Immunoprotecting Effects of Exercise Program against Ovarian Cancer: A Single-Blind, Randomized Controlled Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
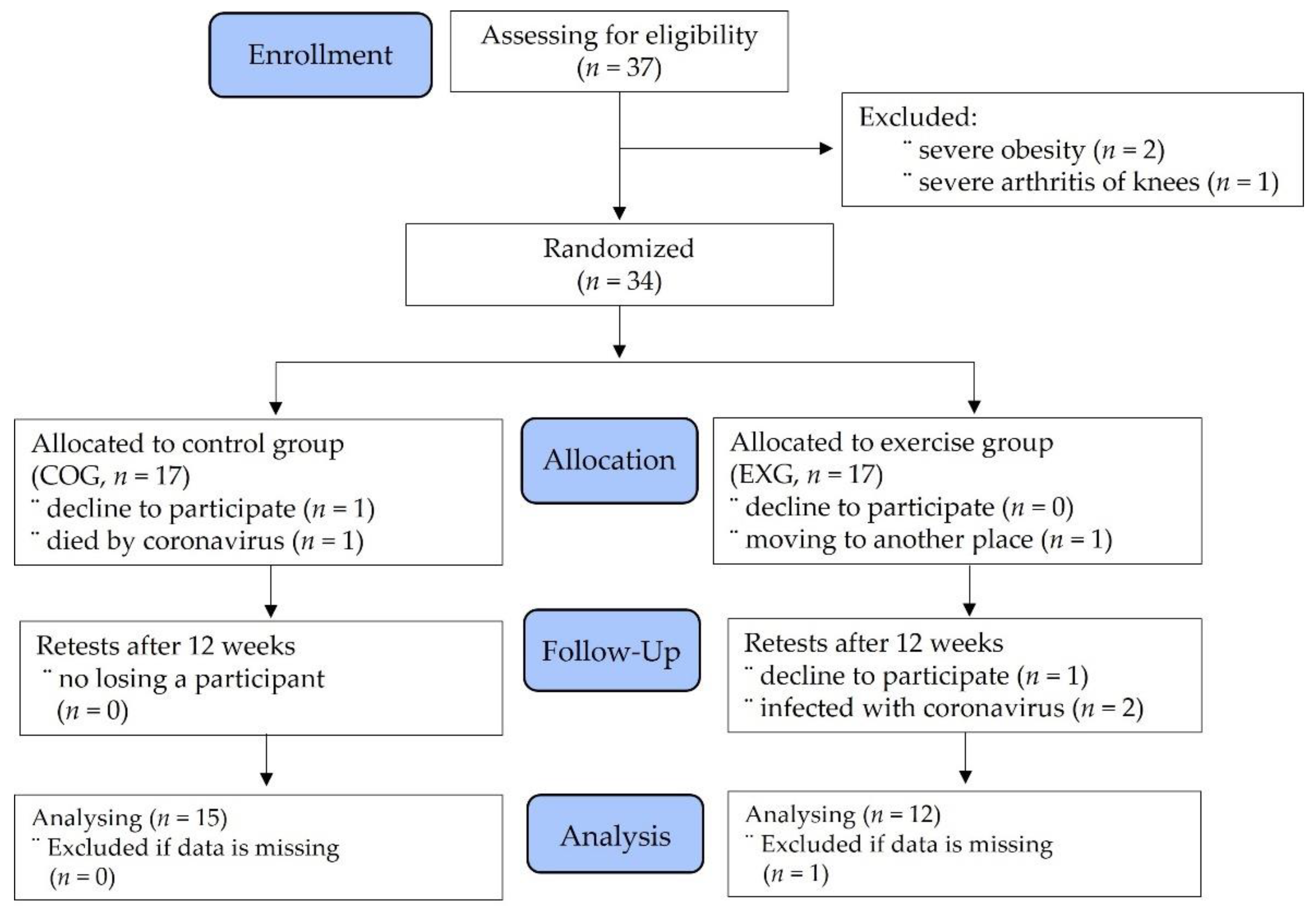
2.1. Participants
2.2. Experimental Design
2.3. Measurement Methods
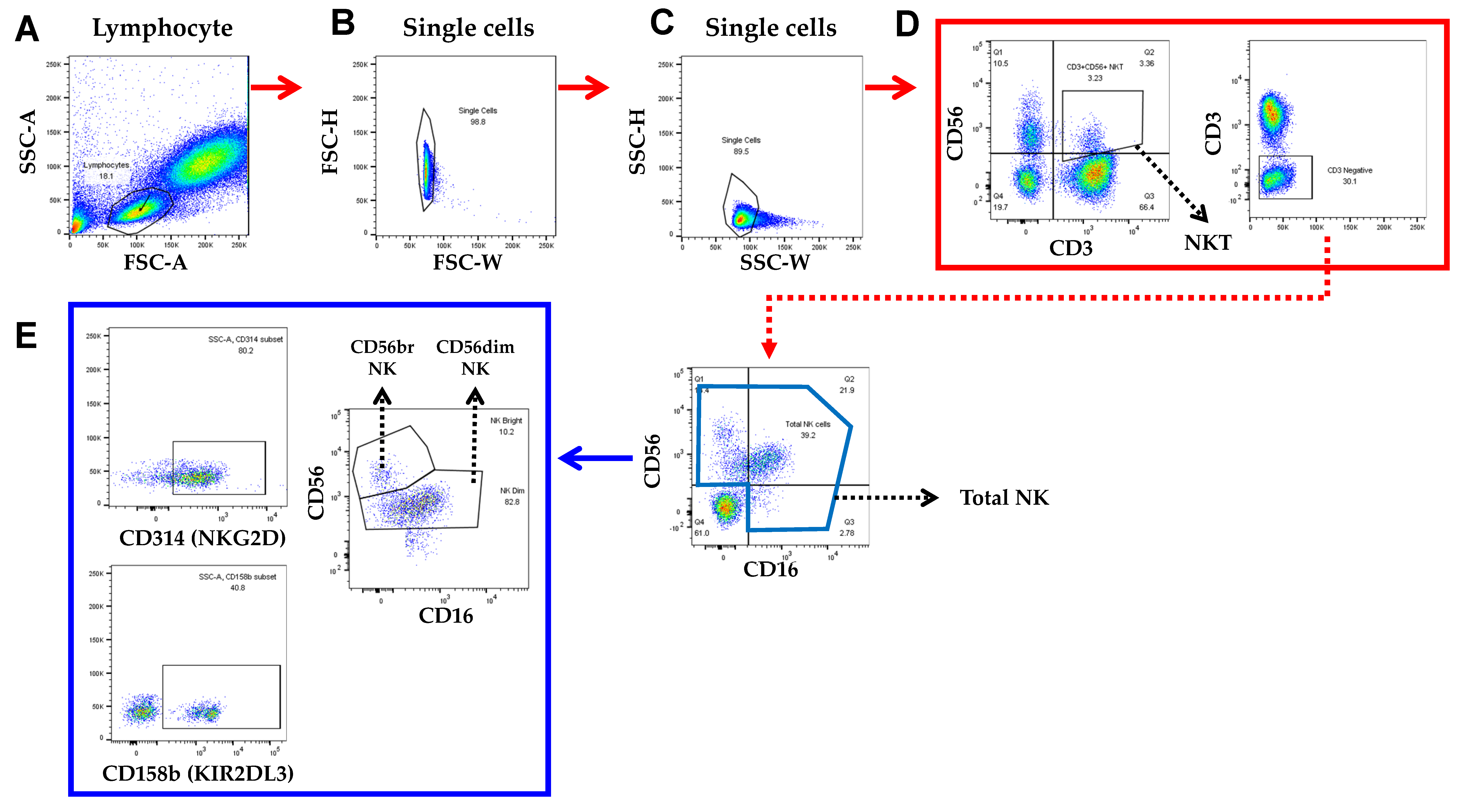
2.3.1. Blood Sample Collection and Measurements of NK Cell-Related Factors
2.3.2. Measurement of Physical Fitness
2.4. Regular Exercise Training Program
2.5. Sample Size Measure and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Similarity of Control Variables before and after the Experiment
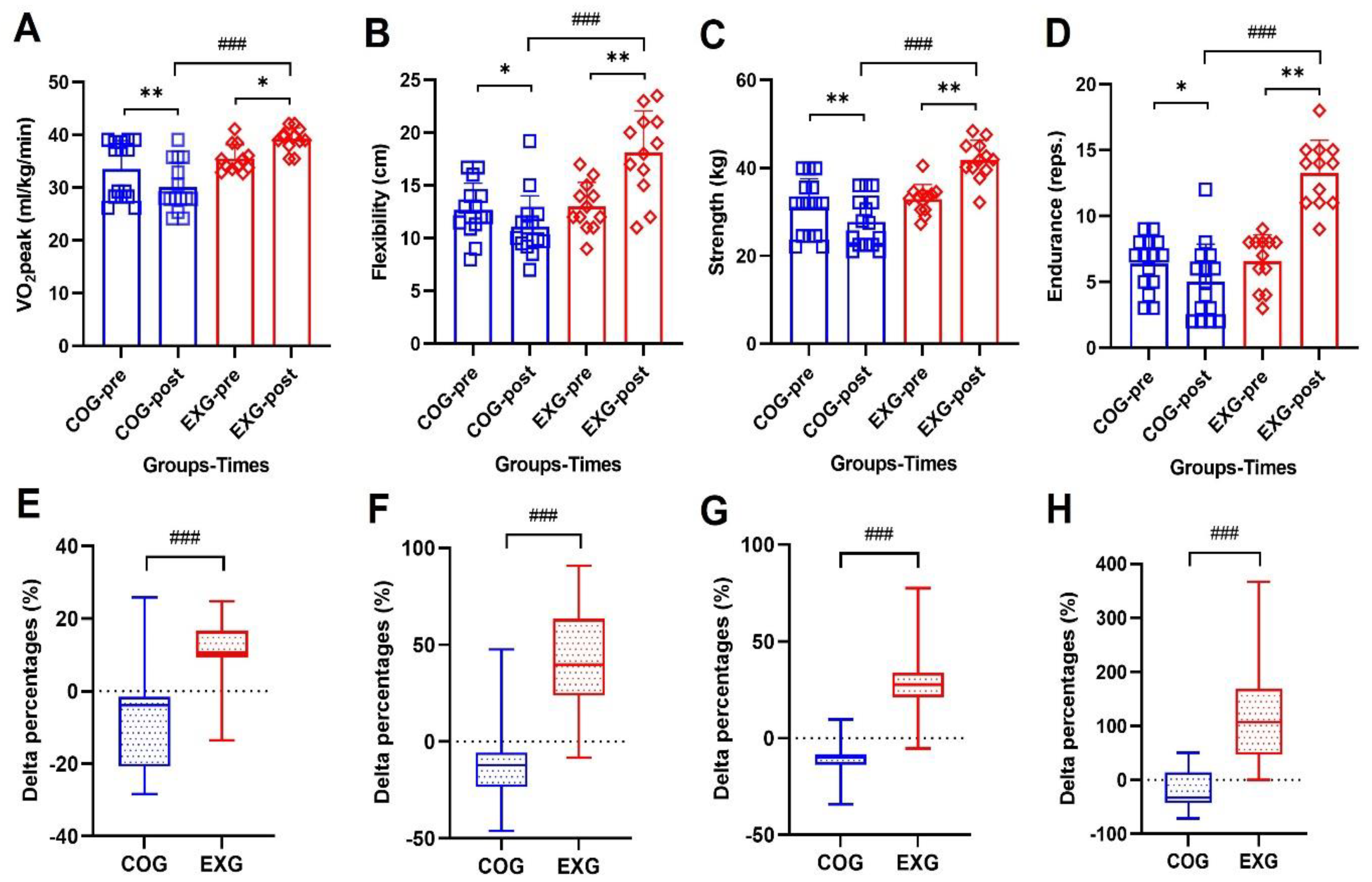
3.3. Positive Changes of Physical Fitness Levels
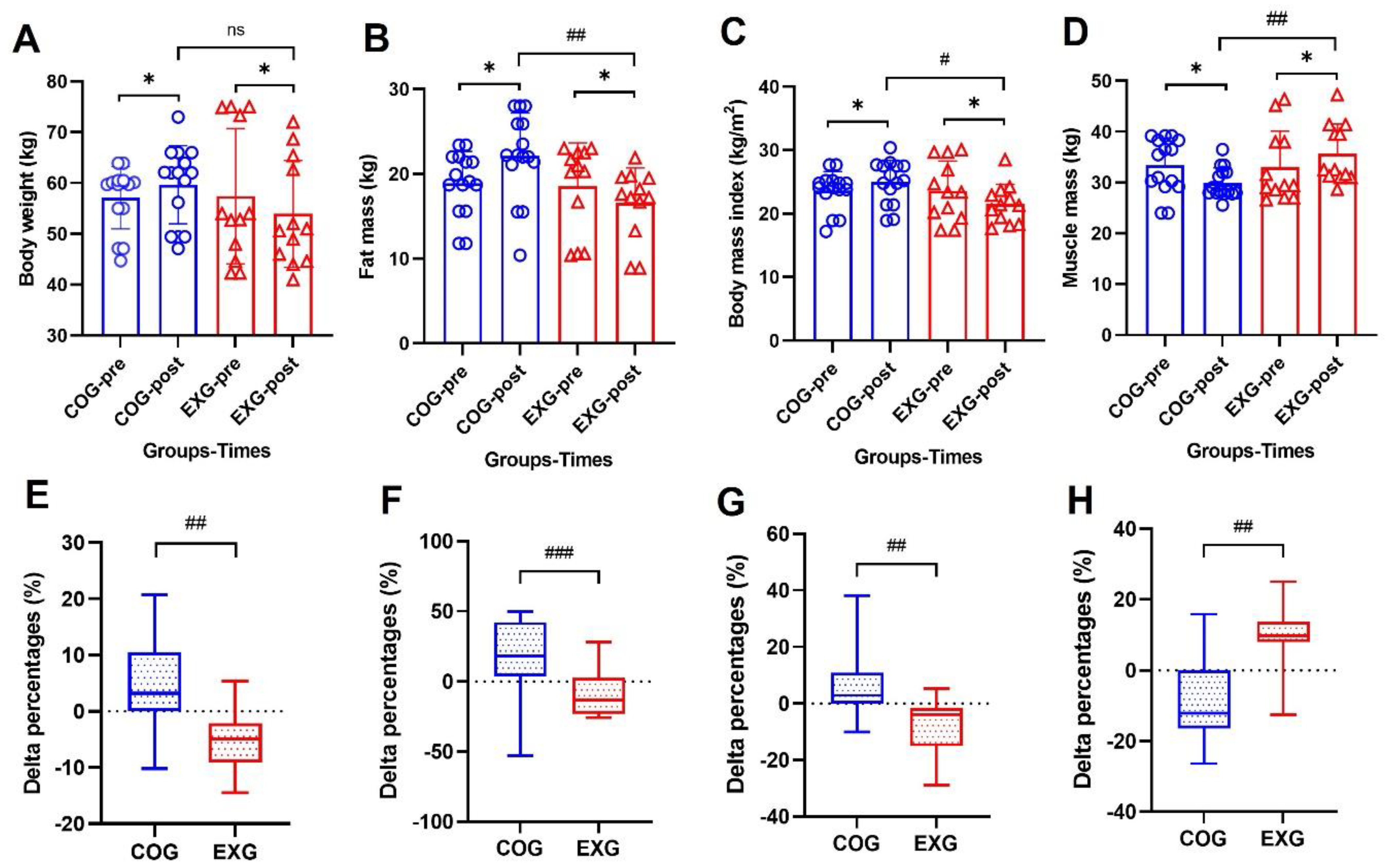
3.4. Body Composition Changes by a Decreased Fat Mass and by an Increased Muscle Mass
3.5. Positive Changes in Neutrophil and Lymphocyte Percentages by Exercise Training
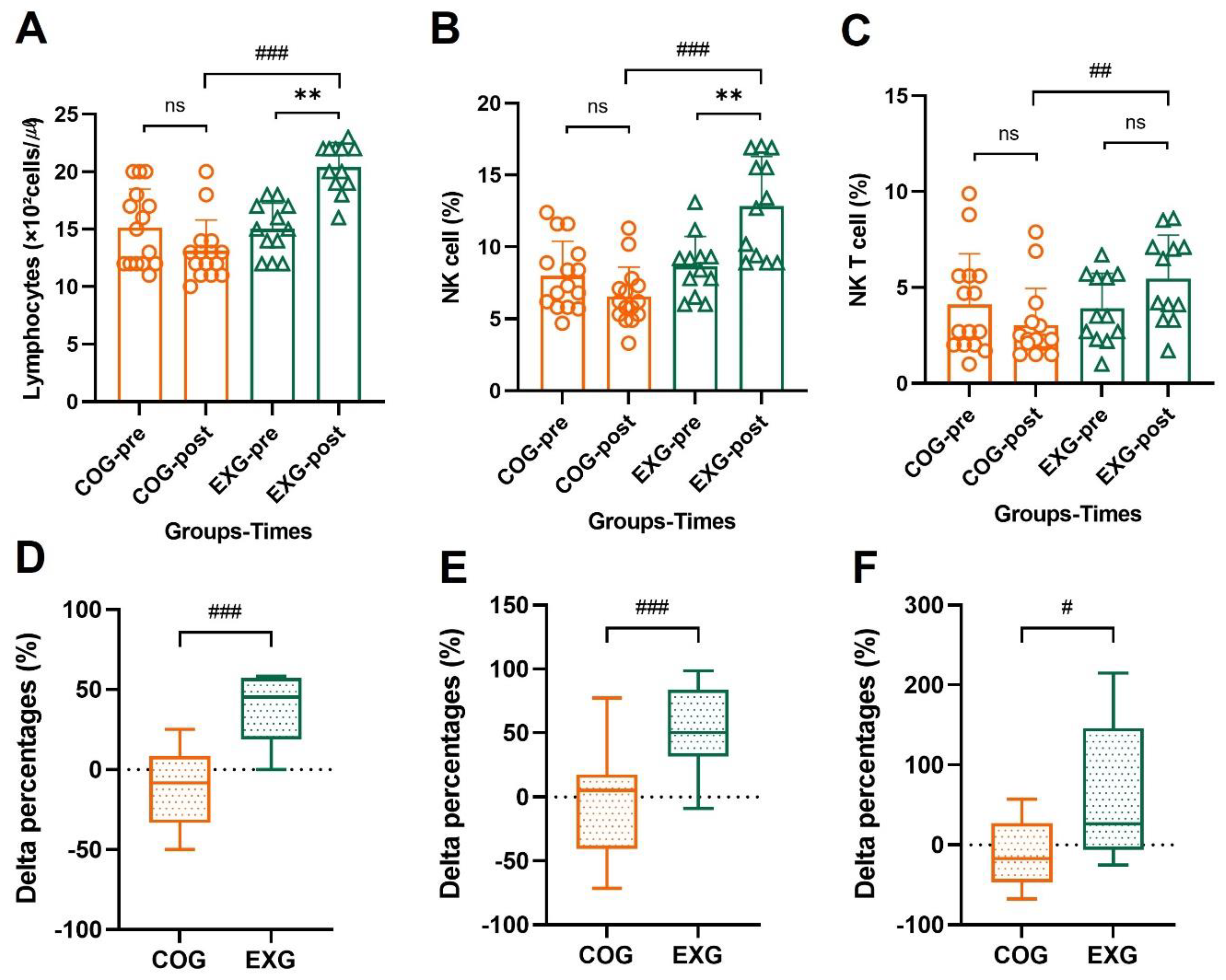
3.6. Differences and Changes in NK Cells and NKT Cells for Lymphocytes by Exercise Training
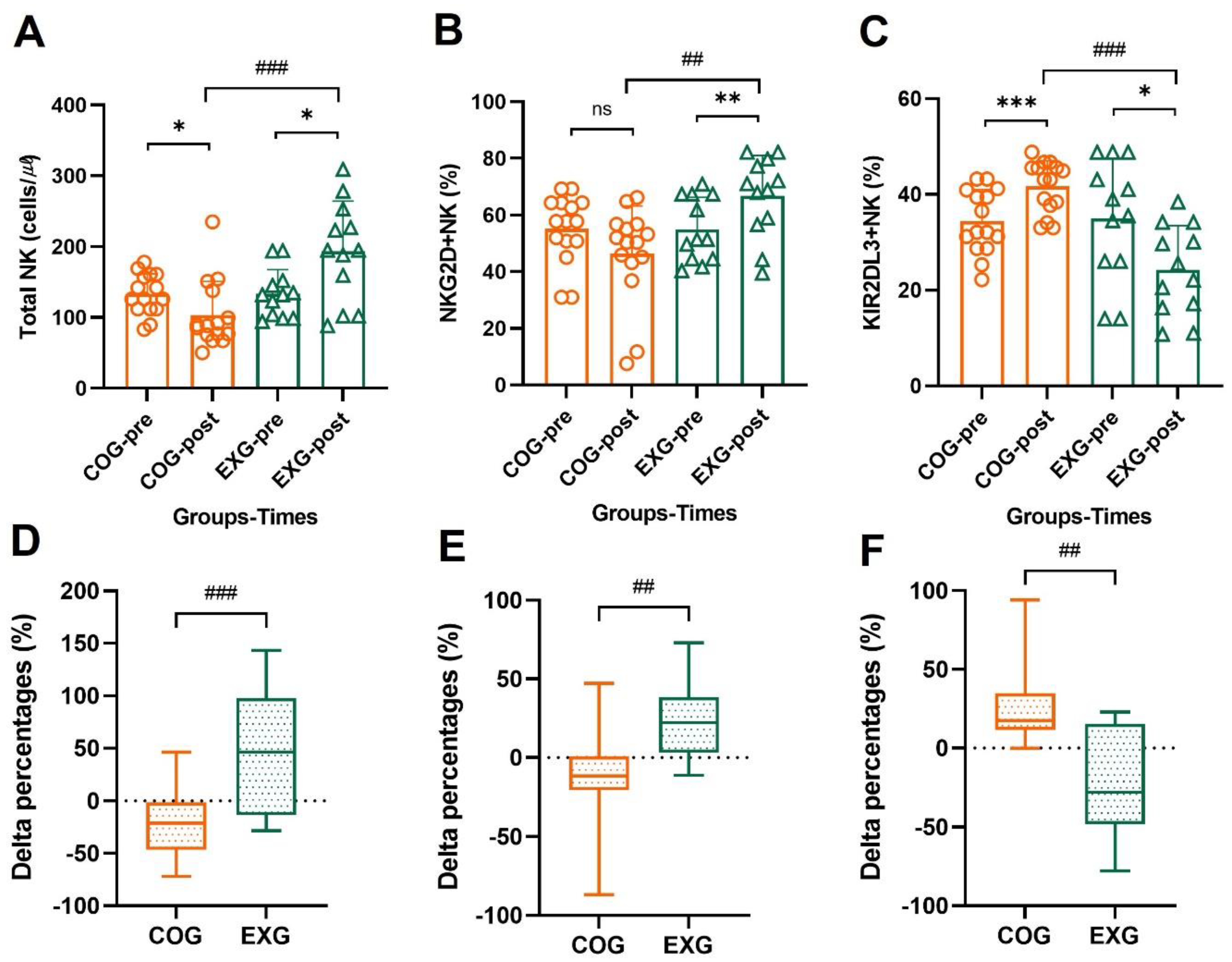
3.7. The Changes of NK Cells’ Activator and Inhibitor
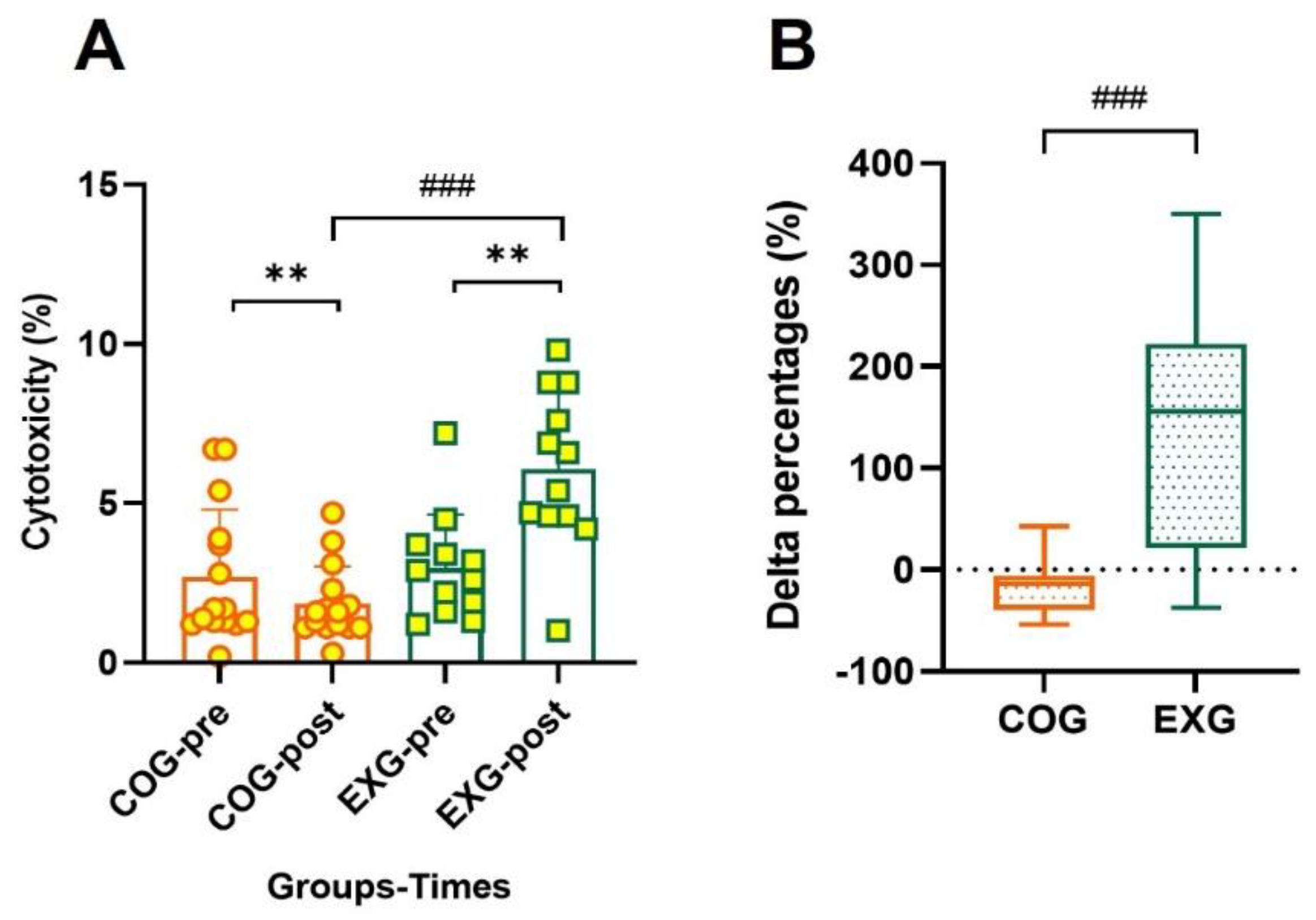
3.8. Positive Changes in Cytotoxicity According to Exercise Training
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics. 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, W.L.; Pak, D.; Celestino, J.; Lu, K.H.; Ning, J.; Lokshin, A.E.; Cheng, Z.; Lu, Z.; Bast, R.C., Jr. Osteopontin, Macrophage Migration Inhibitory Factor and Anti-Interleukin-8 Autoantibodies Complement CA125 for Detection of Early Stage Ovarian Cancer. Cancers 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Pignata, S.; Cannella, L.; Leopardo, D.; Pisano, C.; Bruni, G.S.; Facchini, G. Chemotherapy in epithelial ovarian cancer. Cancer Lett. 2011, 303, 73–83. [Google Scholar] [CrossRef]
- Bankhead, C.R.; Kehoe, S.T.; Austoker, J. Symptoms associated with diagnosis of ovarian cancer: A systematic review. BJOG 2005, 112, 857–865. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Kitase, Y.; Mitra, S.; Erne, C.E.; Novinger, L.J.; Zimmers, T.A.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 685–700. [Google Scholar] [CrossRef]
- Jain, R.; Handorf, E.; Khare, V.; Blau, M.; Chertock, Y.; Hall, M.J. Impact of Baseline Nutrition and Exercise Status on Toxicity and Outcomes in Phase I and II Oncology Clinical Trial Participants. Oncologist 2020, 25, 161–169. [Google Scholar] [CrossRef]
- Lee, J.K.; Jee, Y.S. Effect of Resistance Exercise on Acquired Immunocytes in Cancer Survivors: A Pilot Study. Int. Neurourol. J. 2021, 25, S96–S105. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as Adjunct Therapy in Cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Nausch, N.; Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 2008, 27, 5944–5958. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Campbell, J.P.; Riddell, N.E.; Burns, V.E.; Turner, M.; van Zanten, J.J.; Drayson, M.T.; Bosch, J.A. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 2009, 23, 767–775. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise and Cytokines. Immunol. Cell Biol. 2000, 78, 532–535. [Google Scholar] [CrossRef]
- Courneya, K.S.; Mackey, J.R.; Jones, L.W. Coping with cancer experience: Can physical exercise help? Phys. Sportsmed. 2000, 28, 49–73. [Google Scholar] [CrossRef]
- Dimeo, F.C. Effects of exercise on cancer-related fatigue. Cancer 2001, 92, 1689–1693. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Hankinson, S.E.; Rosner, B.; Holmes, M.D.; Willett, W.C. Physical activity and risk of breast cancer among postmenopausal women. Arch. Intern. Med. 2010, 170, 1758–1764. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Mark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early stage breast cancer. Cancer Causes Control. 2011, 22, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 20, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F.; et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers 2021, 13, 3820. [Google Scholar] [CrossRef]
- Cheng, H.L. A simple, Easy-to-Use Spreadsheet for Automatic Scoring of the International Physical Activity Questionnaire (IPAQ) Short Form. 2016. Available online: https://www.researchgate.net/publication/310953872_A_simple_easy-touse_spreadsheet_for_automatic_scoring_of_the_International_Physical_Activity_Questionnaire_IPAQ_Short_Form (accessed on 6 December 2021).
- Kim, K.; Eun, D.; Jee, Y.S. Higher Impulse Electromyostimulation Contributes to Psychological Satisfaction and Physical Development in Healthy Men. Medicina 2021, 57, 191. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, J.H.; Hong, J.; Choi, Y.T.; Kim, M.H.; Cho, J.H.; Ko, I.G.; Jee, Y.S. A 12-week rehabilitation program improves body composition, pain sensation, and internal/external torques of baseball pitchers with shoulder impingement symptom. J. Exerc. Rehabil. 2014, 10, 35–44. [Google Scholar] [CrossRef]
- Jee, Y.S. The efficacy and safety of whole-body electromyostimulation in applying to human body: Based from graded exercise test. J. Exerc. Rehabil. 2018, 14, 49–57. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Yoo, J.; Jee, Y.S. Effect of playing soccer on stress, sociality, and physical fitness in alienated youth: A retrospective study. J. Exerc. Rehabil. 2020, 16, 154–161. [Google Scholar] [CrossRef]
- Kim, J.D.; Oh, H.W.; Lee, J.H.; Cha, J.Y.; Ko, I.G.; Jee, Y.S. The effect of inversion traction on pain sensation, lumbar flexibility and trunk muscles strength in patients with chronic low back pain. Isokinet. Exerc. Sci. 2013, 21, 237–246. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; French, D.N. Resistance training for health and performance. Curr. Sports Med. Rep. 2002, 1, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tesch, P.A.; Daniels, W.L.; Sharp, D.S. Lactate accumulation in muscle and blood during submaximal exercise. Acta Physiol. Scand. 1982, 114, 441–446. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Anselmi, F.; Fiorentini, C.; Mannucci, R.; Bonifazi, M.; Mondillo, S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 2021, 28, 725–735. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Jee, Y.S. Cancer and exercise immunity: 6th series of scientific evidence. J. Exerc. Rehabil. 2021, 17, 151–152. [Google Scholar] [CrossRef]
- Jee, Y.S. Exercise-induced myokines for cancer patients: 7th in a series of scientific evidence. J. Exerc. Rehabil. 2021, 17, 293–294. [Google Scholar] [CrossRef]
- Grote, S.; Almstedt, H.C.; Tarleton, H.P. Cardiometabolic Health Among Cancer Survivors: A 13-Week Pilot Study of a Combined Aerobic and Resistance Training Program. Oncol. Nurs. Forum. 2016, 43, 306–315. [Google Scholar] [CrossRef][Green Version]
- Ligibel, J. Lifestyle factors in cancer survivorship. J. Clin. Oncol. 2012, 30, 3697–36704. [Google Scholar] [CrossRef]
- Del Giacco, S.R.; Scorcu, M.; Argiolas, F.; Firinu, D.; Del Giacco, G.S. Exercise Training, Lymphocyte subsets and their cytokines production: Experience of an Italian Professional Football Team and their impact on allergy. Biomed. Res. Int. 2014, 2014, 429248. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise and resistance to infection. Can. J. Physiol. Pharmacol. 1998, 76, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pedersen, B.K. Exercise and immune function: Recent development. Sports Med. 1999, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Roessler, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2008, 38, 93–102. [Google Scholar] [CrossRef]
- Schneider, C.M.; Hsieh, C.C.; Sprod, L.K.; Carter, S.D.; Hayward, R. Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integr. Cancer Ther. 2007, 6, 235–241. [Google Scholar] [CrossRef]
- Jee, Y.S. Exercise rehabilitation strategy for the prevention of sarcopenia in cancer populations: 8th in a series of scientific evidence. J. Exerc. Rehabil. 2022, 18, 79–80. [Google Scholar] [CrossRef]
- Del Giacco, S.R.; Tocco, F.; Melis, F.; Crisafulli, A.; Gessa, M.; Santoboni, U.; Caria, M.; Tavera, C.; Del Giacco, S.G.; Concu, A. Responsiveness of human natural killer cells during acute, incremental exercise up to exhaustion. Sport Sci. Health 2004, 1, 36–40. [Google Scholar] [CrossRef]
- Cook, K.D.; Whitmire, J.K. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J. Immunol. 2013, 190, 641–649. [Google Scholar] [CrossRef]
- Lee, Y.W.; Shin, K.W.; Paik, I.-Y.; Jung, W.M.; Cho, S.Y.; Choi, S.T.; Kim, H.D.; Kim, J.Y. Immunological impact of taekwondo competitions. Int. J. Sports Med. 2012, 33, 58–66. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Spence, R.R.; Galvão, D.A.; Newton, R.U. Australian Association for Exercise and Sport Science position stand: Optimising cancer outcomes through exercise. J. Sci. Med. Sport 2009, 12, 428–434. [Google Scholar] [CrossRef] [PubMed]

| Items | Exercise Types | Exercise Training Phases | ||||
|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | Phase IV | |||
| 0–3 Week | 4–6 Week | 7–9 Week | 10–12 Week | |||
| Warm-up | Standing stretching | 10 min | 10 min | 10 min | 10 min | |
| Work-out | Aerobic training | F | 3 days | 3 days | 3 days | 3 days |
| I | 40% VO2 peak | 50% VO2 peak | 60% VO2 peak | 70% VO2 peak | ||
| T | 50 min | 45 min | 40 min | 35 min | ||
| Resistance training | F | 3 days | 3 days | 3 days | 3 days | |
| I | 12RM × 3 sets | 10RM × 3 sets | 8RM × 3 sets | 6RM × 3 sets | ||
| T | 30–40 min | 30–40 min | 30–40 min | 30–40 min | ||
| Cool-down | Sitting/Lying stretching | 15 min | 15 min | 15 min | 15 min | |
| Items | Groups | |||
|---|---|---|---|---|
| Total (n = 27) | COG (n = 15) | EXG (n = 12) | p | |
| Age (y) | 51.07 ± 5.68 | 50.60 ± 5.83 | 51.67 ± 5.68 | 0.548 |
| Height (cm) | 155.74 ± 4.08 | 155.80 ± 3.10 | 155.67 ± 5.21 | 0.981 |
| Body weight (kg) | 57.26 ± 9.76 | 57.14 ± 6.13 | 57.41 ± 13.33 | 0.486 |
| Body mass index (kg/m2) | 23.60 ± 3.78 | 23.61 ± 3.03 | 23.59 ± 4.70 | 0.829 |
| Married, n (%) | 23 (85.2%) | 13 (86.7%) | 10 (83.3%) | 0.905 |
| Employed, n (%) | 8 (29.6%) | 6 (40.0%) | 2 (16.7%) | 0.323 |
| Current smoker, n | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Medications’ number | 1.85 ± 0.99 | 1.87 ± 1.13 | 1.83 ± 0.83 | 0.867 |
| Supplements’ number | 1.89 ± 0.64 | 1.87 ± 0.64 | 1.92 ± 0.67 | 0.867 |
| Comorbidities’ number | 2.58 ± 0.97 | 2.40 ± 0.98 | 2.83 ± 0.94 | 0.236 |
| Ovarian cancer diagnosed year | 4.06 ± 0.49 | 4.12 ± 0.47 | 3.99 ± 0.52 | 0.373 |
| Primary cytoreductive surgery (month) | 46.40 ± 5.93 | 46.18 ± 6.00 | 46.67 ± 6.20 | 1.000 |
| Primary cytoreductive surgery, n (%) | 20 (74.1%) | 11 (73.3%) | 9 (75.0%) | - |
| Interval debulking surgery (month) | 44.71 ± 6.02 | 43.75 ± 5.62 | 46.00 ± 7.55 | 0.857 |
| Interval debulking surgery, n (%) | 7 (25.9%) | 4 (26.7%) | 3 (25.0%) | - |
| Lymph node metastasis, n (%) | 5 (18.5%) | 2 (13.3%) | 3 (25.0%) | 0.614 |
| Organ metastasis, n (%) | 3 (11.1%) | 1 (6.7%) | 2 (16.7%) | 0.683 |
| Other ovarian cancer-related treatment | - | |||
| Surgery, n (%) | 22 (81.5%) | 12 (80.0%) | 10 (83.3%) | 0.905 |
| Radiation therapy, n (%) | 17 (63.0%) | 10 (66.7%) | 7 (58.3%) | 0.719 |
| Chemotherapy, n (%) | 22 (81.5%) | 12 (80.0%) | 10 (83.3%) | 0.905 |
| Items | Time | Groups | ||
|---|---|---|---|---|
| COG (n = 15) | EXG (n = 12) | p | ||
| Daily calorie (kcal/day) | pre | 1618.93 ± 187.75 | 1591.25 ± 166.90 | 0.829 |
| post | 1732.00 ± 263.51 | 1735.50 ± 152.86 | 0.581 | |
| Carbohydrate (g/day) | pre | 117.07 ± 15.50 | 119.83 ± 12.42 | 0.581 |
| post | 118.33 ± 13.91 | 117.50 ± 13.01 | 0.792 | |
| Fiber (g/day) | pre | 19.40 ± 2.82 | 19.17 ± 4.91 | 0.755 |
| post | 20.80 ± 2.98 | 20.08 ± 4.46 | 0.867 | |
| Protein (g/day) | pre | 50.20 ± 6.28 | 51.17 ± 6.95 | 0.905 |
| post | 51.67 ± 5.61 | 50.67 ± 7.88 | 0.581 | |
| Fat (g/day) | pre | 51.93 ± 7.56 | 50.17 ± 9.03 | 0.648 |
| post | 50.80 ± 7.28 | 50.67 ± 9.75 | 0.981 | |
| Vitamin A (ug RAE/day) | pre | 538.13 ± 66.31 | 550.42 ± 62.13 | 0.792 |
| post | 549.13 ± 59.58 | 549.92 ± 55.70 | 0.792 | |
| Vitamin C (mg/day) | pre | 94.40 ± 13.39 | 94.83 ± 12.96 | 0.829 |
| post | 98.13 ± 14.27 | 95.67 ± 17.07 | 0.648 | |
| Calcium (mg/day) | pre | 706.20 ± 112.59 | 690.58 ± 84.11 | 0.683 |
| post | 687.73 ± 137.01 | 680.08 ± 137.37 | 0.981 | |
| Iron (mg/day) | pre | 12.87 ± 3.46 | 11.42 ± 2.71 | 0.300 |
| post | 12.40 ± 3.54 | 11.58 ± 2.39 | 0.683 | |
| Daily physical activity | pre | 264.47 ± 27.83 | 276.00 ± 10.37 | 0.516 |
| (kcal/day) | post | 286.00 ± 31.89 | 283.92 ± 30.30 | 0.792 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-K.; Park, S.; Jee, Y.-S. Immunoprotecting Effects of Exercise Program against Ovarian Cancer: A Single-Blind, Randomized Controlled Trial. Cancers 2022, 14, 2808. https://doi.org/10.3390/cancers14112808
Lee J-K, Park S, Jee Y-S. Immunoprotecting Effects of Exercise Program against Ovarian Cancer: A Single-Blind, Randomized Controlled Trial. Cancers. 2022; 14(11):2808. https://doi.org/10.3390/cancers14112808
Chicago/Turabian StyleLee, Jong-Kyun, Sihwa Park, and Yong-Seok Jee. 2022. "Immunoprotecting Effects of Exercise Program against Ovarian Cancer: A Single-Blind, Randomized Controlled Trial" Cancers 14, no. 11: 2808. https://doi.org/10.3390/cancers14112808
APA StyleLee, J.-K., Park, S., & Jee, Y.-S. (2022). Immunoprotecting Effects of Exercise Program against Ovarian Cancer: A Single-Blind, Randomized Controlled Trial. Cancers, 14(11), 2808. https://doi.org/10.3390/cancers14112808







