Diagnostic Utility of Bronchoalveolar Lavage in Patients with Acute Leukemia under Broad-Spectrum Anti-Infective Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
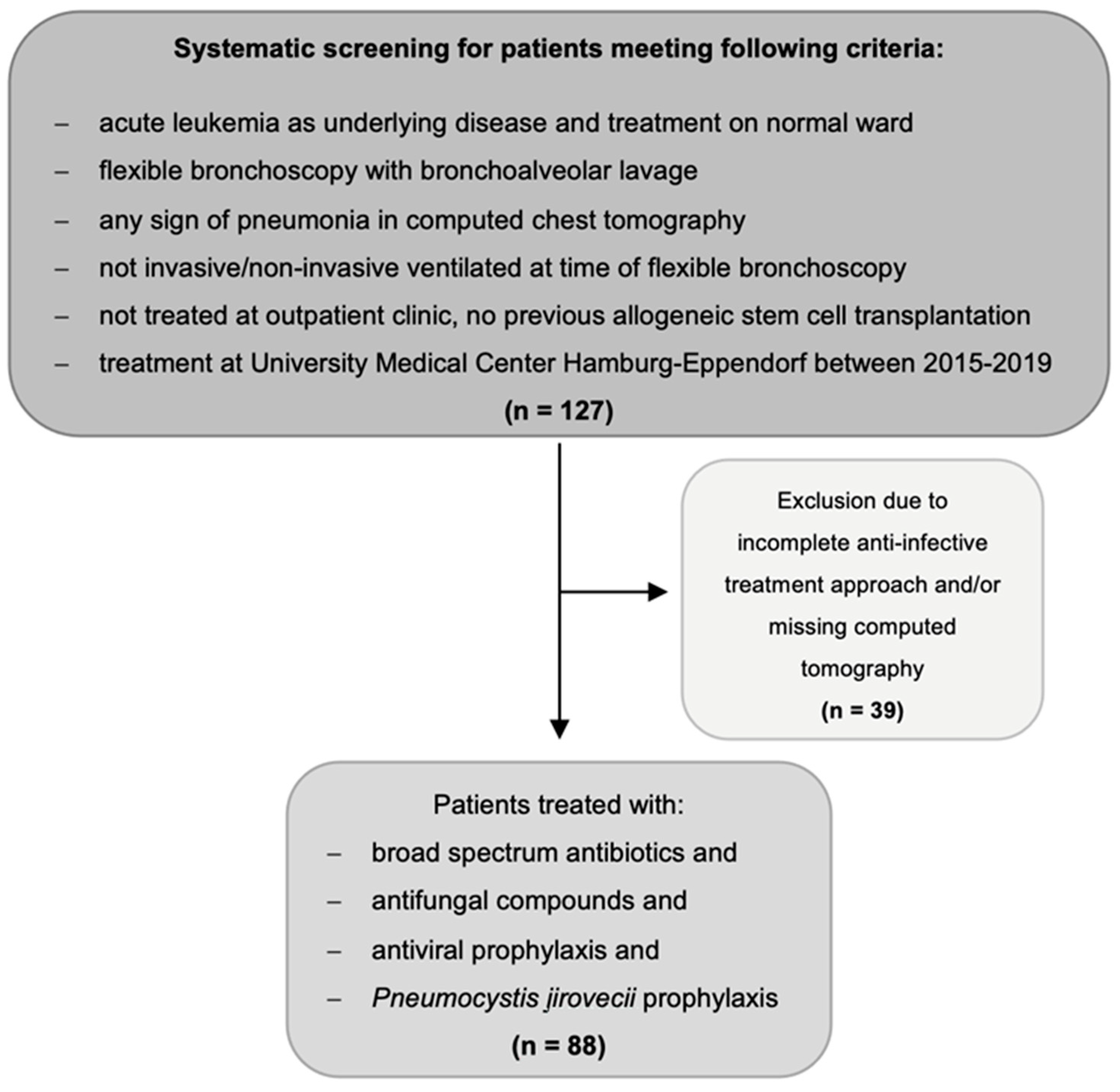
2.1. Study Design and Population
- Aged ≥ 18 years.
- Diagnosed with either AML or acute lymphoblastic leukemia (ALL).
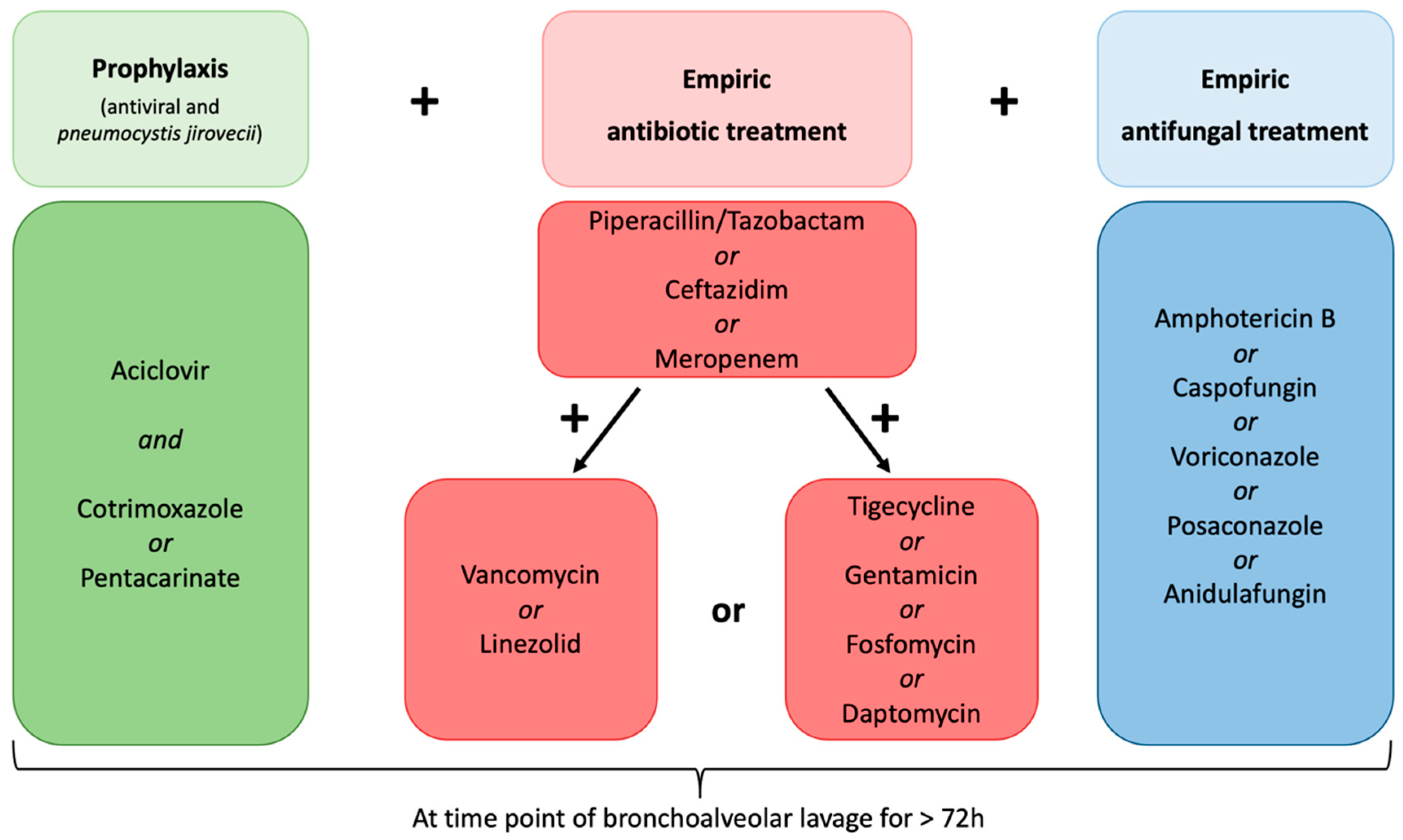
- Diagnosed with multi-drug resistant fever or suspected infection defined as an occurrence under >three days of empiric broad-spectrum antibiotic and empiric antifungal treatment, antiviral prophylaxis, and prophylaxis against Pneumocystis jirovecii (Figure 2).
- Underwent CT scan of the chest showing pulmonary infiltrates within five days prior to FB with BAL.
- Underwent procedure of FB with BAL.
- No previous allogeneic stem cell transplantation.
2.2. Clinical Data Collection
2.3. Procedure of Flexible Bronchoscopy and Bronchoalveolar Lavage
2.4. Microbiological Tests
2.5. Statistical Analysis
2.6. Endpoint
3. Results
3.1. Diagnostic Assessment and Clinical Courses
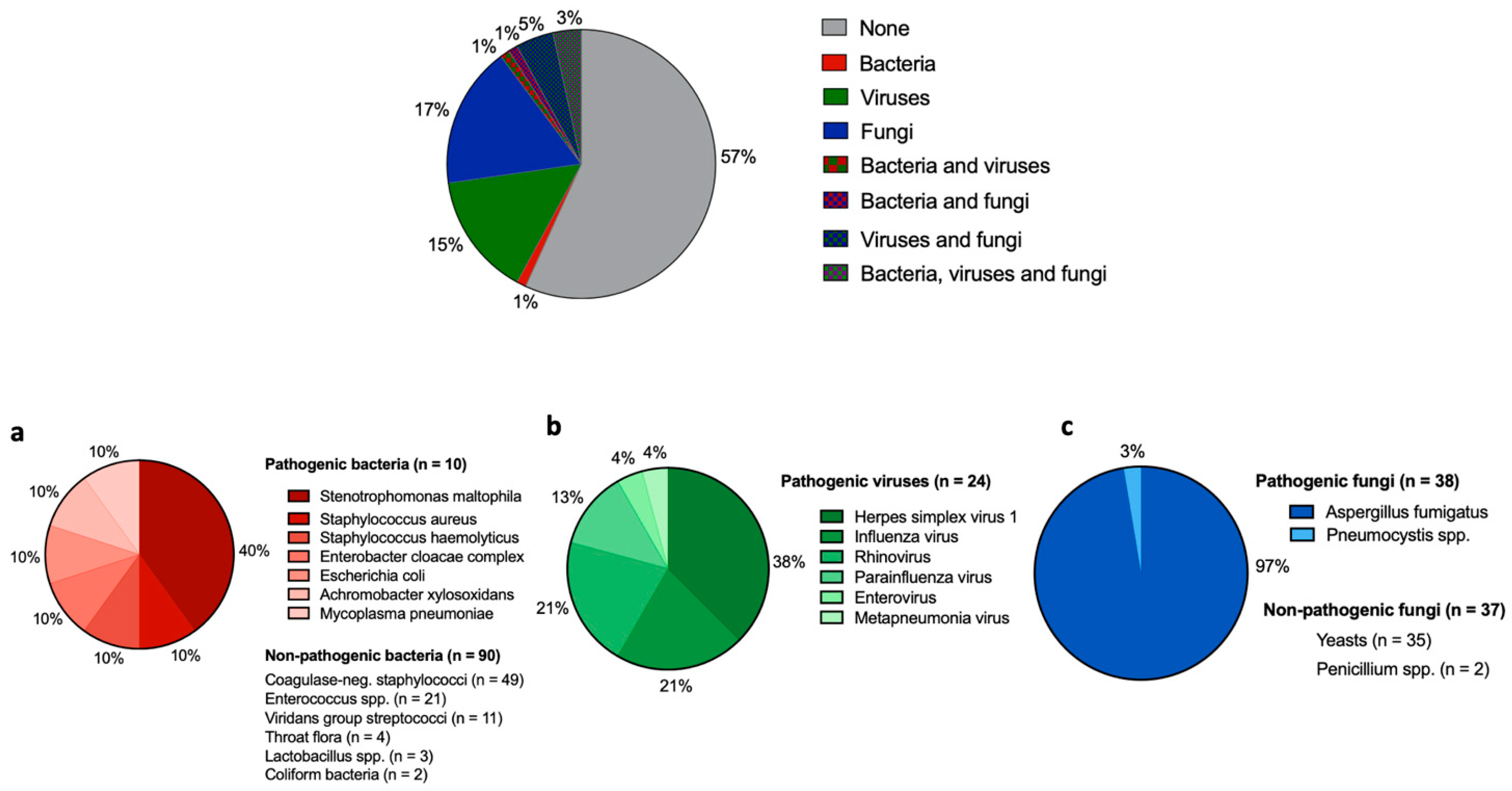
3.2. Microbiological Findings in BAL
3.3. Impact of BAL on Anti-Infective Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. on behalf of the ESMO Guidelines Committee. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27 (Suppl. S5), v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Godnaik, C. Incidence and management of infections in patients with acute leukemia following chemotherapy in general wards. Ecancermedicalscience 2013, 7, 310. [Google Scholar]
- Nesher, L.; Rolston, K.V. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2014, 42, 5–13. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Verga, L.; Busca, A.; Martino, B.; Mitra, M.E.; Fanci, R.; Ballanti, S.; Picardi, M.; Castagnola, C.; Cattaneo, C.; et al. Systemic antifungal treatment after posaconazole prophylaxis: Results from the SEIFEM 2010-C survey. J. Antimicrob. Chemother. 2014, 69, 3142–3147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, 427–431. [Google Scholar] [CrossRef]
- Baden, L.R.; Bensinger, W.; Angarone, M.; Casper, C.; Dubberke, E.R.; Freifeld, A.G.; Garzon, R.; Greene, J.N.; Greer, J.P.; Ito, J.I.; et al. Prevention and treatment of cancer-related infections. J. Natl. Compr. Cancer Netw. 2012, 10, 1412–1445. [Google Scholar] [CrossRef]
- Heussel, C.P.; Kauczor, H.U.; Heussel, G.E.; Fischer, B.; Begrich, M.; Mildenberger, P.; Thelen, M. Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: Use of high-resolution computed tomography. J. Clin. Oncol. 1999, 17, 796–805. [Google Scholar] [CrossRef]
- Patsios, D.; Maimon, N.; Chung, T.; Roberts, H.; Disperati, P.; Minden, M.; Paul, N. Chest low-dose computed tomography in neutropenic acute myeloid leukaemia patients. Respir. Med. 2010, 104, 600–605. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Park, S.Y.; Lee, H.Y.; Lee, K.S.; Shin, K.E.; Moon, J.W. Ultra-Low-Dose Chest CT in Patients with Neutropenic Fever and Hematologic Malignancy: Image Quality and Its Diagnostic Performance. Cancer Res. Treat. 2014, 46, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Piede, J.A.; Ellis, L.R.; High, K.P.; Case, D.; Powell, B. The Evaluation of Bronchoscopy in Acute Leukemia: A Retrospective Chart Review. Blood 2008, 112, 3990. [Google Scholar] [CrossRef]
- Hummel, M.; Rudert, S.; Hof, H.; Hehlmann, R.; Buchheidt, D. Diagnostic yield of bronchoscopy with bronchoalveolar lavage in febrile patients with hematologic malignancies and pulmonary infiltrates. Ann. Hematol. 2008, 87, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Curley, T.; Koenig, K.L.; Mani, S.; Lustberg, M.; Behbehani, G.; Bhatnagar, B.; Blachly, J.S.; Haque, T.Z.; Larkin, K.T.; Long, M.; et al. Diagnostic utility of bronchoscopy in newly diagnosed acute leukemia patients. J. Clin. Oncol. 2020, 38 (Suppl. S15), e19510. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 1 May 2022).
- Martinez, C.; Subira, M.; Lopez, R.; Buj, J.; Sureda, A.; Brunet, S. Clinical usefulness of bronchoalveolar lavage in haematologic patients with suspected pulmonary infection. Eur. J. Haematol. 1995, 55, 344–345. [Google Scholar] [CrossRef]
- Von Eiff, M.; Zuhlsdorf, M.; Roos, N.; Thomas, M.; Buchner, T.; van de Loo, J. Pulmonary infiltrates in patients with haematologic malignancies: Clinical usefulness of non-invasive bronchoscopic procedures. Eur. J. Haematol. 1995, 54, 157–162. [Google Scholar] [CrossRef]
- Marra, R.; Pajano, L.; Pagliari, G.; Frigiera, L.; Storti, S.; Morace, G.; Ardito, F.; Leone, G. The yield of bronchoalveolar lavage in the etiological diagnosis of pneumonia in leukemia and lymphoma patients. Eur. J. Haematol. 1993, 51, 256–258. [Google Scholar] [CrossRef]
- Saito, H.; Anaissie, E.J.; Morice, R.C.; Dekmezian, R.; Bodey, G.P. Bronchoalveolar lavage in the diagnosis of pulmonary infiltrates in patients with acute leukemia. Chest 1988, 94, 745–749. [Google Scholar] [CrossRef]
- Khalid, U.; Akram, M.J.; Butt, F.M.; Ashraf, M.B.; Khan, F. The Diagnostic Utility and Clinical Implications of Bronchoalveolar Lavage in Cancer Patients with Febrile Neutropenia and Lung Infiltrates. Cureus 2020, 12, e10268. [Google Scholar] [CrossRef]
- Peikert, T.; Rana, S.; Edell, E.S. Safety, diagnostic yield, and therapeutic implications of flexible bronchoscopy in patients with febrile neutropenia and pulmonary infiltrates. Mayo Clin. Proc. 2005, 80, 1414–1420. [Google Scholar] [CrossRef]
- Svensson, T.; Lundstrom, K.L.; Hoglund, M.; Cherif, H. Utility of bronchoalveolar lavage in diagnosing respiratory tract infections in patients with hematological malignancies: Are invasive diagnostics still needed? Ups. J. Med. Sci. 2017, 122, 56–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramila, E.; Sureda, A.; Martino, R.; Santamaria, A.; Franquet, T.; Puzo, C.; Montesinos, J.; Perea, G.; Sierra, J. Bronchoscopy guided by high-resolution computed tomography for the diagnosis of pulmonary infections in patients with hematologic malignancies and normal plain chest X-ray. Haematologica 2000, 85, 961–966. [Google Scholar] [PubMed]
- Deotare, U.; Merman, E.; Pincus, D.; Kraguljac, A.P.; Croucher, D.; Kumar, V.; Ibrahimova, N.; Minden, M.D.; Lee, C.; Mehta, S. The utility and safety of flexible bronchoscopy in critically ill acute leukemia patients: A retrospective cohort study. Cancer J. Anaesth. 2018, 65, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Seneviratna, A.; O’Carroll, M.; Lewis, C.A.; Milne, D. Diagnostic yield of bronchoscopic sampling in febrile neutropenic patients with pulmonary infiltrate and haematological disorders. Intern. Med. J. 2012, 42, 536–541. [Google Scholar] [CrossRef]
- Marchesi, F.; Cattaneo, C.; Criscuolo, M.; Delia, M.; Dargenio, M.; Del Principe, M.I.; Spadea, A.; Fracchiolla, N.S.; Melillo, L.; Perruccio, K.; et al. A bronchoalveolar lavage-driven antimicrobial treatment improves survival in hematologic malignancy patients with detected lung infiltrates: A prospective multicenter study of the SEIFEM group. Am. J. Hematol. 2019, 94, 1104–1112. [Google Scholar] [CrossRef]
- Radha, S.; Afroz, T.; Prasad, S.; Ravindra, N. Diagnostic utility of bronchoalveolar lavage. J. Cytol. 2014, 31, 136–138. [Google Scholar] [CrossRef]
| Total Cohort (n = 88) | |
|---|---|
| Median age in years (IQR) | 59 (48–68) |
| Male Sex, n (%) | 59 (67) |
| Underlying disease, n (%) | |
| Acute myleoid leukemia | 76 (86) |
| Acute lymphoblastic leukemia | 12 (14) |
| Time point of FB with BAL, n (%): | |
| Prior any systemic oncological therapy | 1 (1) |
| During induction cycles | 51 (58) |
| During consolidation cycles | 7 (8) |
| During salvage chemotherapy | 21 (24) |
| During low-dose chemotherapy | 8 (9) |
| Aplasia at FB with BAL, n (%): | 83 (94) |
| Duration of aplasia, days (IQR) | 15 (10–25) |
| High-risk neutropenia | 38 (43) |
| Fever at FB with BAL, n (%) | 28 (32) |
| Chest computed tomography, n (%): | 88 (100) |
| Lobar pneumonia | 25 (28) |
| Atypical pneumonia | 63 (72) |
| Invasive ventilation <30 days after the onset of pneumonia, n (%) | 17 (19) |
| Pneumonia-related death <30 days after the onset of pneumonia, n (%) | 12 (14) |
| Anti-infective treatment against bacteria at FB, n (%) | 88 (100) |
| Meropenem | 73 (83 |
| Ceftazidim | 14 (16) |
| Piperacillin/Tazobactam | 1 (1) |
| Vancomycin or Linezolid | 64 (73) |
| Tigecycline | 22 (25) |
| Fosfomycin | 4 (5) |
| Gentamicin | 3 (3) |
| Daptomycin | 1 (1) |
| Anti-infective treatment against fungal infection at FB, n | 88 (100) |
| Amphotericin B | 68 (77) |
| Caspofungin | 9 (10) |
| Voriconazole | 7 (8) |
| Posaconazole | 3 (4) |
| Anidulafungin | 1 (1) |
| Prophylaxis against Pneumocystis jirovecii (Cotrimoxazole n = 89, Pentacarinate n = 2) | 88 (100) |
| Anti-infective treatment against viruses at FB, n (%) | 88 (100) |
| Aciclovir prophylaxis | 88 (100) |
| Oseltamivir | 2 (2) |
| Pathogen Detected in BAL | Prior Anti-Infective Treatment to BAL * | Changes in Anti-Infective Treatment after BAL | |
|---|---|---|---|
| Case 1 | Influenza A | Meropenem, vancomycin, amphotericin B | Oseltamivir |
| Case 2 | Herpes simplex virus 1 ** | Meropenem, vancomycin, amphotericin B | Adaption of aciclovir dosage |
| Case 3 | Herpes simplex virus 1 ** | Meropenem, vancomycin, amphotericin B | Adaption of aciclovir dosage |
| Case 4 | Herpes simplex virus 1 ** | Meropenem, vancomycin, caspofungine | Adaption of aciclovir dosage |
| Case 5 | Influenza A | Meropenem, tigecycline, amphotericin B | Oseltamivir |
| Case 6 | Influenza A | Ceftazidim, tigecycline, amphotericin B | Oseltamivir |
| Case 7 | Herpes simplex virus 1 ** | Ceftazidim, vancomycin, amphotericin B | Adaption of aciclovir dosage |
| Case 8 | Herpes simplex virus 1 ** | Meropenem, tigecycline, amphotericin B | Adaption of aciclovir dosage |
| Case 9 | Herpes simplex virus 1 ** | Meropenem, vancomycin, amphotericin B | Adaption of aciclovir dosage |
| Case 10 | Herpes simplex virus 1 ** and mycoplasma pneumoniae | Meropenem, linezolid, amphotericin B | Azithromycine and adaption of aciclovir dosage |
| Case 11 | Herpes simplex virus 1 ** | Meropenem, tigecycline, voriconazole | Adaption of aciclovir dosage |
| Case 12 | Herpes simplex virus 1 ** and pneumocystis spp. | Meropenem, tigecycline, amphotericin B | Adaption of aciclovir and cotrimoxazole dosage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghandili, S.; von Kroge, P.H.; Simon, M.; Henes, F.O.; Rohde, H.; Hoffmann, A.; Lindeman, N.B.; Bokemeyer, C.; Fiedler, W.; Modemann, F. Diagnostic Utility of Bronchoalveolar Lavage in Patients with Acute Leukemia under Broad-Spectrum Anti-Infective Treatment. Cancers 2022, 14, 2773. https://doi.org/10.3390/cancers14112773
Ghandili S, von Kroge PH, Simon M, Henes FO, Rohde H, Hoffmann A, Lindeman NB, Bokemeyer C, Fiedler W, Modemann F. Diagnostic Utility of Bronchoalveolar Lavage in Patients with Acute Leukemia under Broad-Spectrum Anti-Infective Treatment. Cancers. 2022; 14(11):2773. https://doi.org/10.3390/cancers14112773
Chicago/Turabian StyleGhandili, Susanne, Philipp H. von Kroge, Marcel Simon, Frank O. Henes, Holger Rohde, Armin Hoffmann, Nick Benjamin Lindeman, Carsten Bokemeyer, Walter Fiedler, and Franziska Modemann. 2022. "Diagnostic Utility of Bronchoalveolar Lavage in Patients with Acute Leukemia under Broad-Spectrum Anti-Infective Treatment" Cancers 14, no. 11: 2773. https://doi.org/10.3390/cancers14112773
APA StyleGhandili, S., von Kroge, P. H., Simon, M., Henes, F. O., Rohde, H., Hoffmann, A., Lindeman, N. B., Bokemeyer, C., Fiedler, W., & Modemann, F. (2022). Diagnostic Utility of Bronchoalveolar Lavage in Patients with Acute Leukemia under Broad-Spectrum Anti-Infective Treatment. Cancers, 14(11), 2773. https://doi.org/10.3390/cancers14112773







