.; Fang, J.; et al. MLKL attenuates colon inflammation and colitis-tumorigenesis via suppression of inflammatory responses. Cancer Lett. 2019, 459, 100–111. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Van Lint, S.; Roose, K.; Van Parys, A.; Vandenabeele, P.; Grooten, J.; Tavernier, J.; De Koker, S.; Saelens, X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018, 9, 3417. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, L.; Lin, J.; Zhao, Y.; Zheng, Y. Cationic liposome co-encapsulation of SMAC mimetic and zVAD using a novel lipid bilayer fusion loaded with MLKL-pDNA for tumour inhibition in vivo. J. Drug Target. 2018, 26, 45–54. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Mei, L.; Ruan, J.; Hu, Q.; Peng, J.; Su, H.; Liao, H.; Liu, S.; Liu, W.; et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget 2016, 7, 22219–22233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wu, B.; Guo, Y.S.; Zhou, Y.H.; Fu, Z.G.; Xu, B.Q.; Li, J.H.; Jing, L.; Jiang, J.L.; Tang, J.; et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am. J. Cancer Res. 2015, 5, 3174–3185. [Google Scholar] [PubMed]
- Wang, H.Y.; Zhang, B. Cobalt chloride induces necroptosis in human colon cancer HT-29 cells. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Stephenson, D.J.; Chalfant, C.E.; Brown, R.E. Upregulation of human glycolipid transfer protein (GLTP) induces necroptosis in colon carcinoma cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158–167. [Google Scholar] [CrossRef]
- Cho, E.; Lee, J.K.; Park, E.; Seo, C.H.; Luchian, T.; Park, Y. Antitumor activity of HPA3P through RIPK3-dependent regulated necrotic cell death in colon cancer. Oncotarget 2018, 9, 7902–7917. [Google Scholar] [CrossRef]
- Sun, W.; Wu, X.; Gao, H.; Yu, J.; Zhao, W.; Lu, J.J.; Wang, J.; Du, G.; Chen, X. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free. Radic. Biol. Med. 2017, 108, 433–444. [Google Scholar] [CrossRef]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Ma, C.Y.; Xu, X.M.; Zhang, Y.Q.; Wang, C.G.; Jin, J.; Shen, X.; Gao, J.L.; Li, N.; et al. Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway. Br. J. Pharmacol. 2015, 172, 3929–3943. [Google Scholar] [CrossRef]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef]
- Gomes, A.R.; Pires, A.S.; Abrantes, A.M.; Gonçalves, A.C.; Costa, S.C.; Varela, C.L.; Silva, E.T.; Botelho, M.F.; Roleira, F.M.F. Design, synthesis, and antitumor activity evaluation of steroidal oximes. Bioorganic Med. Chem. 2021, 46, 116360. [Google Scholar] [CrossRef]
- Alvarez-Diaz, S.; Preaudet, A.; Samson, A.L.; Nguyen, P.M.; Fung, K.Y.; Garnham, A.L.; Alexander, W.S.; Strasser, A.; Ernst, M.; Putoczki, T.L.; et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2021, 28, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Guo, J.; Gao, B.; Zhang, W.; Ling, L.; Xu, T.; Pan, C.; Li, L.; Chen, S.; Wang, H.; et al. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci. Signal. 2019, 12, eaaw3423. [Google Scholar] [CrossRef] [PubMed]
- Härtel, H.; Theiß, J.; Abdelaziz, M.O.; Raftery, M.J.; Pecher, G.; Bogner, E. HCMV-Mediated Interference of Bortezomib-Induced Apoptosis in Colon Carcinoma Cell Line Caco-2. Viruses 2021, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Zou, X.; Lan, Y.; Sun, X.; Wang, X.; Zhao, S.; Jiang, C.; Liu, H. Mechanisms underlying 3-bromopyruvate-induced cell death in colon cancer. J. Bioenerg. Biomembr. 2015, 47, 319–329. [Google Scholar] [CrossRef]
- Aaes, T.L.; Verschuere, H.; Kaczmarek, A.; Heyndrickx, L.; Wiernicki, B.; Delrue, I.; De Craene, B.; Taminau, J.; Delvaeye, T.; Bertrand, M.J.M.; et al. Immunodominant AH1 Antigen-Deficient Necroptotic, but Not Apoptotic, Murine Cancer Cells Induce Antitumor Protection. J. Immunol. 2020, 204, 775–787. [Google Scholar] [CrossRef]
- Sun, L.; Moore, E.; Berman, R.; Clavijo, P.E.; Saleh, A.; Chen, Z.; Van Waes, C.; Davies, J.; Friedman, J.; Allen, C.T. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology 2018, 7, e1488359. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.D.; Lee, S.H.; Kwak, C.H.; Chang, Y.C.; Lee, Y.C.; Chung, T.W.; Magae, J.; Kim, C.H. Ascochlorin induces caspase-independent necroptosis in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 239, 111898. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; He, Q.; Liu, S.; He, A.; Yan, J. A pan-RAF inhibitor LY3009120 inhibits necroptosis by preventing phosphorylation of RIPK1 and alleviates dextran sulfate sodium-induced colitis. Clin. Sci. 2019, 133, 919–932. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zheng, M.; Li, Y.M.; Fan, X.Y.; Wang, J.C.; Li, Z.C.; Yang, H.J.; Yu, J.M.; Cui, J.; Jiang, J.L.; et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics 2019, 9, 3659–3673. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y.; Shi, L.; Li, W.; Chen, K.; Li, M.; Chen, X.; Zhang, H.; Li, T.; Matsuzawa-Ishimoto, Y.; et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J. Clin. Investig. 2020, 130, 2111–2128. [Google Scholar] [CrossRef]
- Seneviratne, D.; Ma, J.; Tan, X.; Kwon, Y.K.; Muhammad, E.; Melhem, M.; DeFrances, M.C.; Zarnegar, R. Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis. Gastroenterology 2015, 148, 181–191.e117. [Google Scholar] [CrossRef] [PubMed]
- Conev, N.V.; Dimitrova, E.G.; Bogdanova, M.K.; Kashlov, Y.K.; Chaushev, B.G.; Radanova, M.A.; Petrov, D.P.; Georgiev, K.D.; Bachvarov, C.H.; Todorov, G.N.; et al. RIPK3 expression as a potential predictive and prognostic marker in metastatic colon cancer. Clin. Investig. Medicine. Med. Clin. Exp. 2019, 42, E31–E38. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Ding, A.P.; Qi, W.W.; Zhang, P.H.; Lv, J.; Qiu, W.S.; Sun, Z.Q. Association of Mixed Lineage Kinase Domain-Like Protein Expression With Prognosis in Patients With Colon Cancer. Technol. Cancer Res. Treat. 2017, 16, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, S.; Chen, X.; Xu, H.; Tang, Y. Machine learning identifies two autophagy-related genes as markers of recurrence in colorectal cancer. J. Int. Med. Res. 2020, 48, 300060520958808. [Google Scholar] [CrossRef]
- Ma, D.; Lu, B.; Feng, C.; Wang, C.; Wang, Y.; Luo, T.; Feng, J.; Jia, H.; Chi, G.; Luo, Y.; et al. Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett. 2016, 371, 194–204. [Google Scholar] [CrossRef]
- Akhiani, A.A.; Werlenius, O.; Aurelius, J.; Movitz, C.; Martner, A.; Hellstrand, K.; Thorén, F.B. Role of the ERK pathway for oxidant-induced parthanatos in human lymphocytes. PLoS ONE 2014, 9, e89646. [Google Scholar] [CrossRef]
- Batnasan, E.; Xie, S.; Zhang, Q.; Li, Y. Observation of Parthanatos Involvement in Diminished Ovarian Reserve Patients and Melatonin’s Protective Function Through Inhibiting ADP-Ribose (PAR) Expression and Preventing AIF Translocation into the Nucleus. Reprod. Sci. 2020, 27, 75–86. [Google Scholar] [CrossRef]
- Donizy, P.; Halon, A.; Surowiak, P.; Pietrzyk, G.; Kozyra, C.; Matkowski, R. Correlation between PARP-1 immunoreactivity and cytomorphological features of parthanatos, a specific cellular death in breast cancer cells. Eur. J. Histochem. 2013, 57, e35. [Google Scholar] [CrossRef][Green Version]
- Dinhof, C.; Pirker, C.; Kroiss, P.; Kirchhofer, D.; Gabler, L.; Gojo, J.; Lötsch-Gojo, D.; Stojanovic, M.; Timelthaler, G.; Ferk, F.; et al. p53 Loss Mediates Hypersensitivity to ETS Transcription Factor Inhibition Based on PARylation-Mediated Cell Death Induction. Cancers 2020, 12, 3205. [Google Scholar] [CrossRef]
- Chiu, L.Y.; Ho, F.M.; Shiah, S.G.; Chang, Y.; Lin, W.W. Oxidative stress initiates DNA damager MNNG-induced poly(ADP-ribose)polymerase-1-dependent parthanatos cell death. Biochem. Pharmacol. 2011, 81, 459–470. [Google Scholar] [CrossRef]
- Aredia, F.; Giansanti, V.; Mazzini, G.; Savio, M.; Ortiz, L.M.; Jaadane, I.; Zaffaroni, N.; Forlino, A.; Torriglia, A.; Scovassi, A.I. Multiple effects of the Na+/H+ antiporter inhibitor HMA on cancer cells. Apoptosis Int. J. Program. Cell Death 2013, 18, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Quaas, R.; Hilger, I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Rayes, R.F.; Mouhanna, J.G.; Nicolau, I.; Bourdeau, F.; Giannias, B.; Rousseau, S.; Quail, D.; Walsh, L.; Sangwan, V.; Bertos, N.; et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 2019, 5, e128008. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Lee, E.; Banoth, B.; Malireddi, R.K.S.; Samir, P.; Tuladhar, S.; Mummareddy, H.; Burton, A.R.; Vogel, P.; et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight 2020, 4, e136720. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.D. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. ImmunoHorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007, 131, 966–979. [Google Scholar] [CrossRef]
- Bozkurt, E.; Düssmann, H.; Salvucci, M.; Cavanagh, B.L.; Van Schaeybroeck, S.; Longley, D.B.; Martin, S.J.; Prehn, J.H.M. TRAIL signaling promotes entosis in colorectal cancer. J. Cell Biol. 2021, 220, e202010030. [Google Scholar] [CrossRef]
- Snyder, A.G.; Oberst, A. The Antisocial Network: Cross Talk Between Cell Death Programs in Host Defense. Annu. Rev. Immunol. 2021, 39, 77–101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, B.; Zheng, B.; Xing, C.; Liu, J. Non-Canonical Programmed Cell Death in Colon Cancer. Cancers 2022, 14, 3309. https://doi.org/10.3390/cancers14143309
Pan B, Zheng B, Xing C, Liu J. Non-Canonical Programmed Cell Death in Colon Cancer. Cancers. 2022; 14(14):3309. https://doi.org/10.3390/cancers14143309
Chicago/Turabian StylePan, Bingchen, Bowen Zheng, Chengzhong Xing, and Jingwei Liu. 2022. "Non-Canonical Programmed Cell Death in Colon Cancer" Cancers 14, no. 14: 3309. https://doi.org/10.3390/cancers14143309
APA StylePan, B., Zheng, B., Xing, C., & Liu, J. (2022). Non-Canonical Programmed Cell Death in Colon Cancer. Cancers, 14(14), 3309. https://doi.org/10.3390/cancers14143309






