Simple Summary
Video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS) are known to be safe and efficient surgical procedures to treat lung cancer. Both VATS and RATS allow anatomical resection associated with radical lymph node dissection. However, RATS, unlike VATS, allows the thoracic surgeon to mimic an open approach and to perform lung resection. We hypothesized that the technical advantages of RATS, compared with VATS, would allow more precise resection, with “better lymph node dissection” which could increase survival compared to VATS. Nevertheless, VATS, and RATS nodal up-staging are still debated, with conflicting results and in our study, as well as in the medical literature, RATS failed to show its superiority over VATS in resectable non-small cell lung cancer.
Abstract
Background: Nowadays, video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS) are known to be safe and efficient surgical procedures to treat early-stage non-small cell lung cancer (NSCLC). We assessed whether RATS increased disease-free survival (DFS) compared with VATS for lobectomy and segmentectomy. Methods: This retrospective cohort study included patients treated for resectable NSCLC performed by RATS or VATS, in our tertiary care center from 2012 to 2019. Patients’ data were prospectively recorded and reviewed in the French EPITHOR database. Primary outcomes were 5-year DFS for lobectomy and 3-year DFS for segmentectomy, compared by propensity-score adjusted difference of Kaplan–Meier estimates. Results: Among 844 lung resections, 436 VATS and 234 RATS lobectomies and 46 VATS and 128 RATS segmentectomies were performed. For lobectomy, the adjusted 5-year DFS was 60.9% (95% confidence interval (CI) 52.9–68.8%) for VATS and 52.7% (95%CI 41.7–63.7%) for RATS, with a difference estimated at −8.3% (−22.2–+4.9%, p = 0.24). For segmentectomy, the adjusted 3-year DFS was 84.6% (95%CI 69.8–99.0%) for VATS and 72.9% (95%CI 50.6–92.4%) for RATS, with a difference estimated at −11.7% (−38.7–+7.8%, p = 0.21). Conclusions: RATS failed to show its superiority over VATS for resectable NSCLC.
1. Introduction
Anatomical resection associated with lymph node dissection is the cornerstone of resectable non-small cell lung cancer (NSCLC) treatment [1].
Today, video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS) are indicated (level of recommendation II, grade A) for the resection of early-stage NSCLC, clinical stage I [2,3], because their efficacy and safety have been proven. Compared to thoracotomy, VATS lung resection [4,5,6,7,8,9,10,11] in two randomized controlled trials [7,12,13] or RATS lung resection [11,14,15,16,17,18,19] led to better short-term outcomes, fewer adverse events, shorter hospital stays, and lower morbidity and mortality rates. Regarding short-term outcomes, the superiority of VATS or RATS is still debated in one randomized controlled trial [20] and in systematic reviews and meta-analysis and propensity-matched cohorts [11,14,15,18,21,22,23,24].
For long-term outcomes, overall survival (OS) and disease-free survival (DFS) are the main criteria of oncological quality to evaluate the resection performed for all cancers. No difference was reported when a minimally invasive approach, such as VATS or RATS, was compared with open surgery [11,14,15,18,21,22,23,24]. More than enhanced recovery, VATS and RATS also preserve long-term survival.
Today, few reports [11,14,21,22,23,25,26,27] have compared the long-term survival of RATS, a recent surgical approach, with VATS, a mature, controlled, and well-known approach, for resectable lung cancers. Moreover, no large-scale randomized controlled trial has been done to evaluate the equivalence or superiority of RATS over VATS.
Our objective was to assess whether RATS led to increased 5-year DFS compared with VATS for segmentectomy and lobectomy.
2. Materials and Methods
2.1. Study Type
We conducted an observational, retrospective, and comparative cohort study, in the department of general and thoracic surgery of Rouen University Hospital, France.
2.2. Inclusion/Exclusion Criteria
We included all patients aged ≥18 years old, who had undergone a VATS or RATS lobectomy or segmentectomy with curative intent for a pathological NSCLC of any clinical stage, between 1 January 2012 and 31 December 2019 in our center. Bilobectomy, pneumonectomy, and histologically invalidated NSCLC were excluded.
All cases were discussed in multidisciplinary meeting, in accordance with guidelines, during which the cancer treatment (radiotherapy, chemotherapy, surgery) was chosen. The choice between VATS and RATS was at the discretion of the surgeon and depended on the surgeon’s preference and availability of the robot: 6 days a month on the study period.
We did not exclude patients operated for a second NSCLC (multiple inclusions allowed), patients previously treated for another cancer, or patients with a clinical stage IV NSCLC with a resectable lung lesion associated with curable metastasis.
2.3. Surgical Procedures
VATS was performed by the modified anterior fissureless approach as described by Hansen et al. [28], using the da Vinci Si platform [29] (Intuitive Surgical, Sunnyvale, CA, USA) from 2012 to July 2018, and then the da Vinci X platform from 2018 to 2019. We first used the modified 3-arm technique with three robotic ports and an assistant port, then the modified 4-arm technique on the da Vinci Si platform without the robotic stapler, and then we switched to the da Vinci X platform with the robotic stapler.
From 2015 onwards, we used a multimodality and multidisciplinary approach [30,31,32,33] (Figure 1) combining 3D lung reconstruction (Visible Patient, Strasbourg, France) and lung tumor dye marking [32]. For RATS segmentectomy, we used near-infrared fluorescence with indocyanine green (ICG) to detect the tumor [30,34] and the intersegmental plane [35]; for VATS, we did not have a laser and did not use fluorescence.
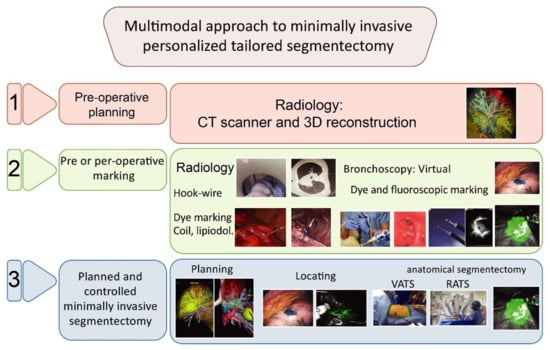
Figure 1.
Multimodal approach to minimally invasive personalized tailored segmentectomy in 3 steps.
Per- and post-operative management were guided by the principles of the enhanced recovery after surgery (ERAS) [36] program. Thoracic drainage was ensured by one chest tube that was removed as soon as possible when there were no air leaks and the amount of drained pleural effusion was less than 300 mL per day.
2.4. Data Collection
Patients were informed of the registration of their data in the French EPITHOR database and gave oral consent to participate in observational research projects. All data were prospectively entered in this database with a high completeness for peri-operative data in the thoracic surgery department. This database was completed and regularly checked by a dedicated data manager. The project protocol was designed in 2020, after most of the data was recorded (2012–2020) and consolidated (2019–2020).
2.5. Surveillance and Follow-Up
Follow-up of thoracic surgeons was recorded in EPITHOR, but patients moving to other centers or followed by pneumologists were quickly lost to follow-up (less than three months of follow-up). Long-term follow-up data was retrospectively completed from 2019 to 2020 via our center’s medical records, letters, and telephone calls to physicians or patients if needed. All-cause mortality was also assessed by examining the French national comprehensive public register of deaths. Only medically confirmed relapses were taken into account.
Patients were considered lost to follow-up, after consolidation of data, if they had a ratio of more than 0.5 between the time without news and the time with news, they had given no news for at least 6 months, were not dead, and had no relapse at last news.
2.6. Outcomes
The primary outcome was 5-year DFS, expressed as a percentage. We hypothesized the superiority of RATS over VATS in both segmentectomy and lobectomy. Secondary outcomes were OS and time to relapse (TTR). Due to an unexpectedly low number of patients followed for 5 years, the 5-year DFS could not be reliably estimated in the segmentectomy subgroup; therefore, the primary analysis of segmentectomy was restricted to 3 years. The DFS was defined as the time to death from any cause or recurrence of the same lung cancer, whichever came first, with censorship at last follow-up (administrative censoring and censoring of losses to follow-up). OS was defined as the time to death from any cause with censorship at last follow-up. TTR was defined as the time to recurrence of the same lung cancer or death caused by the lung cancer, whichever came first, censored at the time of death for patients who died of another cause and at last follow-up for alive patients with no recurrence of the lung cancer. The 5-year DFS, 5-year OS and 5-year TTR were defined as proportions of patients whose, respectively, DFS, OR, and TTR were longer than 5 years.
2.7. TNM Staging
For consistency of TNM stage between patients, we used the 7th Ed. of lung cancer TNM [37]. We converted the 8th Ed. of lung cancer TNM to the 7th Ed. if needed, using the summary of histological reports.
2.8. Statistical Analysis
The consolidated data were exported from EPITHOR and analyzed in R (version 4.0) statistical software. The primary analysis was the comparison of 5-year DFS with propensity-score adjustment performed in lobectomy (first test) and in segmentectomy (second test), each at the 5% significance level without multiple testing procedure. The difference of 5-year DFS was calculated from 5-year Kaplan–Meier estimates with propensity-score weighting, using overlap weights [38], combined with multiple imputation by fully conditional specification and non-parametric percentile bootstrap in a Boot-MI sequence [39]. The multiple imputation was based on predictive mean matching (PMM) for diffusing capacity of the lung carbon monoxide (DLCO) (n = 361/844, 42.8% of missing data) and forced expiratory volume in one second (FEV1) (n = 81/844, 9.6% of missing data); no other variable had missing data. The propensity score was based on a logistic regression explaining the probability of having RATS by age (<65 years, 65–74, 75–84, ≥ 85 years), smoking status (never, former, current), Eastern Cooperative Oncology Group (ECOG) performance status (linear effect), FEV1 (linear effect), DLCO (linear effect), the preoperative clinical tumor nodes metastasis (cTNM) classification (as categorical variable), histological type, and surgeon. These covariables were chosen a priori as possible confounders by indication. The same procedure was used to compare OS and TTR. The unadjusted 30- and 90-day mortality rates were estimated by the beta product confidence procedure (BPCP) [40] and compared by melded confidence intervals with the ‘bpcp’ R package. Comparisons of baseline characteristics between groups were performed by Fisher’s exact tests for qualitative variables, Student’s t-tests for means, and Mann–Whitney’s test for medians.
3. Results
3.1. Description and Comparison of Baseline Characteristics
From 1 January 2012 to 31 December 2019, we performed 1159 major lung resections by minimally invasive or open approach intending to treat a confirmed or a suspected resectable NSCLC. We included 815 patients for whom 844 minimally invasive lung resections were performed, including 670 lobectomies with 234 (34.9%) RATS and 436 (65.1%) VATS and 174 segmentectomies with 128 (73.5%) RATS and 46 (26.5%) VATS for a histologically confirmed NSCLC (Figure 2).
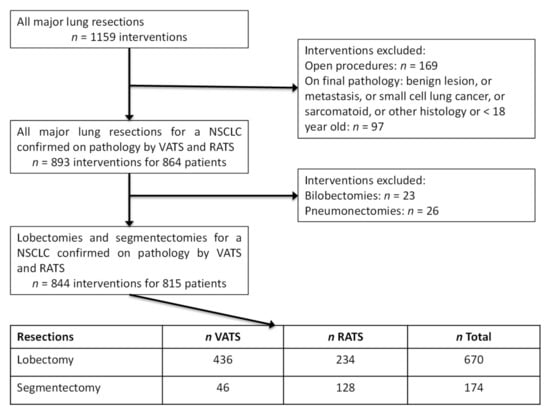
Figure 2.
Flow-chart of our surgical series of resectable NSCLC treated by lobectomy and segmentectomy performed by VATS and RATS from the 1st January 2012 to 31st December 2019.
These 844 procedures were performed by seven surgeons. Two surgeons (#1: n = 290; #2: n = 64) performed 354 (97.8%) RATS procedures. Four surgeons (#1: n = 144; #2: n = 156, #3: n = 110; #4: n = 53) performed 463 (96.1%) VATS procedures. Baseline characteristics of patients with lobectomy and segmentectomy are shown in Table 1.

Table 1.
Pre-operative characteristics of patients who had lobectomy and segmentectomy, according to VATS and RATS procedure.
3.2. Quality of Follow-Up
A total of 48 (5.8%) patients were lost to follow-up. In patients who had lobectomy by VATS or RATS, respectively, 28 (6.4%) and 15 (6.4%) were lost to follow-up, and in the segmentectomy group, respectively, 3 (6.5%) and 2 (1.6%) were lost to follow-up. After exclusion of patients who died and patients with recurrence of lung cancer, the overall median duration of oncological follow-up, capped to 60 months, in VATS and RATS patients was, respectively, 23.9 months (interquartile range (IQR) 7.0–53.8) and 23.4 months (IQR 11.0–42.5) without significant difference (p = 0.96).
3.3. Primary Analysis
After propensity-score weighting, the 5-year DFS of patients who had lobectomy by VATS and RATS, was estimated at 60.9% (95% CI 52.9–68.8%) and 52.7% (95% CI 41.7–63.7%), respectively, with a difference of −8.3% (−22.2 to +4.9%, p = 0.24, first primary analysis). After propensity-score weighting, the 5-year DFS (planned primary analysis) of patients who had segmentectomy by VATS and RATS could not be estimated, but the 3-year DFS was estimated at 84.6% (95% CI 69.8–99.0%) and 72.9% (95% CI 50.6–92.4%), respectively, with a difference of −11.7% (95% CI −38.7 to +7.8%, p = 0.21) (Table 2 and Figure 3).

Table 2.
Long-term survival results of patients who had lobectomy and segmentectomy, according to VATS and RATS procedure.
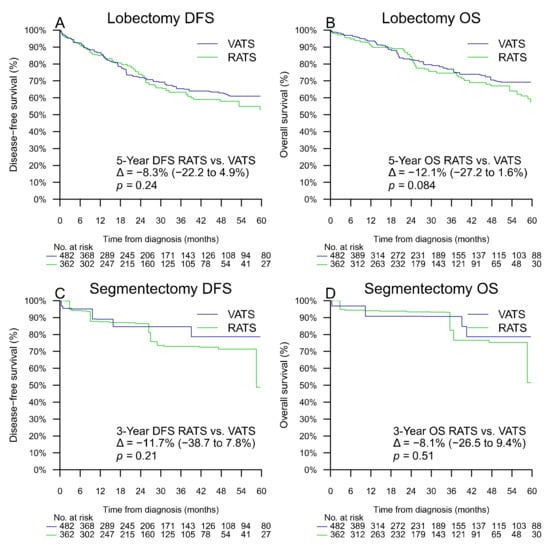
Figure 3.
Propensity-score adjusted DFS for lobectomy (A), OS for lobectomy (B), DFS for segmentectomy (C), and OS for segmentectomy (D). Δ represents the difference of percentage of survival with its 95% confidence interval.
3.4. Per-Operative and Short-Term Post-Operative Outcomes
The unadjusted frequency of nodal up-staging, conversion to thoracotomy, 30 and 90-day mortality rates, mean hospital length of stay, and complication rates and stages were not significantly different between VATS and RATS groups (Table 3) except for conversions to thoracotomy during a segmentectomy (p = 0.04).

Table 3.
Per-operative and post-operative characteristics of patients and tumors.
The operative time was significantly shorter for RATS segmentectomy than for VATS segmentectomy. After a post hoc adjustment for the surgeon in a general linear model, the difference of mean duration for RATS segmentectomy was estimated at +5.8 min for RATS (95% CI −9.3 to +21.0, p = 0.45) compared with VATS. Indeed, the most experienced surgeon performed 78.9% (n = 101/128) of all RATS segmentectomies but only 13.0% (n = 6/46) of all VATS segmentectomies.
3.5. Characteristics of Recurrences
Long-term tumor-related outcomes and recurrences are described in Table 4. Recurrences and treatments were not significantly different between VATS and RATS groups, and 79.1% of patients were free of recurrence in the lobectomy group and 85.0% in the segmentectomy group. Among recurrences occurring within 5 years of the VATS or RATS lobectomy or segmentectomy, 63 (39.9%) were local, 59 (37.3%) were metastatic, and 36 (22.8%) were both local and metastatic recurrences. Between 71.3 and 86.7% of deaths occurring beyond 90 days, were attributed to the operated lung cancer among RATS-VATS and lobectomy-segmentectomy subgroups. A secondary planned subgroup analysis (Table 5) of stage IA tumors found non-significant DFS, OS, and TTR differences, between RATS and VATS.

Table 4.
Long-term tumor-related outcomes of patients with RATS or VATS lobectomy or segmentectomy.

Table 5.
Unadjusted and adjusted comparison of long-term survival in the subgroup of patients with cTNM stage I tumors.
4. Discussion
4.1. Summary of Main Results
In our cohort, we failed to show the long-term oncological superiority of RATS over VATS for resectable lung cancer treated by lobectomy or segmentectomy and lymph node dissection, with a 5-year adjusted estimated difference of DFS of −8.3% (−22.2 to +4.9%, p = 0.24) for lobectomy and a 3-year adjusted difference of DFS of −11.7% (95% CI −38.7 to +7.8%, p = 0.21) for segmentectomy.
4.2. Comparison with Literature
In comparison with systematic review and meta-analysis [11,14,21,22,23], and matched cohort analysis [25,26] (Table 6) that analyzed long-term survival data after comparing VATS and RATS approaches, our results confirm the absence of major superiority of the robotic approach.

Table 6.
Main results of systematic review and meta-analysis, and matched cohort analysis included in our analysis regarding long-term survival following RATS, VATS lobectomy and segmentectomy for NSCLC.
Nevertheless, we can cite two articles that seem to show an advantage of the robotic approach in terms of recurrence rate and DFS. First of all, the meta-analysis of Ma et al. [21], which showed an advantage of RATS for crude recurrence rate (odds ratio (OR): 0.53; 95% CI 0.37–0.74, p < 0.001) for lobectomy but not for segmentectomy, p = 0.18. However, it did not take into account the difference in length of follow-up attributed to the fact that RATS procedures are usually more recent than VATS. Moreover, Ma et al. [21] found no advantage of RATS for 5-year DFS or OS. Next, in the meta-analysis of Wu et al. [23] an advantage was shown for the robotic approach, compared with VATS, for lobectomy, for 5-year DFS (hazard ratio (HR): 0.76; 95% CI: 0.59–0.97, p = 0.03, but without significant superiority of RATS for 5-year OS (HR: 0.77; 95% CI: 0.57–1.05, p = 0.10). Compared with larger VATS series [8,41,42,43], RATS series [15,18,19,44,45,46], systematic reviews and meta-analyses [11,14,21,22,23], and matched cohort analyses [25,26,27] (Table 6), our long-term survival rates for lobectomy are consistent, with 5-year DFS rates of 60.9% for VATS and 52.7% for RATS (propensity score adjusted), p = 0.24, and 5-year OS rates of 76.0% for VATS and 70.4% for RATS, p = 0.54 (propensity score adjusted).
Our multidisciplinary approach [30,31,32,33] (Figure 1 and Figure 3) allows a minimally invasive tailored anatomical segmentectomy with improved surgical margins and oncological effectiveness and safety with preserved long-term survival, a 3-year DFS of 82.4% for VATS, and 78.0% for RATS, p = 0.59 (propensity score adjusted) and a 3-year OS of 87.8% for VATS, and 90.1% for RATS, p = 0.81 (propensity score adjusted). Our long-term survival results are consistent with the literature [21,33,47,48].
Compared with our VATS experience with the anterior fissureless approach technique [28], RATS allows the thoracic surgeon to mimic an open approach to perform lung resection. However, regardless of the approach, the lung resection remains the same, with anatomical resection associated with radical lymph node dissection. Thus, long-term survival in our report and in the literature [11,14,21,22,23,25,26,27], does not seem to be greatly influenced by the surgical approach. We hypothesized that the technical advantages of RATS, compared with VATS, would allow more precise resection, with “better lymph node dissection” which could allow increased survival compared with VATS. Nevertheless, VATS, and RATS nodal up-staging compared with thoracotomy are still debated, with conflicting results about lower upstaging by VATS [11,27,49], higher upstaging by RATS [11,27], or a lack of difference [14,22,23,26], but without a significant impact on long-term survival. In our cohort we did not find significant differences in nodal up-staging rates in VATS and RATS groups; however, the sample size was not large enough to conclude that the staging rates were equivalent. Finally, even if lymph node dissection seemed easier to complete, it is more operator dependent than approach dependent, all three approaches (open surgery, VATS, RATS) allowing a complete quality oncological resection. This could be one of the reasons explaining the lack of significant difference of OS and DFS between RATS and VATS.
4.3. Strengths
Our cohort is one of the largest comparing 5-year outcomes between RATS and VATS, and although underpowered, will add high-quality data to the meta-analysis. Our database is prospectively completed and regularly controlled by our dedicated data manager, guaranteeing a good quality of data.
Although the study was not randomized, the surgical indications for VATS and RATS are mostly the same in our thoracic surgery department, except our preference for RATS for segmentectomy, in a multimodal [30,31,32] approach but lobectomy and segmentectomy were analyzed separately.
4.4. Limitations
We report one of the largest single center surgical cohorts of lobectomy and segmentectomy performed by VATS and RATS. However, the cohort is still too small to draw any firm conclusions regarding the long-term oncological outcomes of VATS and RATS. For 5-year OS the difference between thoracotomy and VATS or RATS is less than 5% according to the largest comparative studies and meta-analyses [11,14,21,22,23,25,26,27].
Another limitation of this study is the follow-up period which was shorter than planned due to the COVID-19 pandemic disrupting our surgical activity. We have now adapted our surgical activity to the pandemic, and a new study could be conducted with longer follow-up which would perhaps allow us to answer our initial research question.
As this study is observational, confounding by indication was possible. The indication of VATS or RATS mainly depended on the surgeon’s preferences and the type of surgery, with a preference for RATS for segmentectomy and for VATS for lobectomy. Since segmentectomy and lobectomy were analyzed separately and the surgeon was included in the propensity score, these main indication biases were canceled. However, we noticed a significant difference of ECOG performance status between RATS and VATS in the lobectomy group suggesting that other confounders were possible. There were adjustments on main prognostic factors, including the ECOG performance status, but a residual confounding bias is possible. However, tumor stages were not significantly different between groups, and propensity-score adjustment had no major effect on DFS and OS differences between VATS and RATS, suggesting that the indication bias may not have had a major impact on results.
The anterior VATS approach was introduced in our department in 2008, and RATS in 2012. Our cohort includes our RATS learning curve, but not that of VATS, which may reduce the comparability of procedures.
4.5. Perspectives
Following international recommendations [3,50,51,52] we used minimally invasive procedures to perform major lung resection for most resectable NSCLC, both for early stages and for advanced cases. Our results regarding short- and long-term survival are encouraging for these “extended” indications of minimally invasive lobectomy, with few conversions to thoracotomy, few postoperative complications, and preserved long-term survival in comparison with the literature [11,14,21,22,23,25,26,27].
For early-stage NSCLC, the time may no longer be ripe for the opposition and confrontation of VATS and RATS, because both techniques can be used by the same surgical team making it possible to optimize the oncological management of patients while also taking into account the logistical and economic constraints [29,53,54] in our hospitals.
We believe that evidence will emerge in the next few years to support robotic surgery as the optimal minimally invasive platform for complex lung resections as segmentectomy and locally advanced NSCLC, as RATS allows surgeons to mimic open surgery while maintaining the advantages of minimally invasive approaches [1].
5. Conclusions
We failed to show the long-term oncological superiority of RATS over VATS in a single center cohort in real-life clinical practice. However, our cohort was too small to detect moderate differences between RATS and VATS. Perhaps the main reason is that both VATS and RATS allow the surgeon to perform oncological resection with complete lymph node dissection and the main limitation is not the tool but the operator. Nevertheless, we plan to compare RATS with VATS in a future multicenter cohort of the French EPITHOR database, once the duration of follow-up is improved.
Author Contributions
Data curation, F.M. (François Montagne), Z.C., F.M. (Frankie Mbadinga), and A.G.; formal analysis, F.M. (François Montagne) and A.G.; investigation, F.M. (François Montagne) and J.-M.B.; methodology, F.M. (François Montagne), F.M. (Frankie Mbadinga), A.G. and J.-M.B.; resources, F.M. (François Montagne), Z.C., F.M. (Frankie Mbadinga), A.G. and J.-M.B.; supervision, J.-M.B.; validation, F.M. (François Montagne), A.G. and J.-M.B.; writing—original draft, F.M. (François Montagne), Z.C., B.B., M.S., F.M. (Frankie Mbadinga), J.S., F.G. and J.-M.B.; writing—review and editing, F.M. (François Montagne), Z.C., B.B., M.S., F.M. (Frankie Mbadinga), J.S., F.G., A.G. and J.-M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the institutional review board of the Rouen University Hospital.
Informed Consent Statement
Patients were informed of the registration of their data in the EPITHOR database and gave a generic oral consent to observational research participation.
Data Availability Statement
The pseudonymized data presented in this study are available on request from the corresponding author. The data are not publicly available due to legal restrictions in France.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Conflicts of Interest
J.M.B. is proctor for Intuitive Surgical, Baxter and Medtronic companies. F.M., Z.C., B.B., M.S., F.M., J.S. and F.G.: none. A.G.: received personal fee for methodological consultancy and statistical teaching from Gleamer company.
References
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives—State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Van Schil, P.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Laursen, L.; Petersen, R.H.; Hansen, H.J.; Jensen, T.K.; Ravn, J.; Konge, L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur. J. Cardio-Thorac. 2016, 49, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Pagès, P.-B.; Delpy, J.-P.; Orsini, B.; Gossot, D.; Baste, J.-M.; Thomas, P.; Dahan, M.; Bernard, A. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy With Thoracotomy: A French Nationwide Study. Ann. Thorac. Surg. 2016, 101, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Falcoz, P.-E.; Puyraveau, M.; Thomas, P.; Decaluwe, H.; Hürtgen, M.; Petersen, R.H.; Hansen, H.; Brunelli, A.; Van Raemdonck, D.; Dahan, M.; et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: A propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur. J. Cardio-Thorac. 2016, 49, 602–609. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Paul, S.; Isaacs, A.J.; Treasure, T.; Altorki, N.K.; Sedrakyan, A. Long term survival with thoracoscopic versus open lobectomy: Propensity matched comparative analysis using SEER-Medicare database. Bmj. Br. Med. J. 2014, 349, g5575. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Sedrakyan, A.; Chiu, Y.-L.; Nasar, A.; Port, J.L.; Lee, P.C.; Stiles, B.M.; Altorki, N.K. Outcomes after lobectomy using thoracoscopy vs thoracotomy: A comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur. J. Cardio-Thorac. 2013, 43, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Altorki, N.K.; Sheng, S.; Lee, P.C.; Harpole, D.H.; Onaitis, M.W.; Stiles, B.M.; Port, J.L.; D’Amico, T.A. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J. Thorac. Cardiovasc. Surg. 2010, 139, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Aiolfi, A.; Nosotti, M.; Micheletto, G.; Khor, D.; Bonitta, G.; Perali, C.; Marin, J.; Biraghi, T.; Bona, D. Pulmonary lobectomy for cancer: Systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery 2021, 169, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Batchelor, T.; Shackcloth, M.; Dunning, J.; Mcgonigle, N.; Brush, T.; Dabner, L.; Harris, R.; Mckeon, H.; Paramasivan, S.; et al. Study protocol for VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer, a UK multicentre randomised controlled trial with an internal pilot (the VIOLET study). Bmj. Open. 2019, 9, e029507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.K.S.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-assisted thoracoscopic versus open lobectomy in patients with early-stage lung cancer: One-year results from a randomized controlled trial (VIOLET). J. Clin. Oncol. 2021, 39, 8504. [Google Scholar] [CrossRef]
- Ng, C.S.; MacDonald, J.K.; Gilbert, S.; Khan, A.Z.; Kim, Y.T.; Louie, B.E.; Marshall, M.B.; Santos, R.S.; Scarci, M.; Shargal, Y.; et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innov. Technol. Tech. Cardiothorac Vasc Surg. 2019, 14, 90–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Shen, Y.; Onaitis, M. Comparative study of anatomic lung resection by robotic vs. video-assisted thoracoscopic surgery. J. Thorac. Dis. 2019, 11, 1243–1250. [Google Scholar] [CrossRef]
- O’Sullivan, K.E.; Kreaden, U.S.; Hebert, A.E.; Eaton, D.; Redmond, K.C. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 526–534. [Google Scholar] [CrossRef]
- Wei, S.; Chen, M.; Chen, N.; Liu, L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 98. [Google Scholar] [CrossRef]
- Kent, M.; Wang, T.; Whyte, R.; Curran, T.; Flores, R.; Gangadharan, S. Open, Video-Assisted Thoracic Surgery, and Robotic Lobectomy: Review of a National Database. Ann. Thorac. Surg. 2014, 97, 236–244. [Google Scholar] [CrossRef]
- Adams, R.D.; Bolton, W.D.; Stephenson, J.E.; Henry, G.; Robbins, E.T.; Sommers, E. Initial Multicenter Community Robotic Lobectomy Experience: Comparisons to a National Database. Ann. Thorac. Surg. 2014, 97, 1893–1900. [Google Scholar] [CrossRef]
- Veronesi, G.; Abbas, A.E.-S.; Muriana, P.; Lembo, R.; Bottoni, E.; Perroni, G.; Testori, A.; Dieci, E.; Bakhos, C.T.; Car, S.; et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front. Oncol. 2021, 11, 726408. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: A meta-analysis. Bmc. Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; D’Souza, D.M.; Richardson, M.; Abdel-Rasoul, M.; Moffatt-Bruce, S.D.; Merritt, R.E. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non–Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin. Lung Cancer 2020, 21, 214–224.e2. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, R.; Yang, S.; Park, B.J.; Li, H. Long-term and short-term outcomes of robot- versus video-assisted anatomic lung resection in lung cancer: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. 2020, 59, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sesti, J.; Langan, R.C.; Bell, J.; Nguyen, A.; Turner, A.; Hilden, P.; Leshchuk, K.; Dabrowski, M.; Paul, S. A Comparative Analysis of Long-Term Survival of Robotic Versus Thoracoscopic Lobectomy. Ann. Thorac. Surg. 2020, 110, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Veluswamy, R.R.; Brown, S.-A.W.; Mhango, G.; Sigel, K.; Nicastri, D.G.; Smith, C.B.; Bonomi, M.; Galsky, M.D.; Taioli, E.; Neugut, A.I.; et al. Comparative Effectiveness of Robotic-Assisted Surgery for Resectable Lung Cancer in Older Patients. Chest 2020, 157, 1313–1321. [Google Scholar] [CrossRef]
- Yang, H.-X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.J.; Petersen, R.H. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach-The Copenhagen experience. Ann. Cardiothorac. Surg. 2012, 1, 70–76. [Google Scholar]
- Gondé, H.; Laurent, M.; Gillibert, A.; Sarsam, O.M.; Varin, R.; Grimandi, G.; Peillon, C.; Baste, J.-M. The affordability of minimally invasive procedures in major lung resection: A prospective study. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 469–475. [Google Scholar] [CrossRef]
- Sarsam, M.; Baste, J.-M.; Lachkar, S. Multidisciplinary approach to minimally invasive lung segmentectomy. J. Vis. Surg. 2020, 6, 50. [Google Scholar] [CrossRef]
- Baste, J.M.; Soldea, V.; Lachkar, S.; Rinieri, P.; Sarsam, M.; Bottet, B.; Peillon, C. Development of a precision multimodal surgical navigation system for lung robotic segmentectomy. J. Thorac. Dis. 2018, 10, S1195–S1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachkar, S.; Baste, J.-M.; Thiberville, L.; Peillon, C.; Rinieri, P.; Piton, N.; Guisier, F.; Salaün, M. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respiration 2018, 95, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chaari, Z.; Montagne, F.; Sarsam, M.; Bottet, B.; Rinieri, P.; Gillibert, A.; Baste, J.M. Midterm survival of imaging-assisted robotic lung segmentectomy for non-small-cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, F.; Jiang, G.; Wang, J. Applications of indocyanine green based near-infrared fluorescence imaging in thoracic surgery. J. Thorac. Dis. 2016, 8, S738–S743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nex, G.; Schiavone, M.; De Palma, A.; Quercia, R.; Brascia, D.; De Iaco, G.; Signore, F.; Panza, T.; Marulli, G. How to identify intersegmental planes in performing sublobar anatomical resections. J. Thorac. Dis. 2020, 12, 3369–3375. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; H Petersen, R.; M Popescu, W.; D Slinger, P. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Giroux, D.; Goldstraw, P. The new TNM classification of lung cancer in practice. Breathe 2011, 7, 348–360. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2018, 188, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomaker, M.; Heumann, C. Bootstrap inference when using multiple imputation. Stat. Med. 2018, 37, 2252–2266. [Google Scholar] [CrossRef]
- Fay, M.P.; Brittain, E.H. Finite sample pointwise confidence intervals for a survival distribution with right-censored data. Stat. Med. 2016, 35, 2726–2740. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-F.J.; Kumar, A.; Klapper, J.A.; Hartwig, M.G.; Tong, B.C.; Harpole, D.H.; Berry, M.F.; D’Amico, T.A. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann. Surg. 2019, 269, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Boffa, D.J.; Kosinski, A.S.; Furnary, A.P.; Kim, S.; Onaitis, M.W.; Tong, B.C.; Cowper, P.A.; Hoag, J.R.; Jacobs, J.P.; Wright, C.D.; et al. Minimally Invasive Lung Cancer Surgery Performed by Thoracic Surgeons as Effective as Thoracotomy. J. Clin. Oncol. 2018, 36, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Huang, J.-Y.; Lin, C.-H.; Ko, J.-L.; Chou, C.-T.; Wu, Y.-C.; Lin, S.-H.; Liaw, Y.-P. Thoracoscopic Lobectomy Produces Long-Term Survival Similar to That with Open Lobectomy in Cases of Non–Small Cell Lung Carcinoma: A Propensity-Matched Analysis Using a Population-Based Cancer Registry. J. Thorac. Oncol. 2016, 11, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, L.; Sedda, G.; Maisonneuve, P.; Tessitore, A.; Casiraghi, M.; Petrella, F.; Galetta, D. A Brief Report on Survival After Robotic Lobectomy for Early-Stage Lung Cancer. J. Thorac. Oncol. 2019, 14, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Ghanim, A.F.; Dylewski, M.; Veronesi, G.; Spaggiari, L.; Park, B.J. The long-term survival of robotic lobectomy for non–small cell lung cancer: A multi-institutional study. J. Thorac. Cardiovasc. Surg. 2018, 155, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann. Cardiothorac. Surg. 2012, 1, 24–26. [Google Scholar]
- Lutz, J.A.; Seguin-Givelet, A.; Grigoroiu, M.; Brian, E.; Girard, P.; Gossot, D. Oncological results of full thoracoscopic major pulmonary resections for clinical Stage I non-small-cell lung cancer. Eur. J. Cardio-Thorac. 2018, 55, 263–270. [Google Scholar] [CrossRef]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur. J. Cardio-Thorac. 2018, 55, 427–433. [Google Scholar] [CrossRef]
- Licht, P.B.; Jørgensen, O.D.; Ladegaard, L.; Jakobsen, E. A National Study of Nodal Upstaging After Thoracoscopic Versus Open Lobectomy for Clinical Stage I Lung Cancer. Ann. Thorac. Surg. 2013, 96, 943–950. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Schil, P.E.V.; Balduyck, B.; Waele, M.D.; Hendriks, J.M.; Hertoghs, M.; Lauwers, P. Surgical treatment of early-stage non-small-cell lung cancer. Eur. J. Cancer 2013, 11, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Dahan, M.; Riquet, M.; Massart, G.; Falcoz, P.E.; Brouchet, L.; Le Pimpec Barthes, F.; Doddoli, C.; Martinod, E.; Fadel, E.; et al. Pratiques chirurgicales dans le traitement du cancer primitif non à petites cellules du poumon Recommandations de la SFCTCV: Pratiques chirurgicales dans le traitement du cancer du poumon. Rev. Mal. Respir. 2008, 25, 1031–1036. [Google Scholar] [CrossRef]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Gac, C.L.; Gondé, H.; Gillibert, A.; Laurent, M.; Selim, J.; Bottet, B.; Rémi, V.; Jean-Marc, B. Medico-economic impact of robot-assisted lung segmentectomy: What is the cost of the learning curve? Interact. Cardio Vasc. Thorac. Surg. 2019, 30, 255–262. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).