Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents—A Prospective Cohort Study of the AGMT Study-Group
Abstract
:Simple Summary
Abstract
1. Introduction
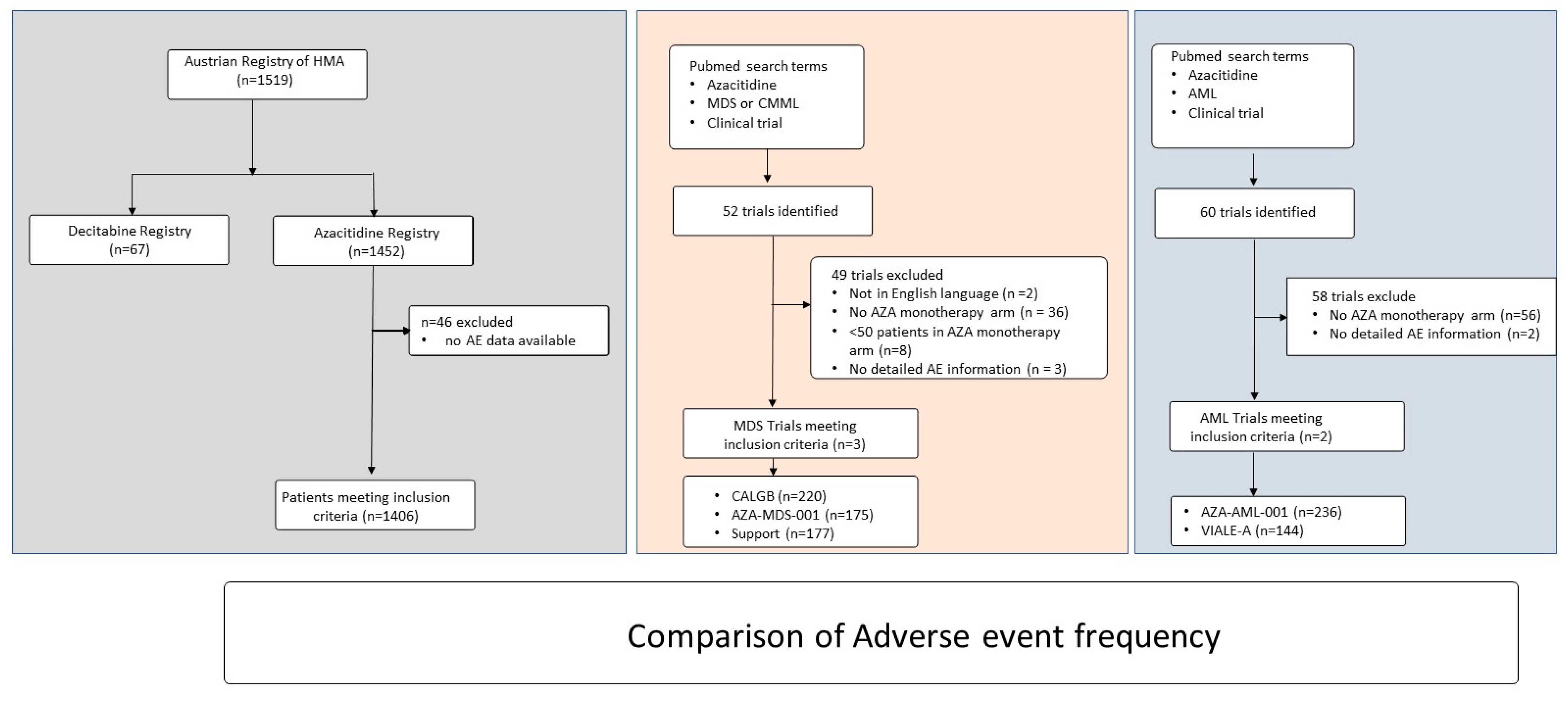
2. Methods
Statistics
3. Results
3.1. Baseline Characteristics of Patients Included in the Austrian Registry
3.2. Treatment Characteristics and Treatment Outcomes
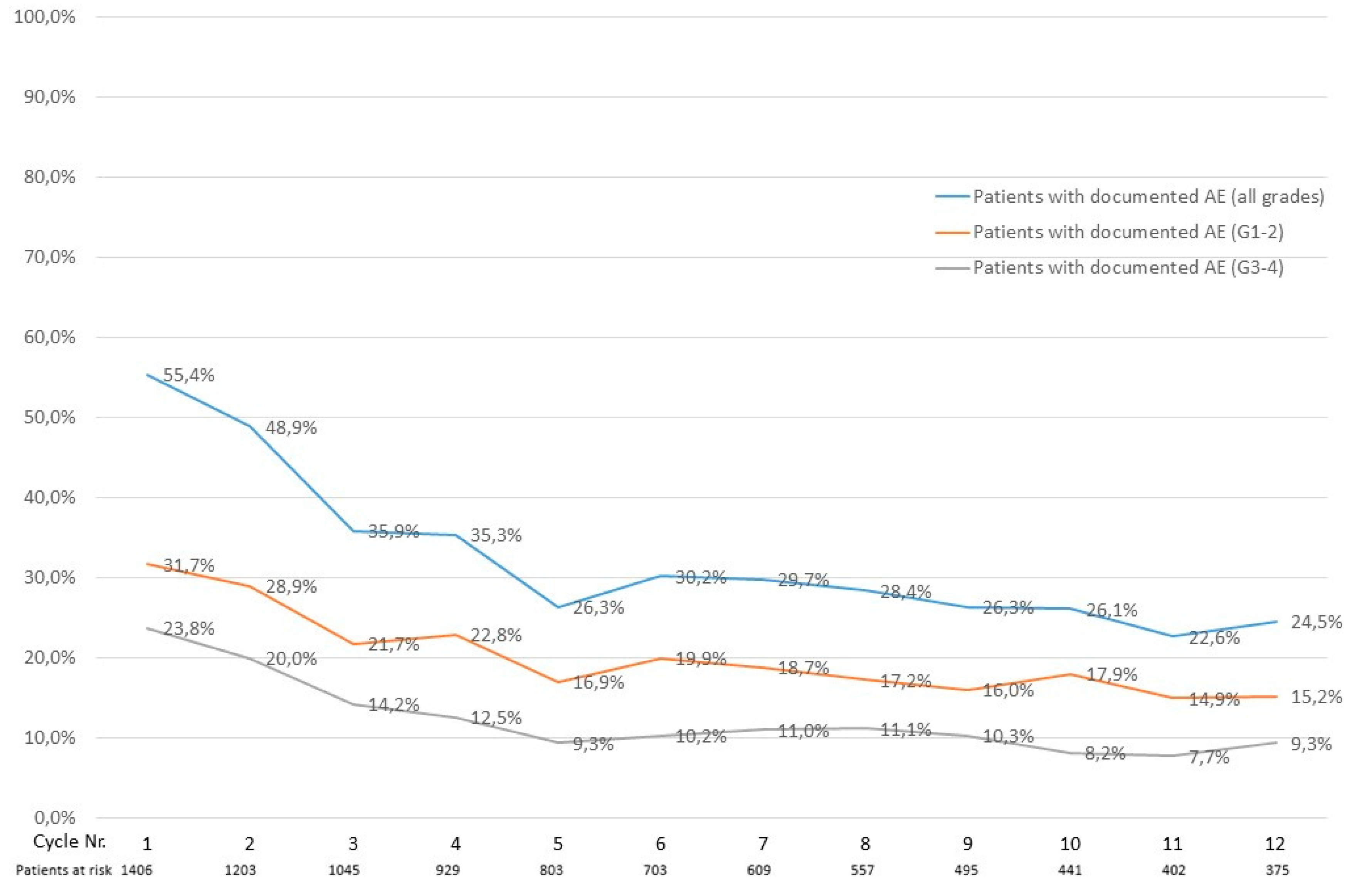
3.3. Documented Adverse Events in the Austrian Registry
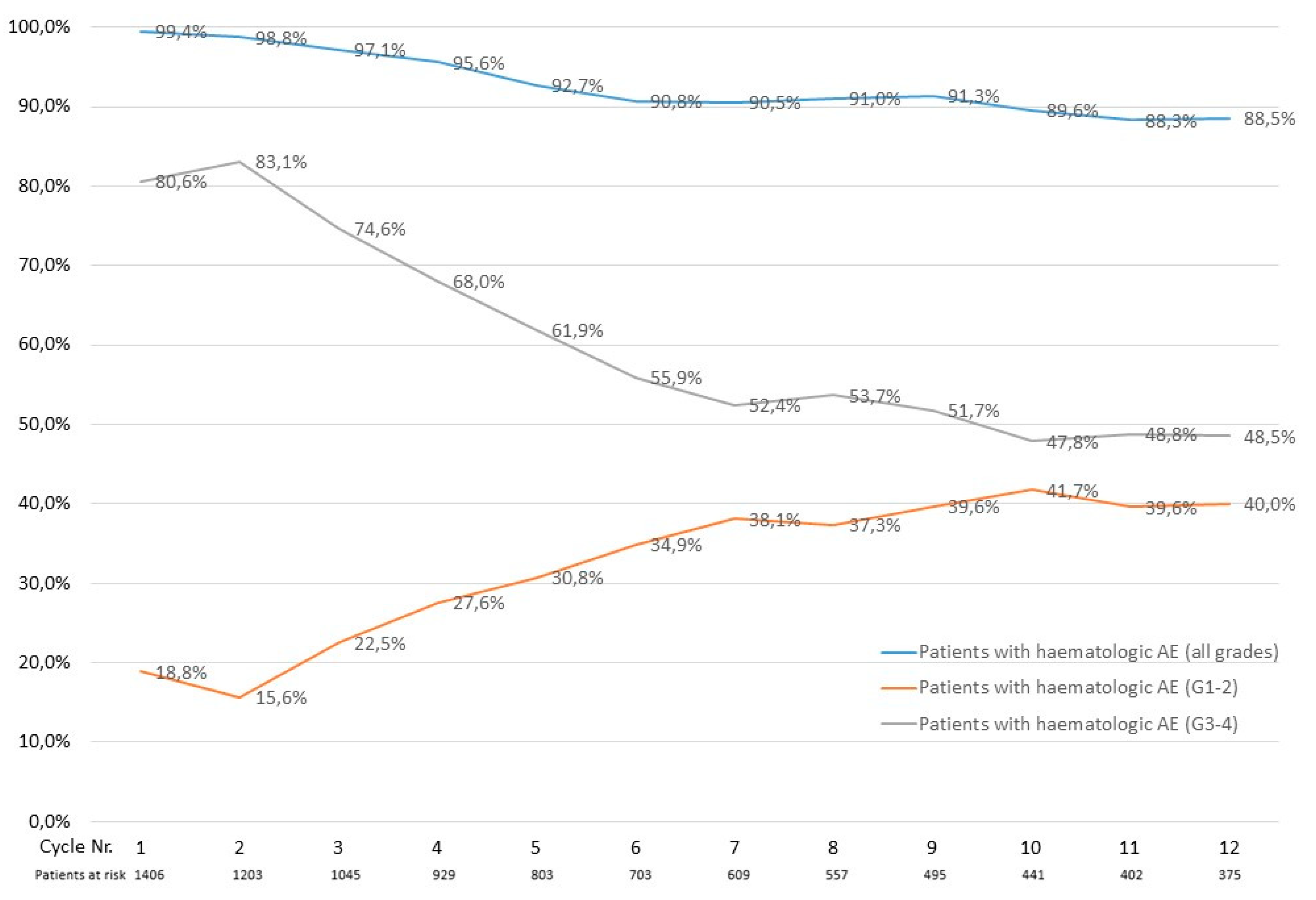
3.4. Calculated Treatment-Emergent Adverse Events in the Austrian Registry
3.5. Documented Infections in the Austrian Registry
3.6. Impact of Adverse Events on Azacitidine Treatment
3.7. Treatment and Outcome of Adverse Events
3.8. Comparison of Adverse Event Frequency in Patients with Myelodysplastic Syndromes or Chronic Myelomonocytic Leukemia with Data from Clinical Trials
3.9. Comparison of Adverse Event Frequency in Patients with Acute Myeloid Leukemia with Data from Clinical Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleyer, L.; Neureiter, D.; Faber, V.; Greil, R. Myelodysplastic Syndromes (MDS). In Chronic Myeloid Neoplasias and Clonal Overlap Syndromes; Springer: Vienna, Austria, 2010; pp. 153–222. [Google Scholar]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050794s011lbl.pdf (accessed on 26 April 2022).
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Sill, H.; Schlick, K.; Thaler, J.; Halter, B.; Machherndl-Spandl, S.; Zebisch, A.; et al. Azacitidine front-line in 339 patients with myelodysplastic syndromes and acute myeloid leukaemia: Comparison of French-American-British and World Health Organization classifications. J. Hematol. Oncol. 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleyer, L.; Stauder, R.; Burgstaller, S.; Schreder, M.; Tinchon, C.; Pfeilstocker, M.; Steinkirchner, S.; Melchardt, T.; Mitrovic, M.; Girschikofsky, M.; et al. Azacitidine in patients with WHO-defined AML—Results of 155 patients from the Austrian Azacitidine Registry of the AGMT-Study Group. J. Hematol. Oncol. 2013, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Beyne-Rauzy, O.; Turlure, P.; Vey, N.; Recher, C.; Dartigeas, C.; Legros, L.; et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011, 117, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Dinmohamed, A.G.; van Norden, Y.; Visser, O.; Posthuma, E.F.M.; Huijgens, P.C.; Sonneveld, P.; van de Loosdrecht, A.A.; Jongen-Lavrencic, M. Effectiveness of azacitidine for the treatment of higher-risk myelodysplastic syndromes in daily practice: Results from the Dutch population-based PHAROS MDS registry. Leukemia 2015, 29, 2449–2451. [Google Scholar] [CrossRef]
- Bernal, T.; Martínez-Camblor, P.; Sánchez-García, J.; de Paz, R.; Luño, E.; Nomdedeu, B.; Ardanaz, M.T.; Pedro, C.; Amigo, M.L.; Xicoy, B.; et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: Results from the Spanish registry. Leukemia 2015, 29, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Grinblatt, D.L.; Sekeres, M.A.; Komrokji, R.S.; Swern, A.S.; Sullivan, K.A.; Narang, M. Patients with myelodysplastic syndromes treated with azacitidine in clinical practice: The AVIDA registry. Leuk. Lymphoma 2015, 56, 887–895. [Google Scholar] [CrossRef]
- Pleyer, L.; Burgstaller, S.; Girschikofsky, M.; Linkesch, W.; Stauder, R.; Pfeilstocker, M.; Schreder, M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: Results from the Austrian Azacitidine Registry of the AGMT-Study Group. Ann. Hematol. 2014, 93, 1825–1838. [Google Scholar] [CrossRef] [Green Version]
- Mozessohn, L.; Cheung, M.C.; Fallahpour, S.; Gill, T.; Maloul, A.; Zhang, L.; Lau, O.; Buckstein, R. Azacitidine in the “real-world”: An evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br. J. Haematol. 2018, 181, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.G.; Vasilatou, D.; Kontos, C.K.; Foukas, P.; Kefala, M.; Ioannidou, E.-D.; Bouchla, A.; Bazani, E.; Dimitriadis, G.; Pappa, V. Treatment with 5-Azacytidine improves clinical outcome in high-risk MDS patients in the “real life” setting: A single center observational study. Hematology 2016, 21, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voso, M.T.; Niscola, P.; Piciocchi, A.; Fianchi, L.; Maurillo, L.; Musto, P.; Pagano, L.; Mansueto, G.; Criscuolo, M.; Aloe-Spiriti, M.A.; et al. Standard dose and prolonged administration of azacitidine are associated with improved efficacy in a real-world group of patients with myelodysplastic syndrome or low blast count acute myeloid leukemia. Eur. J. Haematol. 2016, 96, 344–351. [Google Scholar] [CrossRef]
- Stahl, M.; DeVeaux, M.; Montesinos, P.; Itzykson, R.; Ritchie, E.K.; Sekeres, M.A.; Barnard, J.D.; Podoltsev, N.A.; Brunner, A.M.; Komrokji, R.S.; et al. Hypomethylating agents in relapsed and refractory AML: Outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Díez-Campelo, M.; Lorenzo, J.I.; Itzykson, R.; Rojas, S.M.; Berthon, C.; Luño, E.; Beyne-Rauzy, O.; Perez-Oteyza, J.; Vey, N.; Bargay, J.; et al. Azacitidine improves outcome in higher-risk MDS patients with chromosome 7 abnormalities: A retrospective comparison of GESMD and GFM registries. Br. J. Haematol. 2018, 181, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Falantes, J.; Pleyer, L.; Thépot, S.; Almeida, A.M.; Maurillo, L.; Martínez-Robles, V.; Stauder, R.; Itzykson, R.; Pinto, R.; Venditti, A.; et al. Real life experience with frontline azacitidine in a large series of older adults with acute myeloid leukemia stratified by MRC/LRF score: Results from the expanded international E-ALMA series (E-ALMA+). Leuk. Lymphoma 2018, 59, 1113–1120. [Google Scholar] [CrossRef]
- Thépot, S.; Itzykson, R.; Seegers, V.; Recher, C.; Raffoux, E.; Quesnel, B.; Delaunay, J.; Cluzeau, T.; Marfaing Koka, A.; Stamatoullas, A.; et al. Azacitidine in untreated acute myeloid leukemia: A report on 149 patients. Am. J. Hematol. 2014, 89, 410–416. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Itzykson, R.; Santini, V.; Chaffaut, C.; Lionel, A.; Thepot, S.; Giagounidis, A.; Morabito, M.; Droin, N.; Luebbert, M.; Sapena, R.; et al. Decitabine Versus Hydroxyurea for Advanced Proliferative CMML: Results of the Emsco Randomized Phase 3 Dacota Trial. Blood 2020, 136, 53–54. [Google Scholar] [CrossRef]
- Pleyer, L.; Leisch, M.; Kourakli, A.; Padron, E.; Maciejewski, J.P.; Xicoy Cirici, B.; Kaivers, J.; Ungerstedt, J.; Heibl, S.; Patiou, P.; et al. Outcomes of patients with chronic myelomonocytic leukaemia treated with non-curative therapies: A retrospective cohort study. Lancet Haematol. 2021, 8, e135–e148. [Google Scholar] [CrossRef]
- Leisch, M.; Jansko, B.; Zaborsky, N.; Greil, R.; Pleyer, L. Next Generation Sequencing in AML—On the Way to Becoming a New Standard for Treatment Initiation and/or Modulation? Cancers 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.L.; Larson, R.A. Cancer and Leukemia Group B Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 24, 3895–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0; National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Dickinson, M.; Cherif, H.; Fenaux, P.; Mittelman, M.; Verma, A.; Portella, M.S.O.; Burgess, P.; Ramos, P.M.; Choi, J.; Platzbecker, U.; et al. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood 2018, 132, 2629–2638. [Google Scholar] [CrossRef]
- Pappa, V.; Anagnostopoulos, A.; Bouronikou, E.; Briasoulis, E.; Kotsianidis, I.; Pagoni, M.; Zikos, P.; Tsionos, K.; Viniou, N.; Meletis, J.; et al. A retrospective study of azacitidine treatment in patients with intermediate-2 or high risk myelodysplastic syndromes in a real-world clinical setting in Greece. Int. J. Hematol. 2017, 105, 184–195. [Google Scholar] [CrossRef]
- Ramos, F.; Thépot, S.; Pleyer, L.; Maurillo, L.; Itzykson, R.; Bargay, J.; Stauder, R.; Venditti, A.; Seegers, V.; Martínez-Robles, V.; et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: Clinical use and outcome prediction. Leuk. Res. 2015, 39, 296–306. [Google Scholar] [CrossRef]
- Pleyer, L.; Döhner, H.; Dombret, H.; Seymour, J.; Schuh, A.; Beach, C.; Swern, A.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. Int. J. Mol. Sci. 2017, 18, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert Baden, L.; Almyroudis, N.G.; Buss, B.; Cooper, B.; Dadwal, S.; Dubberke, E.R.; El Boghdadly, Z. NCCN Guidelines Version 1. 2021 Prevention and Treatment of Cancer-Related Infections; NCCN: Philadelphia, PA, USA, 2021. [Google Scholar]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef]
- Santini, V.; Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Silverman, L.R.; List, A.; Gore, S.D.; Seymour, J.F.; Backstrom, J.; Beach, C.L. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur. J. Haematol. 2010, 85, 130–138. [Google Scholar] [CrossRef]
- Framework for FDA’s Real-World Evidence Program; US Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Smedby, K.E.; Eloranta, S. Real-world evidence in safety assessment of new treatments. Lancet Haematol. 2018, 5, e510–e511. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, R.; Bowman, L.; Landray, M. Randomization versus Real-World Evidence. N. Engl. J. Med. 2020, 383, e21. [Google Scholar] [CrossRef]
- Juliusson, G.; Lazarevic, V.; Hörstedt, A.-S.; Hagberg, O.; Höglund, M.; Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood 2012, 119, 3890–3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total Cohort (n = 1406) | MDS (n = 504) | CMML (n = 133) | AML (n = 769) | p Value | |
|---|---|---|---|---|---|
| Initial diagnosis: MDS, n (%) | 622 (44.2) | 470 (93.3) | 15 (11.3) | 137 (17.8) | NA |
| CMML | 133 (9.5) | 4 (0.8) | 106 (79.7) | 23 (3.0) | |
| AML 1 | 583 (41.5) | 7 (1.4) | 1 (0.8) | 575 (74.8) | |
| CMPD | 41 (2.9) | 6 (1.2) | 4 (3.0) | 31 (4.0) | |
| Unknown | 27 (1.9) | 17 (3.4) | 7 (5.3) | 3 (0.4) | |
| Diagnosis at azacitidine start: MDS, n (%) | 504 (35.8) | 504 (100) | 0 (0.0) | 0 (0.0) | NA |
| CMML | 133 (9.5) | 0 (0.0) | 133 (100) | 0 (0.0) | |
| AML1 | 769 (54.7) | 0 (0.0) | 0 (0.0) | 796 (100) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Mean age (SD), years | 71.9 (9·88) | 71.8 (9.48) | 73.0 (7.96) | 71.8 (10.42) | 0.5375 0.8778 |
| Median (IQR) | 73.0 (67.0–78.0) | 72.0 (66.0–78.0) | 74.0 (69.0–79.0) | 73.0 (67.0–79.0) | |
| Min-max | 23.0–99.0 | 36.0–99.0 | 38.0–87.0 | 23.0–93.0 | |
| ≥75 years, n (%) | 605 (43.0) | 216 (42.9) | 60 (45.1) | 329 (42.8) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Sex: Female, n (%) | 549 (39.0) | 175 (34.7) | 52 (39.1) | 322 (41.9) | 0.0380 |
| Male | 857 (61.0) | 329 (65.3) | 81 (60.9) | 447 (58.1) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ECOG-PS: 0–1, n (%) | 1073 (76.3) | 404 (80.1) | 108 (81.2) | 561 (72.9) | 0.0121 |
| 2–4 | 333 (23.7) | 100 (19.9) | 25 (18.8) | 208 (27.1) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| HCT-CI risk group: Low risk, n (%) | 434 (30.9) | 168 (33.3) | 42 (31.6) | 224 (29.1) | 0.3392 |
| Intermediate risk | 500 (35.6) | 180 (35.7) | 42 (31.6) | 278 (36.2) | |
| High risk | 470 (33.4) | 154 (30.6) | 49 (36.8) | 267 (34.7) | |
| Unknown | 2 (0.1) | 2 (0.4) | 0 (0.0) | 0 (0.0) | |
| Treatment-related disease: No, n (%) | 1212 (86.2) | 423 (83.9) | 118 (88.7) | 671 (87.3) | 0.0406 |
| Yes | 187 (13.3) | 81 (16.1) | 11 (8.3) | 95 (12.4) | |
| Unknown | 7 (0.5) | 0 (0.0) | 4 (3.0) | 3 (0.4) | |
| IPSS cytogenetic risk: Good, n (%) | 878 (62.4) | 323 (64.1) | 93 (69.9) | 462 (60.1) | 0.1855 |
| Intermediate | 203 (14.4) | 63 (12.5) | 21 (15.8) | 119 (15.5) | |
| Poor | 151 (10.7) | 62 (12.3) | 7 (5.3) | 82 (10.7) | |
| Not evaluable | 90 (6.4) | 27 (5.4) | 8 (6.0) | 55 (7.2) | |
| Unknown | 84 (6.0) | 29 (5.8) | 11 (8.3) | 51 (6.6) | |
| R-IPSS cytogenetic risk: Very good, n (%) | 33 (2.3) | 11 (2.2) | 0 (0.0) | 22 (2.9) | 0.2126 |
| Good | 856 (61.0) | 318 (63.1) | 93 (69.9) | 447 (58.1) | |
| Intermediate | 223 (15.9) | 70 (13.9) | 22 (16.5) | 131 (17.0) | |
| Poor | 99 (7.0) | 41 (8.1) | 6 (4.5) | 52 (6.8) | |
| Very poor | 19 (1.4) | 8 (1.6) | 0 (0.0) | 11 (1.4) | |
| Not evaluable | 90 (6.4) | 27 (5.4) | 8 (6.0) | 55 (7.2) | |
| Unknown | 84 (6.0) | 29 (5.8) | 4 (3.1) | 51 (6.6) | |
| IPSS risk group: Lower-risk 2, n (%) | 351 (24.9) | 208 (41.2) | 73 (54.8) | 70 (9.1) | <0.0001 |
| Higher-risk 3 | 889 (63.2) | 229 (45.4) | 47 (35.3) | 613 (79.7) | |
| Unknown | 166 (11.8) | 67 (13.3) | 13 (9.7) | 86 (11.2) | |
| Red blood cell transfusion dependence: Yes, n (%) | 821 (58.4) | 266 (52.8) | 85 (63.9) | 470 (61.1) | 0.0051 |
| No | 585 (41.6) | 238 (47.2) | 48 (36.1) | 299 (38.9) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Platelet transfusion dependence: Yes, n (%) | 1115 (79.3) | 414 (82.1) | 115 (86.5) | 586 (76.2) | 0.0038 |
| No | 291 (20.7) | 90 (17.9) | 18 (13.5) | 183 (23.8) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total Cohort (n = 1406) | MDS (n = 504) | CMML (n = 133) | AML (n = 769) | p Value | |
|---|---|---|---|---|---|
| Calculated adverse events 1,2 | |||||
| Neutropenia:1 Grade1–2, n (%) | 97 (6.9) | 39 (7.7) | 15 (11.3) | 43 (5.6)253 (32.9) | 0.0090 |
| Grade 3–4 | 508 (36.1) | 208 (41.3) | 47 (35.3) | ||
| Lymphopenia:1 Grade 1–2, n (%) | 719 (51.1) | 217 (43.0) | 38 (28.6) | 464 (60.3) | <0.0001 |
| Grade 3–4 | 263 (18.7) | 163 (32.3) | 25 (18.9) | 75 (9.8) | |
| Anemia:1 Grade 1–2, n (%) | 247 (17.6) | 70 (13.9) | 23 (17.3) | 154 (20.0) | 0.0080 |
| Grade 3–4 | 610 (43.4) | 241 (47.8) | 64 (48.1) | 305 (39.7) | |
| Thrombopenia:1 Grade 1–2, n (%) | 168 (11.9) | 60 (11.9) | 24 (18.0) | 84 (10.9) | 0.1074 |
| Grade 3–4 | 517 (36.8) | 196 (38.9) | 45 (33.8) | 276 (35.9) | |
| Bilirubin increase:2 Grade1–2, n (%) | 248 (17.6) | 124 (24.6) | 33 (24.8) | 91 (11.8) | <0.0001 |
| Grade 3–4 | 157 (11.1) | 81 (16.0) | 13 (9.7) | 63 (8.1) | |
| GOT increase:2 Grade 1–2, n (%) | 250 (17.7) | 112 (22.2) | 24 (18.0) | 114 (14.8) | 0.1380 |
| Grade 3–4 | 178 (12.6) | 55 (10.9) | 20 (15.0) | 103 (13.3) | |
| GPT increase:2 Grade 1–2, n (%) | 322 (22.9) | 141 (27.9) | 21 (15.7) | 160 (20.8) | 0.0122 |
| Grade 3–4 | 221 (15.7) | 70 (13.8) | 16 (12.0) | 135 (17.5) | |
| Creatinine increase:2 Grade 1–2, n (%) | 330 (23.4) | 148 (29.3) | 54 (40.6) | 128 (16.6) | <0.0001 |
| Grade 3–4 | 270 (19.2) | 109 (21.6) | 43 (32.3) | 118 (15.3) | |
| Documented adverse events | |||||
| Pyrexia: Grade 1–2, n (%) | 287 (20.4) | 92 (18.3) | 13 (9.8) | 182 (23.7) | 0.0003 |
| Grade 3–4 | 43 (3.1) | 15 (3.0) | 1 (0.8) | 27 (3.5) | |
| Febrile neutropenia: Grade 1–2, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.0001 |
| Grade 3–4 | 470 (33.4) | 196 (38.9) | 24 (18.0) | 250 (32.5) | |
| Pneumonia Grade 1–2, n (%) | 207 (14.7) | 79 (15.7) | 15 (11.3) | 113 (14.7) | 0.4446 |
| Grade 3–4 | 80 (5.7) | 24 (4.8) | 4 (3.0) | 52 (6.8) | |
| Upper resp. infection: Grade 1–2, n (%) | 227 (16.1) | 93 (18.5) | 22 (16.5) | 112 (14.6) | 0.1811 |
| Grade 3–4 | 13 (0.9) | 3 (0.6) | 1 (0.8) | 9 (1.2) | |
| Nausea: Grade 1–2, n (%) | 137 (9.7) | 56 (11.1) | 13 (9.8) | 68 (8.8) | 0.4103 |
| Grade 3–4 | 3 (0.2) | 1 (0.2) | 0 (0.0) | 2 (0.3) | |
| Diarrhea: Grade 1–2, n (%) | 129 (9.2) | 44 (8.7) | 13 (9.8) | 72 (9.4) | 0.9005 |
| Grade 3–4 | 10 (0.7) | 4 (0.8) | 2 (1.5) | 4 (0.5) | |
| Constipation: Grade 1–2, n (%) | 117 (8.3) | 49 (9.7) | 13 (9.8) | 55 (7.2) | 0.2184 |
| Grade 3–4 | 2 (0.1) | 1 (0.2) | 0 (0.0) | 1 (0.1) | |
| Urinary tract infection: Grade 1–2, n (%) | 106 (7.5) | 40 (7.9) | 11 (8.3) | 55 (7.2) | 0.8263 |
| Grade 3–4 | 12 (0.9) | 6 (1.2) | 0 (0.0) | 6 (0.8) | |
| Skin/mucosal infection: Grade 1–2, n (%) | 102 (7.3) | 40 (7.9) | 7 (5.3) | 55 (7.2) | 0.5643 |
| Grade 3–4 | 14 (1.0) | 9 (1.8) | 0 (0.0) | 5 (0.7) | |
| Bacterial infection other: Grade 1–2, n (%) | 81 (5.8) | 22 (4.4) | 6 (4.5) | 53 (6.9) | 0.1350 |
| Grade 3–4 | 24 (1.7) | 12 (2.4) | 2 (1.5) | 10 (1.3) | |
| Injection site reaction: Grade 1–2, n (%) | 316 (22.5) | 132 (26.2) | 31 (23.3) | 153 (19.9) | 0.0304 |
| Grade 3–4 | 10 (0.7) | 2 (0.4) | 0 (0.0) | 8 (1.0) | |
| Fatigue: Grade 1–2, n (%) | 422 (30.0) | 159 (31.5) | 45 (33.8) | 218 (28.3) | 0.2859 |
| Grade 3–4 | 48 (3.4) | 11 (2.2) | 4 (3.0) | 33 (4.3) | |
| Pain: Grade 1–2, n (%) | 368 (26.2) | 133 (26.4) | 41 (30.8) | 194 (25.2) | 0.3947 |
| Grade 3–4 | 42 (3.0) | 9 (1.8) | 5 (3.8) | 28 (3.6) | |
| CALGB [26] 1 | AZA-MDS-001 [3] | Support [31] | Austrian Registry | p Value | |
|---|---|---|---|---|---|
| Phase | III | III | III | Registry | NA |
| Year published | 2006 | 2009 | 2018 | 2022 | NA |
| Included diagnosis | Newly diagnosed MDS and CMML | Newly diagnosed MDS and CMML 2 | Newly diagnosed MDS 3 | Newly diagnosed/RR MDS and CMML | NA |
| Allowed IPSS risk categories | Low-high | Int 2-high | Int 1-high | Low-high | NA |
| Allowed pretreatments | None | None | None | No restrictions | NA |
| Study design | AZA vs. BSC | AZA vs. CCR | AZA +/− eltrombopag | AZA | NA |
| Total patients in azacitidine arm, n | 220 | 175 | 177 | 637 | NA |
| Median age, yrs | 67 | 69 | 70 | 73 | |
| Sex: Male, n (%) | 107 (48.6) | 132 (74) | 124 (70) | 410 (64.4%) | <0.0001 |
| ECOG-PS: 0–1, n (%) | 149 (67.7) | 164 (93.7) | 177 (100.0) 4 | 512 (80.4) | <0.0001 |
| 2–4 | 24 (10.9) | 11 (6.3) | 0 (0.0) | 125 (19.6) | |
| Treatment related disease: Yes, n (%) | NR | NR | NR | 92 (14.4) | NA |
| IPSS risk group: Lower risk, n (%) | NR | 5 (3.0) | 61 (34.0) | 281 (44.1) | <0.0001 |
| Higher risk | NR | 158 (89.0) | 116 (66.0) | 276 (43.3) | |
| IPSS cytogenetic risk: Good risk, n (%) | NR | 83 (46.0) | 81 (46.0) | 416 (65.3) | <0.0001 |
| Intermediate risk | NR | 37 (21.0) | 39 (22.0) | 84 (13.1) | |
| Poor risk | NR | 50 (28.0) | 57 (32.0) | 69 (10.8) | |
| Azacitidine treatment cycles, median | NR | 9 | 6 | 7.25 | NA |
| Q1–Q3 | NR | 4–15 | NR | 3.5–16.0 | |
| Median overall survival, months | 20 | 24.5 | 18.7 | 13.9 | NA |
| Treatment discount. due to AE: Yes, n (%) | NR | 8 (5.0) | 24 (13.5) | 33 (5.1) | 0.0001 |
| Neutropenia: Grade1–4, n (%) | 71 (32.3) | 115 (65.7) | 46 (26.0) | 309 (48.5) | <0·0001 |
| Grade 3–4 | NR | 107 (61.1) | 46 (26.0) | 255 (40.0) | <0.0001 |
| Anemia: Grade 1–4, n (%) | 153 (69.5) | 90 (51.4) | 26 (14.7) | 398 (62.5) | <0·0001 |
| Grade 3–4 | NR | 24 (13.7) | 20 (11.3) | 305 (47.9) | <0.0001 |
| Thrombopenia: Grade 1–4, n (%) | 144 (65.5) | 122 (69.7) | NR | 325 (51.0) | <0.0001 |
| Grade 3–4 | NR | 102 (58.3) | NR | 241 (37.8) | <0.0001 |
| Pyrexia: Grade 1–4, n (%) | 114 (51.8) | 53 (30.3) | 46 (26.0) | 121 (19.0) | <0·0001 |
| Grade 3–4 | NR | 8 (4.6) | 5 (2.8) | 16 (2.5) | 0.3579 |
| Febrile neutropenia: Grade 1–4, n (%) | 36 (16.4) | 24 (13.7) | 38 (21.5) | 220 (34.5) | <0·0001 |
| Grade 3–4 | NR | 22 (12.6) | 32 (18.1) | 220 (34.5) | <0.0001 |
| Pneumonia Grade 1–4, n (%) | 24 (10.9) | 22 (12.6) | 25 (14.1) | 122 (19.2) | 0·0100 |
| Grade 3–4 | NR | 18 (10.3) | 10 (5.6) | 28 (4.4) | 0.0115 |
| Upper resp. infection: Grade 1–4, n (%) | 28 (12.7) | 16 (9.1) | NR | 119 (18.7) | 0.0033 |
| Grade 3–4 | NR | 3 (1.7) | NR | 4 (0.6) | 0.1685 |
| Urinary tract infection: Grade 1–4, n (%) | NR | 15 (8.6) | NR | 57 (8.9) | 0.8765 |
| Grade 3–4 | NR | 3 (1.7) | NR | 6 (0.9) | 0.3873 |
| Nausea: Grade 1–4, n (%) | 155 (70.5) | 84 (48.0) | 46 (26.0) | 70 (11.0) | <0·0001 |
| Grade 3–4 | NR | 3 (1.7) | 1 (0.6) | 1 (0.2) | 0.0362 |
| Diarrhea: Grade 1–4, n (%) | 80 (36.4) | 38 (21.7) | 25 (14.1) | 63 (9.9) | <0·0001 |
| Grade 3–4 | NR | 1 (0.6) | 1 (0.6) | 6 (0.9) | 0.8208 |
| Constipation: Grade 1–4, n (%) | 74 (33.6) | 88 (50.3) | 57 (32.2) | 63 (9.9) | <0·0001 |
| Grade 3–4 | NR | 2 (1.1) | 2 (1.1) | 1 (0.2) | 0.1151 |
| Injection site reaction: Grade 1–4, n (%) | 30 (13.6) | 51 (29.1) | NR | 165 (25.9) | 0.0002 |
| Grade 3–4 | NR | 1 (0.6) | NR | 2 (0.3) | 0.6190 |
| Fatigue: Grade 1–4, n (%) | NR | 43 (24.0) | 25 (14.1) | 219 (34.4) | <0.0001 |
| Grade 3–4 | NR | 6 (3.4) | 1 (0.6) | 15 (2.4) | 0.1775 |
| AZA-AML 001 [4] | VIALE A [24] | Austrian Registry | p Value | |
|---|---|---|---|---|
| Phase | III | III | Registry | NA |
| Year published | 2015 | 2020 | 2022 | NA |
| Included diagnosis | Newly diagnosed AML | Newly diagnosed AML | Newly diagnosed/RR AML | NA |
| Allowed pretreatments | None | None | No restrictions | NA |
| Study design | AZA vs.·CCR | AZA +/− venetoclax | AZA | NA |
| Total patients in azacitidine arm, n | 236 | 145 | 769 | NA |
| Median age, yrs | 75 | 76 | 73 | NA |
| Sex: Male, n (%) | 139 (57.7) | 87 (60) | 447 (58.1) | 0.9077 |
| ECOG-PS: 0–1, n (%) | 236 (100.0) | 81 (56.0) | 561 (72.9) | <0.0001 |
| 2–4 | 0 (0.0) | 64 (44.0) | 208 (27.1) | |
| Treatment related disease: Yes, n (%) | 8 (3.3) | 9 (6.2) | 95 (12.4) | 0.0008 |
| MRC cytogenetic risk: Low risk, n (%) | 0 (0.0) | 0 (0.0) | 23 (3.0) | 0.0003 |
| Intermediate risk | 155 (64.3) | 89 (61.0) | 481 (62.5) | |
| Poor risk | 85 (35.3) | 56 (39.0) | 159 (20.7) | |
| Azacitidine treatment cycles, median | 6 | 4.5 | 4.0 | NA |
| Min-max | 1–28 | 1.0–26.0 | 1.0–75.0 | |
| Median overall survival, months | 10.4 | 9.6 | 7.3 | NA |
| Treatment discount. due to AE: Yes, n (%) | 89 (37.0) | 5 (3.4) | 40 (5.2) | <0.0001 |
| Neutropenia: Grade1–4, n (%) | 71 (30.1) | 42 (29.2) | 296 (38.5) | 0.0140 |
| Grade 3–4 | 62 (26.3) | 41 (28.5) | 253 (32.9) | 0.1226 |
| Anemia: Grade 1–4, n (%) | 48 (20.3) | 30 (20.8) | 459 (59.7) | <0.0001 |
| Grade 3–4 | 37 (15.7) | 29 (20.1) | 305 (39.7) | <0.0001 |
| Thrombopenia: Grade 1–4, n (%) | 64 (27.1) | 58 (40.3) | 360 (46.8) | <0.0001 |
| Grade 3–4 | 56 (23.7) | 55 (38.2) | 276 (35.9) | 0.0011 |
| Pyrexia: Grade 1–4, n (%) | 89 (37.7) | 32 (22.2) | 209 (27.2) | 0.0010 |
| Grade 3–4 | 18 (7.6) | 2 (1.4) | 27 (3.5) | 0.0043 |
| Febrile neutropenia: Grade 1–4, n (%) | 76 (32.2) | 27 (18.8) | 250 (32.5) | 0.0030 |
| Grade 3–4 | 66 (28.0) | 27 (18.8) | 250 (32.5) | 0.0032 |
| Pneumonia Grade 1–4, n (%) | 57 (24.2) | 39 (27.1) | 165 (21.5) | 0.2800 |
| Grade 3–4 | 45 (19.1) | 36 (25.0) | 52 (6.8) | <0.0001 |
| Upper resp. infection: Grade 1–4, n (%) | NR | NR | 121 (15.7) | NA |
| Grade 3–4 | NR | NR | 9 (1.2) | |
| Urinary tract infection: Grade 1–4, n (%) | NR | NR | 61 (7.9) | NA |
| Grade 3–4 | NR | NR | 6 (0.8) | |
| Nausea: Grade 1–4, n (%) | 94 (39.8) | 50 (34.7) | 70 (9.1) | <0.0001 |
| Grade 3–4 | 0 (0.0) | 1 (0.7) | 2 (0.3) | 0.7020 |
| Diarrhea: Grade 1–4, n (%) | 87 (36.9) | 48 (33.3) | 76 (9.9) | <0.0001 |
| Grade 3–4 | 0 (0.0) | 4 (2.8) | 4 (0.5) | 0.0145 |
| Constipation: Grade 1–4, n (%) | 99 (41.9) | 46 (38.9) | 65 (8.5) | <0.0001 |
| Grade 3–4 | 0 (0.0) | 2 (1.4) | 1 (0.1) | 0.0610 |
| Injection site reaction: Grade 1–4, n (%) | NR | NR | 161 (20.9) | NA |
| Grade 3–4 | NR | NR | 8 (1.0) | |
| Fatigue: Grade 1–4, n (%) | 54 (22.9) | 24 (16.7) | 251 (32.6) | <0.0001 |
| Grade 3–4 | 0 (0.0) | 2 (1.4) | 33 (4.3) | 0.0050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leisch, M.; Pfeilstöcker, M.; Stauder, R.; Heibl, S.; Sill, H.; Girschikofsky, M.; Stampfl-Mattersberger, M.; Tinchon, C.; Hartmann, B.; Petzer, A.; et al. Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents—A Prospective Cohort Study of the AGMT Study-Group. Cancers 2022, 14, 2459. https://doi.org/10.3390/cancers14102459
Leisch M, Pfeilstöcker M, Stauder R, Heibl S, Sill H, Girschikofsky M, Stampfl-Mattersberger M, Tinchon C, Hartmann B, Petzer A, et al. Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents—A Prospective Cohort Study of the AGMT Study-Group. Cancers. 2022; 14(10):2459. https://doi.org/10.3390/cancers14102459
Chicago/Turabian StyleLeisch, Michael, Michael Pfeilstöcker, Reinhard Stauder, Sonja Heibl, Heinz Sill, Michael Girschikofsky, Margarete Stampfl-Mattersberger, Christoph Tinchon, Bernd Hartmann, Andreas Petzer, and et al. 2022. "Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents—A Prospective Cohort Study of the AGMT Study-Group" Cancers 14, no. 10: 2459. https://doi.org/10.3390/cancers14102459
APA StyleLeisch, M., Pfeilstöcker, M., Stauder, R., Heibl, S., Sill, H., Girschikofsky, M., Stampfl-Mattersberger, M., Tinchon, C., Hartmann, B., Petzer, A., Schreder, M., Kiesl, D., Vallet, S., Egle, A., Melchardt, T., Piringer, G., Zebisch, A., Machherndl-Spandl, S., Wolf, D., ... Pleyer, L. (2022). Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents—A Prospective Cohort Study of the AGMT Study-Group. Cancers, 14(10), 2459. https://doi.org/10.3390/cancers14102459









