Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
Statistical Methods
3. Results
3.1. Clinicopathological Features
3.2. Overall Survival
3.3. Local Recurrence
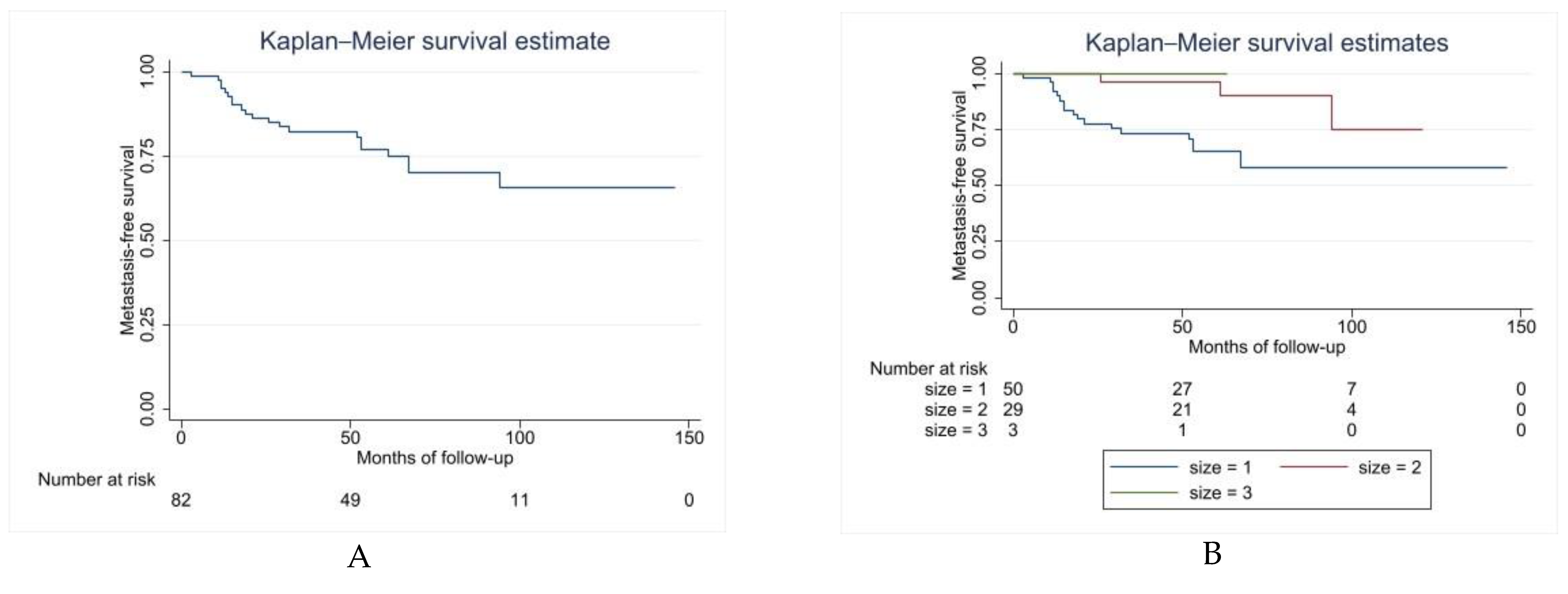
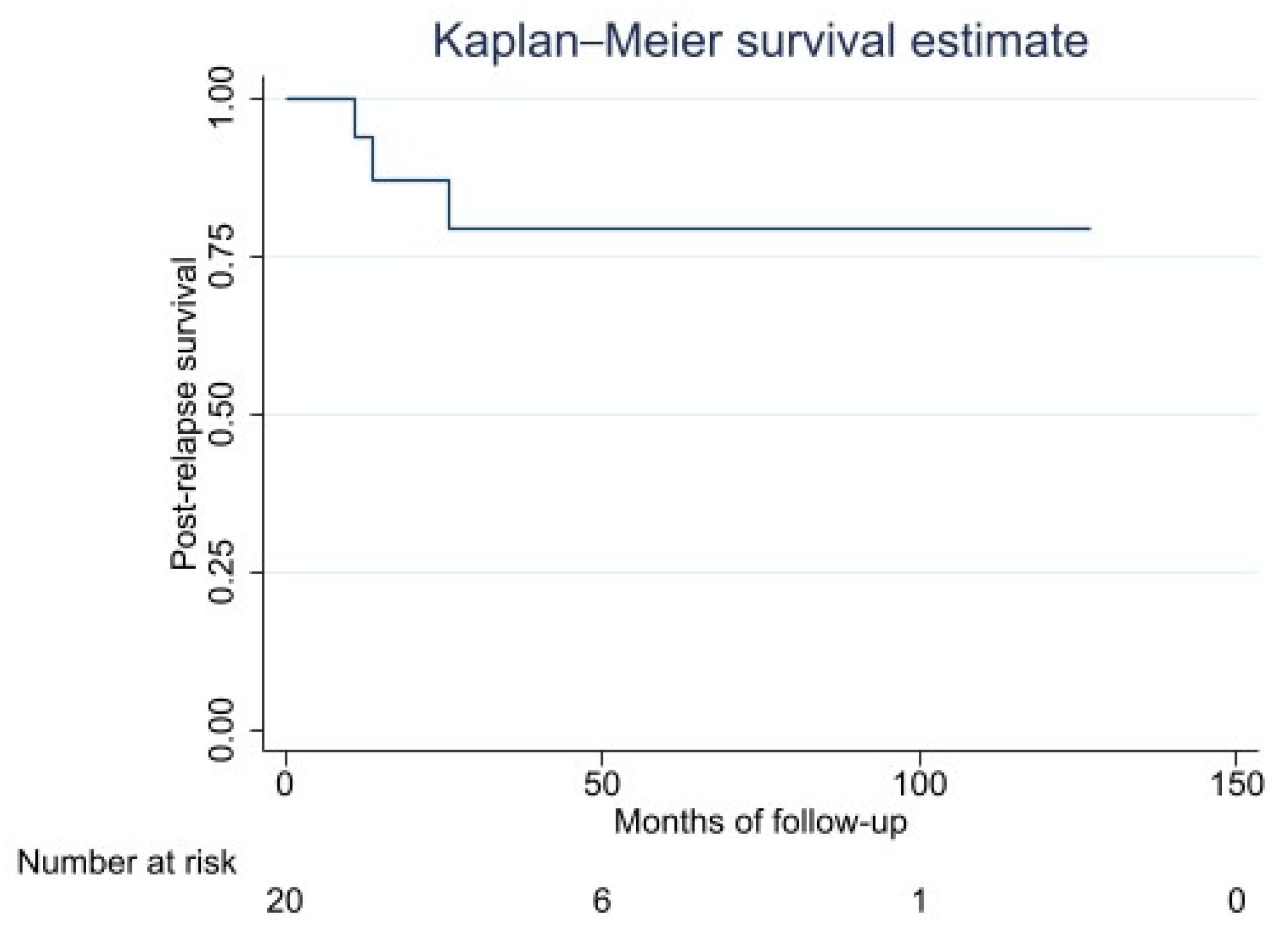
3.4. Distant Metastasis: Metastasis-Free Survival, Metastatic Pattern, and Post-Relapse Survival
3.5. Disease-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dujardin, F.; Debled, M.; Guillemet, C.; Simonet, J.; Hamidou, H.; Cambon-Michot, C.; Dubray, B.; Vera, P. Diagnosis and treatment of soft-tissuesoft tissue tumors. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2006, 92, 637–650. [Google Scholar] [CrossRef]
- Abaricia, S.; Hirbe, A.C. Diagnosis and Treatment of Myxoid Liposarcomas: Histology Matters. Curr. Treat. Options Oncol. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Losada, J.; Sánchez-Martín, M.; Rodríguez-García, M.A.; Pérez-Mancera, P.A.; Pintado, B.; Flores, T.; Battaner, E.; Sánchez-Garćia, I. Liposarcoma initiated by FUS/TLS-CHOP: The FUS/TLS domain plays a critical role in the pathogenesis of liposarcoma. Oncogene 2000, 19, 6015–6022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.A.; Easley, K.A.; Goldblum, J.R. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am. J. Surg. Pathol. 1996, 20, 171–180. [Google Scholar] [CrossRef]
- Dürr, H.R.; Rauh, J.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Roeder, F.; Jansson, V.; Klein, A. Myxoid liposarcoma: Local relapse and metastatic pattern in 43 patients. BMC Cancer 2018, 18, 304. [Google Scholar] [CrossRef]
- Hoffman, A.; Ghadimi, M.P.H.; Demicco, E.G.; Creighton, C.J.; Torres, K.; Colombo, C.; Peng, T.; Lusby, K.; Ingram, D.; Hornick, J.L.; et al. Localized and Metastatic Myxoid/Round Cell Liposarcoma Clinical and Molecular Observations. Cancer 2013, 119, 1868–1877. [Google Scholar] [CrossRef]
- Muratori, F.; Bettini, L.; Frenos, F.; Mondanelli, N.; Greto, D.; Livi, L.; Franchi, A.; Roselli, G.; Scorianz, M.; Capanna, R.; et al. Myxoid Liposarcoma: Prognostic Factors and Metastatic Pattern in a Series of 148 Patients Treated at a Single Institution. Int. J. Surg. Oncol. 2018, 2018, 8928706. [Google Scholar] [CrossRef] [Green Version]
- Moreau, L.-C.; Turcotte, R.; Ferguson, P.; Wunder, J.; Clarkson, P.; Masri, B.; Isler, M.; Dion, N.; Werier, J.; Ghert, M.; et al. Myxoid\round cell liposarcoma (MRCLS) revisited: An analysis of 418 primarily managed cases. Ann. Surg. Oncol. 2012, 19, 1081–1088. [Google Scholar] [CrossRef]
- Schwab, J.H.; Boland, P.; Guo, T.; Brennan, M.F.; Singer, S.; Healey, J.H.; Antonescu, C.R. Skeletal metastases in myxoid liposarcoma: An unusual pattern of distant spread. Ann. Surg. Oncol. 2007, 14, 1507–1514. [Google Scholar] [CrossRef]
- Haniball, J.; Sumathi, V.P.; Kindblom, L.G.; Abudu, A.; Carter, S.R.; Tillman, R.M.; Jeys, L.; Spooner, D.; Peake, D.; Grimer, R.J. Prognostic factors and metastatic patterns in primary myxoid/round-cell liposarcoma. Sarcoma 2011, 2011, 538085. [Google Scholar] [CrossRef]
- Fiore, M.; Grosso, F.; Lo Vullo, S.; Pennacchioli, E.; Stacchiotti, S.; Ferrari, A.; Collini, P.; Lozza, L.; Mariani, L.; Casali, P.G.; et al. Myxoid/round cell and pleomorphic liposarcomas: Prognostic factors and survival in a series of patients treated at a single institution. Cancer 2007, 109, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Spillane, A.J.; Fisher, C.; Thomas, J.M. Myxoid liposarcoma--the frequency and the natural history of nonpulmonary soft tissue metastases. Ann. Surg. Oncol. 1999, 6, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.Y.; Springfield, D.S.; Mankin, H.J. Frequent incidence of extrapulmonary sites of initial metastasis in patients with liposarcoma. Cancer 1995, 75, 1120–1127. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, X.-C.; Xu, M.; Yang, Y. Surgical Outcomes and Prognostic Factors of Myxoid Liposarcoma in Extremities: A Retrospective Study. Orthop. Surg. 2019, 11, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Lemeur, M.; Mattei, J.-C.; Souteyrand, P.; Chagnaud, C.; Curvale, G.; Rochwerger, A. Prognostic factors for the recurrence of myxoid liposarcoma: 20 cases with up to 8 years follow-up. Orthop. Traumatol. Surg. Res. 2015, 101, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin. Orthop. Relat. Res. 2003, 415, 4–18. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Wu, J.; Qian, S.; Jin, L. Prognostic factors of patients with extremity myxoid liposarcomas after surgery. J. Orthop. Surg. Res. 2019, 14, 90. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Tsukushi, S.; Nakashima, H.; Ishiguro, N. Clinicopathologic prognostic factors of pure myxoid liposarcoma of the extremities and trunk wall. Clin. Orthop. Relat. Res. 2010, 468, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.J.; Yi, S.Y.; Kim, K.H.; Cho, Y.J.; Beum, S.H.; Lee, Y.H.; Suh, J.S.; Hur, H.; Kim, K.S.; Kim, S.H.; et al. Prognostic model to predict survival outcome for curatively resected liposarcoma: A multi-institutional experience. J. Cancer 2016, 7, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.M.; Kattan, M.W.; Antonescu, C.R.; Brennan, M.F.; Singer, S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann. Surg. 2006, 244, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Corson, J.M.; Demetri, G.D.; Healey, E.A.; Marcus, K.; Eberlein, T.J. Prognostic factors predictive of survival for truncal and retroperitoneal soft tissue sarcoma. Ann. Surg. 1995, 221, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic Factors for Patients with Localized Soft tissue Sarcoma Treated with Conservation Surgery and Radiation Therapy An Analysis of 1225 Patients. 2003, 97, 2530–2543. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Engstrim, K.; Bergh, P.; Gustafson, P.; Hultborn, R.; Johansson, H.; Lofvenberg, R.; Zaikova, O.; Trovik, C.; Wahlstrom, O.; Bauer, H.C.F.; et al. Liposarcoma Outcome Based on the Scandinavian Sarcoma Group Register. Cancer 2008, 113, 1649–1656. [Google Scholar] [CrossRef]
- Kilpatrick, S.E.; Doyon, J.; Choong, P.F.M.; Sim, F.H.; Nascimento, A.G. The Clinicopathologic Spectrum of Myxoid and Round Cell Liposarcoma A Study of 95 Cases. Int. J. Am. Cancer Soc. 1996, 77, 1450–1458. [Google Scholar] [CrossRef]
- Pitson, G.; Robinson, P.; Wilke, D.; Kandel, R.A.; White, L.; Griffin, A.M.; Bell, R.S.; Catton, C.N.; Wunder, J.S.; O’Sullivan, B. Radiation response: An additional unique signature of myxoid liposarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 522–526. [Google Scholar] [CrossRef]
- Salduz, A.; Alpan, B.; Valiyev, N.; Özmen, E.; İribaş, A.; Ağaoğlu, F.; Bayram, A.; Bilgiç, B.; Özger, H. Neoadjuvant radiotherapy for myxoid liposarcomas: Oncologic outcomes and histopathologic correlations. Acta Orthop. Traumatol. Turc. 2017, 51, 355–361. [Google Scholar] [CrossRef]
- Guadagnolo, B.A.; Zagars, G.K.; Ballo, M.T.; Patel, S.R.; Lewis, V.O.; Benjamin, R.S.; Pollock, R.E. Excellent Local Control Rates and Distinctive Patterns of Failure in Myxoid Liposarcoma Treated With Conservation Surgery and Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 760–765. [Google Scholar] [CrossRef]
- De Vreeze, R.S.A.; de Jong, D.; Haas, R.L.; Stewart, F.; van Coevorden, F. Effectiveness of Radiotherapy in Myxoid Sarcomas Is Associated With a Dense Vascular Pattern. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Kachare, S.D.; Brinkley, J.; Vohra, N.A.; Zervos, E.E.; Wong, J.H.; Fitzgerald, T.L. Radiotherapy Associated With Improved Survival for High-Grade Sarcoma of the Extremity. J. Surg. Oncol. 2015, 112, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.P.; Yu, X.L.; Zhang, Z.; Jia, L.J.; Feng, Y.; Yang, Z.Z.; Chen, X.X.; Wang, J.; Ma, S.L.; Guo, X.M. The efficacy of postoperative radiotherapy in localized primary soft tissue sarcoma treated with conservative surgery. Radiat. Oncol. 2016, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Tirumani, S.H.; Tirumani, H.; Jagannathan, J.P.; Shinagare, A.B.; Hornick, J.L.; Ramaiya, N.H.; Wagner, A.J. Metastasis in dedifferentiated liposarcoma: Predictors and outcome in 148 patients. Eur. J. Surg. Oncol. 2015, 41, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Bosenberg, M.W.; Mentzel, T.; McMenamin, M.E.; Oliveira, A.M.; Fletcher, C.D.M. Pleomorphic liposarcoma: Clinicopathologic analysis of 57 cases. Am. J. Surg. Pathol. 2004, 28, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Ten Heuvel, S.E.; Hoekstra, H.J.; van Ginkel, R.J.; Bastiaannet, E.; Suurmeijer, A.J.H. Clinicopathologic prognostic factors in myxoid liposarcoma: A retrospective study of 49 patients with long-term follow-up. Ann. Surg. Oncol. 2007, 14, 222–229. [Google Scholar] [CrossRef]


| Factor | Number of Patients | % |
|---|---|---|
| Patients | 82 | 100 |
| Gender | ||
| Male | 53 | 65 |
| Female | 29 | 35 |
| Location | ||
| Thigh | 51 | 62 |
| Leg | 24 | 29 |
| Buttock | 6 | 7 |
| Arm | 1 | 1 |
| Tumor size | ||
| >10 cm | 50 | 61 |
| 5–10 cm | 29 | 35 |
| <5 cm | 3 | 4 |
| Surgery | ||
| Excision | 80 | 98 |
| Amputation | 2 | 2 |
| Margin | ||
| Wide/radical | 63 | 77 |
| Marginal | 16 | 20 |
| Intralesional | 3 | 4 |
| Radiotherapy | ||
| Pre-operative | 57 | 68 |
| Post-operative | 19 | 23 |
| None | 6 | 9 |
| Chemotherapy | ||
| Pre-operative | 45 | 55 |
| Post-operative | 13 | 16 |
| Pre- and post-operative | 2 | 2 |
| None | 22 | 27 |
| Factor | Level | Kaplan–Maier Estimates (95% CI) 5-Year | p > chi2 |
|---|---|---|---|
| Gender | F | 100% (ND) | 0.592 |
| M | 93% (80–97) | ||
| Age | >60 | 100% (ND) | 0.787 |
| <60 | 94% (83–98) | ||
| Size | >10 cm | 93% (79–97) | 0.2919 |
| 5–10 cm | 100% | ||
| <5 cm | 100% | ||
| Location | Thigh | 93% (79–97) | 0.8938 |
| Leg | 100% | ||
| Buttock | 100% | ||
| Other | 100% | ||
| Margin | Wide/Radical | 96% (85–99) | 0.0806 |
| Marginal | 93% (59–99) | ||
| Intralesional | 100% | ||
| Chemotherapy | Yes | 94% (81–97) | 0.8497 |
| No | 100% | ||
| Radiotherapy | Yes | 96% (87–99) | 0.1279 |
| No | 83% (27–97) |
| Factor | Level | p > chi2 | HR (95%CI) |
|---|---|---|---|
| Gender | F (ref) | 0.1270 | |
| M | |||
| Age | >60 (ref) | 0.8263 | |
| <60 | |||
| Size | >10 cm (ref) | * 0.0337 | ** 0.248 (0.07–0.84) p = 0.026 |
| 5–10 cm | |||
| <5 cm | |||
| Location | Tight (ref) | 0.3708 | |
| Leg | |||
| Buttock | |||
| Other | |||
| Margin | Wide (ref) | 0.1099 | |
| Marginal | |||
| Intralesional | |||
| Radical | |||
| Chemotherapy | Yes (ref) | 0.0961 | |
| No | |||
| Radiotherapy | Yes (ref) | 0.1161 | |
| No |
| Factor | Level | p > chi2 | HR (95% CI) |
|---|---|---|---|
| Gender | F (ref) | 0.1462 | |
| M | |||
| Age | >60 (ref) | 0.7886 | |
| <60 | |||
| Size | >10 cm (ref) | 0.0199 | ** 0.1773 p = 0.020 95% CI: 0.4–0.76 |
| 5–10 cm | |||
| <5 cm | |||
| Location | Tight (ref) | 0.3236 | |
| Leg | |||
| Buttock | |||
| Arm | |||
| Margin | Wide/radical (ref) | 0.1097 | |
| Marginal | |||
| Intralesional | |||
| Chemotherapy | Yes (ref) | 0.1081 | |
| No | |||
| Radiotherapy | Yes (ref) | 0.2820 | |
| No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuzzato, G.; Laranga, R.; Ostetto, F.; Bubbico, E.; Vara, G.; Bianchi, G. Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern. Cancers 2022, 14, 2657. https://doi.org/10.3390/cancers14112657
Tuzzato G, Laranga R, Ostetto F, Bubbico E, Vara G, Bianchi G. Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern. Cancers. 2022; 14(11):2657. https://doi.org/10.3390/cancers14112657
Chicago/Turabian StyleTuzzato, Gianmarco, Roberta Laranga, Federico Ostetto, Elisa Bubbico, Giulio Vara, and Giuseppe Bianchi. 2022. "Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern" Cancers 14, no. 11: 2657. https://doi.org/10.3390/cancers14112657
APA StyleTuzzato, G., Laranga, R., Ostetto, F., Bubbico, E., Vara, G., & Bianchi, G. (2022). Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern. Cancers, 14(11), 2657. https://doi.org/10.3390/cancers14112657






