Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
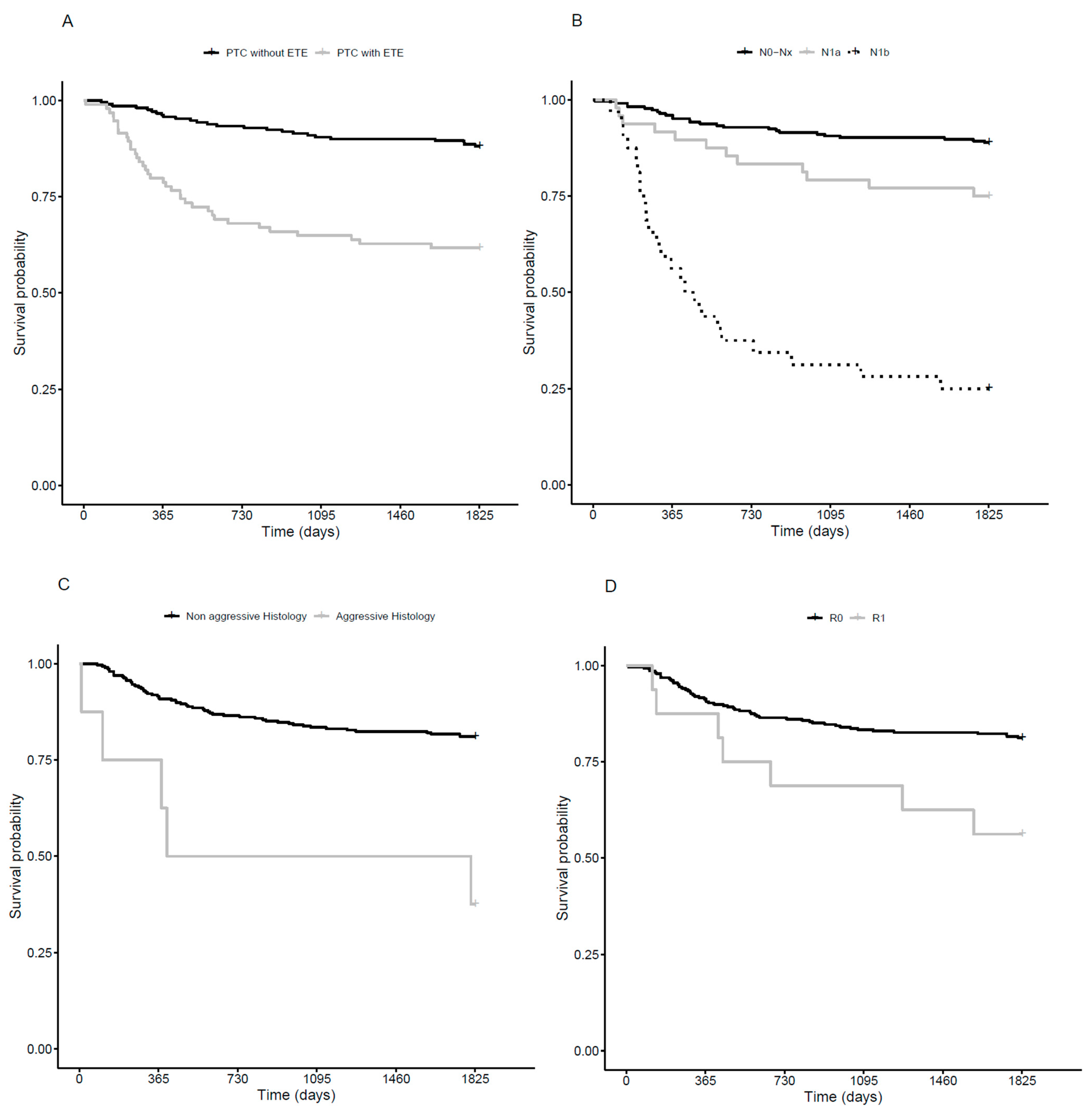
3.2. Disease Free Survival
3.3. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, N. A Population-Based Analysis of Survival Factors in Differentiated and Medullary Thyroid Carcinoma. Otolaryngol. Head Neck Surg. 2003, 128, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Veiga, L.; Neta, G.; Aschebrook-Kilfoy, B.; Ron, E.; Devesa, S. Thyroid Cancer Incidence Patterns in Sao Paulo, Brazil, and the U.S. SEER Program, 1997–2008. Thyroid 2013, 23, 748–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, J.P.; Loree, T.R.; Dharker, D.; Strong, E.W.; Begg, C.; Vlamis, V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am. J. Surg. 1992, 164, 658–661. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node- Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (8th ed.): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Xing, M. Genetic-guided Risk Assessment and Management of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 109–124. [Google Scholar] [CrossRef]
- Sorrenti, S.; Carbotta, G.; Di Matteo, F.M.; Catania, A.; Pironi, D.; Tartaglia, F.; Tarroni, D.; Gagliardi, F.; Tripodi, D.; Watanabe, M.; et al. Evaluation of Clinicopathological and Molecular Parameters on Disease Recurrence of Papillary Thyroid Cancer Patient: A Retrospective Observational Study. Cancers 2020, 12, 3637. [Google Scholar] [CrossRef]
- Hay, I.D.; Johnson, T.R.; Thompson, G.B.; Sebo, T.J.; Reinalda, M.S. Minimal extrathyroid extension in papillary thyroid carcinoma does not result in increased rates of either cause-specific mortality or postoperative tumor recurrence. Surgery 2016, 159, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Radowsky, J.S.; Howard, R.S.; Burch, H.B.; Stojadinovic, A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014, 24, 241–244. [Google Scholar] [CrossRef]
- Ito, Y.; Tomoda, C.; Uruno, T.; Takamura, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Kuma, K.; Miyauchi, A. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: Massive but not minimal extension affects the relapse-free survival. World J. Surg. 2006, 30, 780–786. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Diker-Cohen, T.; Hirsch, D.; Shimon, I.; Bachar, G.; Akirov, A.; Duskin-Bitan, H.; Robenshtok, E. Impact of Minimal Extra-Thyroid Extension in Differentiated Thyroid Cancer: Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2018, 3, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Chéreau, N.; Buffet, C.; Trésallet, C.; Tissier, F.; Golmard, J.L.; Leenhardt, L.; Menegaux, F. Does Extracapsular Extension Impact the Prognosis of Papillary Thyroid Microcarcinoma? Ann. Surg. Oncol. 2014, 21, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Roshan, D.; Abraham, E.; Wang, L.; Garibotto, N.; Wykes, J.; Campbell, P.; Ebrahimi, A. An Analysis of The American Joint Committee on Cancer 8th Edition T Staging System for Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, Z.; Sun, W.; Zhang, H. The new T3b category has clinical significance? SEER-based study. Clin. Endocrinol. 2021, 94, 449–459. [Google Scholar] [CrossRef]
- Parvathareddy, K.; Siraj, A.K.; Qadri, Z.; DeVera, F.; Siddiqui, K.; Al-Sobhi, S.; Al-Daye, F.; Al-Kuraya, K.S. Microscopic Extrathyroidal Extension Results in Increased Rate of Tumor Recurrence and Is an Independent Predictor of Patient’s Outcome in Middle Eastern Papillary Thyroid Carcinoma Sandeep. Front. Oncol. 2021, 11, 724432. [Google Scholar] [CrossRef]
- Nixon, I.J.; Ganly, I.; Patel, S.; Palmer, F.L.; Whitcher, M.M.; Tuttle, R.M.; Shaha, A.R.; Shah, J.P. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery 2011, 150, 1242–1249. [Google Scholar] [CrossRef] [Green Version]
- Forleo, R.; Grani, G.; Alfò, M.; Zilioli, V.; Giubbini, R.; Zatelli, M.C.; Gagliardi, I.; Piovesan, A.; Ragni, A.; Morelli, S.; et al. Minimal Extrathyroidal Extension in Predicting 1-Year Outcomes: A Longitudinal Multicenter Study of Low-to-Intermediate-Risk Papillary Thyroid Carcinoma (ITCO#4). Thyroid 2021, 12, 1814–1821. [Google Scholar] [CrossRef]
- Weber, M.; Binse, I.; Oebbecke, K.; Brandenburg, T.; Herrmann, K.; Theurer, S.; Weber, F.; Ehrlich, A.; Schmid, K.W.; Führer-Sakel, D.; et al. Analysis of risk factors and prognosis in differentiated thyroid cancer with focus on minimal extrathyroidal extension. BMC Endocr. Disord. 2021, 21, 161. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.G.; Jeon, M.J.; Kim, H.K.; Yi, H.S.; Kim, E.S.; Kim, B.H.; Kim, W.B.; Shong, Y.K.; Kan, H.C.; et al. Modification of the Tumor-Node-Metastasis Staging System for Differentiated Thyroid Carcinoma by Considering Extra-Thyroidal Extension and Lateral Cervical Lymph Node Metastasis. Endocrinol. Metab. 2020, 35, 149–156. [Google Scholar] [CrossRef]
- Park, J.S.; Chang, J.W.; Liu, L.; Jung, S.N.; Koo, B.S. Clinical implications of microscopic extrathyroidal extension in patients with papillary thyroid carcinoma. Oral Oncol. 2017, 72, 183–187. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, E.K.; Chung, W.Y.; Yoon, J.H.; Kwak, J.Y. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: Is it a real prognostic factor? Ann. Surg. Oncol. 2011, 18, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.G.; Forleo, R.; Maino, F.; Fralassi, N.; Barbato, F.; Palmitesta, P.; Pilli, T.; Capezzone, M.; Brilli, L.; Ciuolin, C.; et al. Small papillary thyroid carcinoma with minimal extrathyroidal extension should be managed as ATA low-risk tumor. J. Endocrinol. Investig. 2018, 41, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourão, G.; Calsolari, M.R. Risk of recurrence in patients with papillary thyroid carcinoma and minimal extrathyroidal extension not treated with radioiodine. J. Endocrinol. Investig. 2019, 42, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, R.; Song, L.; Chen, W.; Jiang, K.; Tang, H.; Wei, T.; Li, Z.; Gong, R.; Lei, J.; et al. Implications of Extrathyroidal Extension Invading Only the Strap Muscles in Papillary Thyroid Carcinomas. Thyroid 2020, 30, 57–64. [Google Scholar] [CrossRef]
- Mete, O.; Rotstein, L.; Asa, S.L. Controversies in thyroid pathology: Thyroid capsule invasion and extrathyroidal extension. Ann. Surg. Oncol. 2010, 17, 386–391. [Google Scholar] [CrossRef]
- Tam, S.; Amit, M.; Boonsripitayanon, M.; Busaidy, N.L.; Cabanillas, M.E.; Waguespack, S.G.; Gross, N.D.; Grubbs, E.G.; Williams, M.D.; Lai, S.Y.; et al. Effect of Tumor Size and Minimal Extrathyroidal Extension in Patients with Differentiated Thyroid Cancer. Thyroid 2018, 28, 982–990. [Google Scholar] [CrossRef]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.; Ba, G.G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Thomas, E.G.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81 (Suppl. 1), 1–122. [Google Scholar] [CrossRef]
- Pacini, F.; Basolo, F.; Bellantone, R.; Boni, G.; Cannizzaro, M.A.; De Palma, M.; Durante, C.; Elisei, R.; Fadda, G.; Frasoldati, A.; et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: Joint statements of six Italian societies. J. Endocrinol. Investig. 2018, 41, 849–876. [Google Scholar] [CrossRef] [Green Version]
- Zerdoud, S.; Giraudet, A.L.; Leboulleux, S.; Leenhardt, L.; Bardet, S.; Clerc, J.; Toubert, M.E.; Al Ghuzlan, A.; Lamy, P.J.; Bournaud, C.; et al. Radioactive iodine therapy, molecular imaging and serum biomarkers for differentiated thyroid cancer: 2017 guidelines of the French Societies of Nuclear Medicine, Endocrinology, Pathology, Biology, Endocrine Surgery and Head and Neck Surgery. Ann. Endocrinol. 2017, 78, 162–175. [Google Scholar] [CrossRef]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef] [PubMed]
| mETE (n = 93) | No mETE (n = 210) | p Value | ||
|---|---|---|---|---|
| Mean age, years (range) | 57 (25–86) | 57 (29–90) | 0.695 | |
| Female, n (%) | 73 (78.5) | 170 (80.9) | 0.620 | |
| Aggressive pathology, n (%) | 5 (5.3) | 3 (1.4) | 0.061 | |
| Lymph node involvement, n (%) | <0.001 | |||
| N0/Nx | 51 (54.8) | 173 (82.3) | ||
| N1a | 24 (25.8) | 23 (11.0) | ||
| N1b | 18 (19.4) | 14 (6.7) | ||
| Margin resection, n (%) | R0 R1 | 80 (86) 13 (13.9) | 208 (99) 2.0 (0.9) | <0.001 |
| Tumor size, mm | Mean Range | 16.3 3–39 | 19.1 1–39 | 0.006 |
| Metastasis at diagnosis, n (%) | 6 (6.4) | 6 (2.8) | 0.198 |
| Hazard Ratio | [95% CI] | p Value | ||
|---|---|---|---|---|
| mETE | No Yes | 1 2.55 | [1.48; 4.40] | <0.001 |
| Lymph node involvement | N0/Nx N1a N1b | 1 1.67 8.94 | [0.81; 3.46] [4.92; 16.26] | <0.001 |
| Rem. (n) | Bio/In (n) | Struct (n) | Bio/In (OR) | Struct (OR [95% CI]) | p Value * | ||
|---|---|---|---|---|---|---|---|
| Age, years | ≤55 >55 | 168 73 | 24 10 | 16 8 | 1 0.96 [0.44; 2.11] | 1 1.15 [0.47; 2.81] | 0.945 |
| mETE | No Yes | 180 61 | 18 16 | 8 16 | 1 2.62 [1.26; 5.46] | 1 5.90 [2.41; 14.47] | <0.001 |
| Aggressive pathology | No Yes | 238 3 | 33 1 | 20 4 | 1 2.40 [0.24; 23.8] | 1 15.87 [3.32; 75.88] | 0.005 |
| Lymph node involvement | N0/Nx N1a N1b | 192 35 14 | 16 8 10 | 14 3 7 | 1 2.74 [1.09; 6.90] 8.57 [3.29; 22.35] | 1 1.18 [0.32; 4.30] 6.86 [2.38; 19.74] | <0.001 |
| Margin resection | R0 R1 | 231 10 | 32 2 | 21 3 | 1 1.44 [0.30; 6.89] | 1 3.30 [0.84; 12.43] | 0.289 |
| Tumor size, mm | ≤10 10–20 >20 | 41 105 95 | 9 15 10 | 4 12 8 | 1 0.48 [0.18; 1.27] 0.65 [0.26; 1.60] | 1 0.86 [0.25; 3.03] 1.17 [0.36; 1.60] | 0.641 |
| Rem. (n) | Bio/In. (n) | Struct. (n) | Bio/In (OR) | Struct. (OR) | p Value * | ||
|---|---|---|---|---|---|---|---|
| mETE | No Yes | 180 61 | 18 16 | 8 16 | 1 1.83 [0.83; 4.06] | 1 4.92 [1.87; 12.97] | 0.003 |
| Aggressive pathology | No Yes | 238 3 | 33 1 | 20 4 | 1 1.90 [0.17; 20.86] | 1 12.84 [2.23; 73.97] | 0.020 |
| Lymph node involvement | N0/Nx N1a N1b | 192 35 14 | 6 8 10 | 14 3 7 | 1 2.27 [0.87; 5.92] 7.28 [2.72; 19.48] | 1 0.59 [0.14; 2.48] 3.98 [1.23; 12.84 ] | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzehouane, N.; Roy, P.; Decaussin-Petrucci, M.; Bertholon-Grégoire, M.; Bully, C.; Perrin, A.; Lasolle, H.; Lifante, J.-C.; Borson-Chazot, F.; Bournaud, C. Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC). Cancers 2022, 14, 2591. https://doi.org/10.3390/cancers14112591
Bouzehouane N, Roy P, Decaussin-Petrucci M, Bertholon-Grégoire M, Bully C, Perrin A, Lasolle H, Lifante J-C, Borson-Chazot F, Bournaud C. Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC). Cancers. 2022; 14(11):2591. https://doi.org/10.3390/cancers14112591
Chicago/Turabian StyleBouzehouane, Nadia, Pascal Roy, Myriam Decaussin-Petrucci, Mireille Bertholon-Grégoire, Chantal Bully, Agnès Perrin, Helene Lasolle, Jean-Christophe Lifante, Françoise Borson-Chazot, and Claire Bournaud. 2022. "Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC)" Cancers 14, no. 11: 2591. https://doi.org/10.3390/cancers14112591
APA StyleBouzehouane, N., Roy, P., Decaussin-Petrucci, M., Bertholon-Grégoire, M., Bully, C., Perrin, A., Lasolle, H., Lifante, J.-C., Borson-Chazot, F., & Bournaud, C. (2022). Prognostic Impact of Microscopic Extra-Thyroidal Extension (mETE) on Disease Free Survival in Patients with Papillary Thyroid Carcinoma (PTC). Cancers, 14(11), 2591. https://doi.org/10.3390/cancers14112591







