A Systematic Review of the Guidelines on Venous Thromboembolism Prophylaxis in Gynecologic Oncology
Abstract
Simple Summary
Abstract
1. Introduction
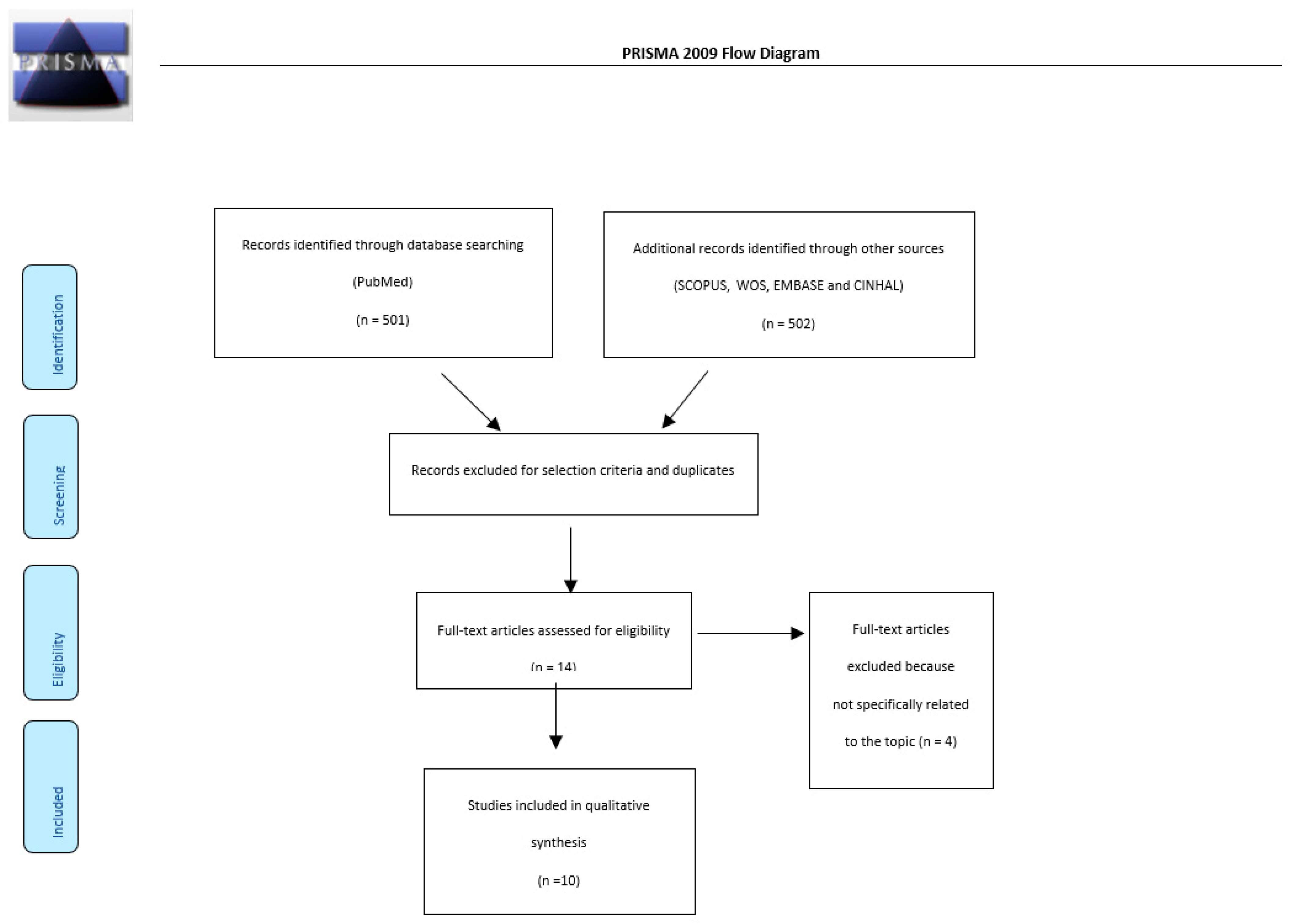
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Checking
2.3. Quality Assessment
3. Results
- The National Comprehensive Cancer Network (NCCN), 2020 [24];
- The American Society of Clinical Oncology (ASCO), 2020 [25];
- The International Initiative on Thrombosis and Cancer and the International Society on Thrombosis and Haemostasis (ITAC/ISTH) with the support of the French National Cancer Institute, 2019 [26];
- The Italian Association of Medical Oncology (AIOM) 2019 [27];
- The Spanish Society in Medical Oncology (SEOM) clinical guideline of venous thromboembolism and cancer, 2019 [28];
- The Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology (BCSH), 2015 [29];
- The Canadian consensus recommendations on the treatment of VTE in cancer patients, 2015 [30];
- The European Society of Medical Oncology (ESMO) 2011 [31];
- The Italian Society for Haemostasis and Thrombosis (SISET), 2011 [32].
- The American Society of Hematology (ASH), 2021 [33]
3.1. National Comprehensive Cancer Network (NCCN)
- UFH OR LMWH prophylaxis in all oncological inpatients;
- Post-surgical prophylaxis for at least 4 weeks;
- Intermittent pneumatic compression (IPC) was suggested for inpatients with cancer not suitable for pharmacological prophylaxis;
- Fondaparinux could be safely used;
- DOACs (apixaban and rivaroxaban) were suggested for discharged medical patients and outpatients under chemotherapy following the Khorana score, from 3 to 6 months;
- CVC patients should not be routinely treated with anticoagulant prophylaxis unless they were at high risk for VTE.
3.2. The American Society of Clinical Oncology (ASCO)
3.3. The International Initiative on Thrombosis and Cancer and the International Society on Thrombosis and Haemostasis (ITAC/ISTH), with the Support of the French National Cancer Institute
3.4. The Italian Association of Medical Oncology (AIOM)
3.5. Spanish Society of Medical Oncology (SEOM) Clinical Guideline of Venous Thromboembolism and Cancer
3.6. European Society of Medical Oncology (ESMO)
3.7. The Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology (BCSH)
3.8. The Canadian Consensus Recommendation on the Treatment of VTE in Cancer Patients
3.9. Italian Society for Haemostasis and Thrombosis (SISET)
3.10. The American Society of Hematology (ASH)
4. Discussion
- Hospitalized medical patients;
- Hospitalized surgical patients, preoperative and postoperative;
- Ambulatory outpatients on systemic therapy or undergoing CVC.
4.1. Prevention of VTE in the Hospitalized Medical Patient with Cancer
4.2. Prevention of VTE in the Surgical Patient with Cancer
4.3. Prevention of VTE in the Ambulatory Patient Undergoing Systemic Therapy
- Acute damage on a vessel’s wall;
- Vessels’ delayed endothelial integrity damage; and
- The reduction of coagulation processes’ regulatory proteins, such as decreased protein C and S levels, i.e., reducing the antithrombin III (ATIII) level.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTE | Venous thromboembolic events |
| CVC | Central venous catheter |
| UFH | Unfractionated heparin |
| LMWH | Low-molecular-weight heparin |
| IPC | Intermittent pneumatic compression |
| DOACs | Direct oral anticoagulants |
| MMX | Mammography |
| PICC | Peripherally inserted central catheters |
| PORT | Centrally inserted totally implanted vascular access |
References
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous Thromboembolism: A Public Health Concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Lip, G.Y. Virchow’s Triad Revisited: Blood Constituents. Pathophysiol. Haemost. Thromb. 2003, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of Initial and Recurrent Thromboembolic Disease Among Patients with Malignancy Versus Those without Malignancy: Risk Analysis Using Medicare Claims Data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef]
- Falanga, A.; Donati, M.B. Pathogenesis of Thrombosis in Patients with Malignancy. Int. J. Hematol. 2001, 73, 137–144. [Google Scholar] [CrossRef]
- Rickles, F.R.; Falanga, A. Molecular Basis for the Relationship Between Thrombosis and Cancer. Thromb. Res. 2001, 102, V215–V224. [Google Scholar] [CrossRef]
- Ashrani, A.A.; Heit, J.A.; Noble, S.I.R.; Johnson, M.J.; Lee, A.Y.Y. Epidemiology of venous thromboembolism. Adv. Dis. A Clin. Guid. 2008, 12, 464–474. [Google Scholar] [CrossRef][Green Version]
- Lip, G.Y.; Chin, B.S.; Blann, A.D. Cancer and the prothrombotic state. Lancet Oncol. 2002, 3, 27–34. [Google Scholar] [CrossRef]
- Rickles, F.R.; Levine, M.; Edwards, R.L. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992, 11, 237–248. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Wharin, C.; Tagalakis, V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Rev. 2014, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.T.; Evans, L.; Lambrecht, L.; Wan, J. Deep venous thrombosis in gynecological oncology: Incidence and clinical symptoms study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2012, 119, 648–655. [Google Scholar] [CrossRef]
- Satoh, T.; Matsumoto, K.; Tanaka, Y.O.; Akiyama, A.; Nakao, S.; Sakurai, M.; Ochi, H.; Onuki, M.; Minaguchi, T.; Sakurai, H.; et al. Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb. Res. 2013, 131, e127–e132. [Google Scholar] [CrossRef]
- Tasaka, N.; Minaguchi, T.; Hosokawa, Y.; Takao, W.; Itagaki, H.; Nishida, K.; Akiyama, A.; Shikama, A.; Ochi, H.; Satoh, T. Prevalence of venous thromboembolism at pretreatment screening and associated risk factors in 2086 patients with gynecological cancer. J. Obstet. Gynaecol. Res. 2020, 46, 765–773. [Google Scholar] [CrossRef]
- Barber, E.L.; Clarke-Pearson, D.L. The limited utility of currently available venous thromboembolism risk assessment tools in gynecological oncology patients. Am. J. Obstet. Gynecol. 2016, 215, 445.e1–445.e9. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; Hariton, E.; Clemmer, J.; Clark, R.M.; Hall, T.; Boruta, D.M.; Schorge, J.O.; del Carmen, M.G. Incidence and Effects on Mortality of Venous Thromboembolism in Elderly Women With Endometrial Cancer. Obstet. Gynecol. 2015, 125, 1362–1370. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Thomas, E.D.; Slaughter, K.N.; Farrell, R.; Ding, K.; Farris, R.E.; Lauer, J.K.; Perry, L.J.; McMeekin, D.S.; Moore, K.N. The survival detriment of venous thromboembolism with epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 73–77. [Google Scholar] [CrossRef]
- Lavikainen, L.I.; Guyatt, G.H.; Lee, Y.; Couban, R.J.; Luomaranta, A.L.; Sallinen, V.J.; Kalliala, I.E.J.; Karanicolas, P.J.; Cartwright, R.; Aaltonen, R.L.; et al. Systematic reviews of observational studies of Risk of Thrombosis and Bleeding in General and Gynecologic Surgery (ROTBIGGS): Introduction and methodology. Syst. Rev. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Graul, A.; Latif, N.; Zhang, X.; Dean, L.T.; Morgan, M.; Giuntoli, R.; Burger, R.; Kim, S.; Ko, E. Incidence of Venous Thromboembolism by Type of Gynecologic Malignancy and Surgical Modality in the National Surgical Quality Improvement Program. Int. J. Gynecol. Cancer 2017, 27, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. Can. Med. Assoc. J. 2010, 182, E839–E842. [Google Scholar] [CrossRef] [PubMed]
- Siering, U.; Eikermann, M.; Hausner, E.; Hoffmann-Eßer, W.; Neugebauer, E.A. Appraisal Tools for Clinical Practice Guidelines: A Systematic Review. PLoS ONE 2013, 8, e82915. [Google Scholar] [CrossRef]
- Hoffmann-Eßer, W.; Siering, U.; Neugebauer, E.A.; Lampert, U.; Eikermann, M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument—a third of AGREE II users apply a cut-off for guideline quality. J. Clin. Epidemiology 2017, 95, 120–127. [Google Scholar] [CrossRef]
- Sanchez, O.; Clerc, S.; Pinot, J.; Pastré, J.; Planquette, B. Maladie veineuse thromboembolique. Rev. Des Mal. Respir. Actual. 2020, 12, 317–324. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, E566–E581. [Google Scholar] [CrossRef]
- Barni, S.; Petrella, M.C.; Falanga, A.; Verso, M.; Piccioli, A.; Antonuzzo, A.; Imberti, D.; Tarantini, L.; Labianca, R. Linee Guida AIOM Tromboembolismo Venoso Nei Pazienti Con Tumori Solidi. 2019. Available online: https://www.aiom.it/wp-content/uploads/2018/11/2018_LG_AIOM_Tromboembolismo.pdf (accessed on 7 July 2021).
- Martín, A.J.M.; Díaz, E.G.; Escobar, I.G.; Montero, R.M.; Martínez-Marín, V.; Olmos, V.P.; Segura, P.P.; Verdúguez, T.Q.; Fernández, M.S. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin. Transl. Oncol. 2020, 22, 171–186. [Google Scholar] [CrossRef]
- Watson, H.G.; Keeling, D.M.; Laffan, M.; Tait, R.C.; Makris, M.; on behalf of the British Committee for Standards in Haematology. Guideline on aspects of cancer-related venous thrombosis. Br. J. Haematol. 2015, 170, 640–648. [Google Scholar] [CrossRef]
- Easaw, J.; Shea–Budgell, M.; Wu, C.; Czaykowski, P.; Kassis, J.; Kuehl, B.; Lim, H.; MacNeil, M.; Martinusen, D.; McFarlane, P.; et al. Canadian Consensus Recommendations on the Management of Venous Thromboembolism in Patients with Cancer. Part 1: Prophylaxis. Curr. Oncol. 2015, 22, 133–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974, Erratum in Blood Adv. 2021, 5, 1953. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Falanga, A.; Roila, F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, vi85–vi92. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; Armani, U.; Carpenedo, M.; Falanga, A.; Fulfaro, F.; Imberti, D.; Laurora, R.; Molinari, A.C.; Prisco, D.; Silingardi, M.; et al. Prevention of venous thromboembolism in patients with cancer: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET)1. Thromb. Res. 2011, 129, e171–e176. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; on behalf of the COMPASS–CAT Working Group. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS–Cancer-Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef]
- Cella, C.A.; Di Minno, G.; Carlomagno, C.; Arcopinto, M.; Cerbone, A.M.; Matano, E.; Tufano, A.; Lordick, F.; De Simone, B.; Muehlberg, K.S.; et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncol. 2017, 22, 601–608. [Google Scholar] [CrossRef]
- Turpie, A.G. Thrombosis prophylaxis in the acutely ill medical patient: Insights from the prophylaxis in medical patients with enoxaparin (MEDENOX) trial. Am. J. Cardiol. 2000, 86, 48–52. [Google Scholar] [CrossRef]
- Carrier, M.; Khorana, A.; Moretto, P.; Le Gal, G.; Karp, R.; Zwicker, J.I. Lack of Evidence to Support Thromboprophylaxis in Hospitalized Medical Patients with Cancer. Am. J. Med. 2014, 127, 82–86.e1. [Google Scholar] [CrossRef]
- Bergqvist, D.; Agnelli, G.; Cohen, A.T.; E Nilsson, P.; Le Moigne-Amrani, A.; Dietrich-Neto, F. The Enoxacan II Investigators Prolonged prophylaxis against venous thromboembolism with enoxaparin in patients undergoing cancer surgery: Long-term survival analysis. Phlebol. J. Venous Dis. 2006, 21, 195–198. [Google Scholar] [CrossRef]
- ENOXACAN Study Group. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: A double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br. J. Surg. 1997, 84, 1099–1103. [Google Scholar]
- Illuminati, G.; Calio’, F.G.; Pizzardi, G.; Amatucci, C.; Masci, F.; Palumbo, P. Fondaparinux for intra and perioperative anticoagulation in patients with heparin-induced thrombocytopenia candidates for peripheral vascular surgery: Report of 4 cases. Int. J. Surg. Case Rep. 2016, 28, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, S.R.; Brennecke, A.; Behbakht, K.; Tayebnejad, A.; Breed, C.A.; Babayan, L.M.; Cheng, G.; Ramzan, A.A.; Wheeler, L.J.; Corr, B.R.; et al. Safety and Efficacy of Apixaban vs Enoxaparin for Preventing Postoperative Venous Thromboembolism in Women Undergoing Surgery for Gynecologic Malignant Neoplasm: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e207410. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Filliter, C.; Azoulay, L.; Tagalakis, V. Effectiveness and safety of direct oral anticoagulants in patients with cancer associated venous thromboembolism. Thromb. Res. 2021, 202, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-P.; Xiong, Y.-T.; Fan, Z.-Q.; Yan, L.-J.; Wang, J.-Y.; Gu, Z.-J. Efficacy of intermittent pneumatic compression for venous thromboembolism prophylaxis in patients undergoing gynecologic surgery: A systematic review and meta-analysis. Oncotarget 2016, 8, 20371–20379. [Google Scholar] [CrossRef]
- Togna, G.I.; Togna, A.R.; Franconi, M.; Caprino, L. Cisplatin Triggers Platelet Activation. Thromb. Res. 2000, 99, 503–509. [Google Scholar] [CrossRef]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandalà, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Liakishev, A.A. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. Results of the RE-COVER study. Kardiologiia 2010, 50, 80–81. [Google Scholar]
| Cpg | Scope and Purpose | Stakeholder Involvement | Rigor of Development | Clarity of Presentation | Applicability | Editorial Independence | Overall Assessment |
|---|---|---|---|---|---|---|---|
| NCCN 2020 | 100 (21/21) | 100 (21/21) | 100 (56/56) | 100 (21/21) | 100 (28/28) | 100 (14/14) | 100 (7/7) |
| ASCO 2020 | 100 (21/21) | 100 (21/21) | 1000 (56/56) | 100 (21/21) | 100 (28/28) | 100 (14/14) | 100 (7/7) |
| ITAC/ISTH 2019 | 100 (21/21) | 100 (21/21) | 100 (56/56) | 100 (21/21) | 100 (28/28) | 100 (14/14) | 100 (7/7) |
| AIOM 2019 | 100 (21/21) | 100 (21/21) | 96.4 (54/56) | 100 (21/21) | 100 (28/28) | 92.8 (13/14) | 100 (7/7) |
| SEOM 2019 | 95.2 (20/21) | 90.4 (19/21) | 94.6 (53/56) | 95.2 (20/21) | 82.1 (23/28) | 100 (14/14) | 85.7 (6/7) |
| BCSH 2015 | 90.4 (19/21) | 76.1 (16/21) | 87.5 (49/56) | 100 (21/21) | 92.8 (26/28) | 100 (14/14) | 85.7 (6/7) |
| Canadian Consensus 2015 | 90.4 (19/21) | 90.4 (19/21) | 87.5 (49/56) | 95.2 (20/21) | 85.7 (24/28) | 92.8 (13/14) | 71.4 (5/7) |
| ESMO 2011 | 100 (21/21) | 95.2 (20/21) | 91.1 (51/56) | 100 (21/21) | 100 (28/28) | 85.7 (12/14) | 85.7 (6/7) |
| SISET 2011 | 100 (21/21) | 95.2 (20/21) | 85.7 (49/56) | 80.9 (17/21) | 89.2 (25/28) | 85.7 (12/14) | 71.4 (5/7) |
| ASH 2021 | 100 (21/21) | 100(21/21) | 100 (56/56) | 95.2(20/21) | 100(28/28) | 92.8(13/14) | 100(7/7) |
| Guideline | Prevention in Hospitalized Medical Patients | Prevention in Surgical Patients | Timing and Duration | Prevention in Ambulatory Patients under Systemic Therapy | Prevention in CVC Cancer Patients |
|---|---|---|---|---|---|
| NCCN 2020 | LMWH or UFH or fondaparinux in inpatients: DOACs (apixaban or rivaroxaban) after discharge in all hospitalized gynecological cancer patients, up to 6 months if Khorana score >2 after discharge | LMWH, UFH, and fondaparinux in major abdominal or pelvic surgery | for 30 days after admission | DOACs (apixaban or rivaroxaban) up to 6 months only indicated in high-risk patients (Khorana score) | not indicated as routine prophylaxis |
| ASCO 2020 | LMWH or UFH or fondaparinux in all hospitalized gynecological cancer patients and after discharge in high-risk patients (Khorana score) | LMWH: Mechanical methods can help prophylaxis but should be used as monotherapy only if medical prophylaxis contraindicated. Fondaparinux or UFH can be considered in major cancer surgery (pelvic, abdominal). | should be started preoperatively, last for 7 days, and extended to 4 weeks after major procedures | LMWH or DOACs (apixaban or rivaroxaban) only indicated in high-risk patients (Khorana Score) | \ |
| ITAC/ISTH 2019 | LMWH or fondaparinux in gynecological cancer patients with reduced mobility | LMWH or UFH: Mechanical methods should not be used as monotherapy; in case of major laparotomy and all laparoscopies, should be started preoperatively | for 30 days after admission | DOACs (apixaban or rivaroxaban or edoxaban) only indicated high-risk patients (Khorana Score, COMPASS-KAT score, ONKOTEV score) | not indicated as routine prophylaxis; should be inserted on the right side and use PORT instead of PICC |
| AIOM 2019 | LMWH or UFH or fondaparinux in all hospitalized gynecological cancer patients | LMWH: Mechanical methods suggested but should be used as monotherapy only if prophylaxis is contraindicated. Fondaparinux or UFH can be considered in major cancer surgery (pelvic, abdominal) | should be started preoperatively, last for 7 days, and extended to 4 weeks in high-risk groups | LMWH or DOACs (apixaban or rivaroxaban) only indicated in high-risk patients (Khorana Score) | not indicated as routine prophylaxis |
| SEOM 2019 | LMWH in gynecological cancer patients with concomitant acute medical illness | LMWH and mechanical or only mechanical if LMWH contraindicated in all surgical interventions, should be started preoperatively | should be started preoperatively, last for at least 7 days, and extended to 4 weeks in high-risk patients | LMWH or DOACs for 12 weeks after initiation therapy only indicated in high-risk patients (Khorana or other models suggested) | not indicated as routine prophylaxis |
| ESMO 2011 | LMWH or UFH or fondaparinux in gynecological cancer patients with reduced mobility or acute medical illness | UFH or LMWH in all surgical gynecological cancer patients | for 30 days after admission | LMWH indicated only in high-risk patients | not indicated as routine prophylaxis |
| BCSH 2015 | LMWH in all inpatient gynecological cancer patient | in case of major abdominal or pelvic surgery | \ | LMWH or warfarin only indicated in high-risk patients (Khorana Score); not indicated in hormonal replacement therapy if no previous VTE | not indicated as routine prophylaxis |
| Canadian Consensus 2015 | LMWH in all inpatient gynecological cancer patients | all surgical cancer patients | for 30 days after admission | LMWH only indicated in high-risk patients (Khorana Score>2) | not indicated as routine prophylaxis |
| SISET 2011 | LMWH of fondaparinux: mechanical prophylaxis if high risk of bleeding or anticoagulant contraindications in gynecological cancer patient with concomitant acute medical illness | LMWH, UFH, and fondaparinux in all surgical gynecological cancer patients | should be started preoperatively, last for 7 days, and extended to 4 weeks in high-risk patients | not indicated | not indicated as routine prophylaxis |
| ASH 2021 | LMWH (UFH) in patients with severe renal impairment, mechanical prophylaxis if high risk for bleeding, mechanical and pharmacological prophylaxis if very high risk for TVE | LMWH or fondaparinux for patients at low bleeding risk, mechanical prophylaxis as monotherapy if LMWH contraindicated, combination of pharmacological and mechanical methods for patients at high risk of thrombosis | Should be started postoperatively, extended up to 4 weeks | LWHM only for patients receiving systemic therapy at intermediate and high risk for thrombosis (Khorana score complemented by clinical judgment and experience) | not indicated as routine prophylaxis |
| Patient Chacteristic | Risk Score |
|---|---|
| Site of primary cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Prechemotherapy platelet count 350 × 109/L or higher | 1 |
| Hemoglobin level less than 10 g/dL or use of red cells’ growth factors | 1 |
| Prechemotherapy leukocyte count higher than 11 × 109/L | 1 |
| Predictors for VTE | Score |
|---|---|
| Cancer-related risk factors | |
| - Anti-hormonal therapy for women with hormone receptor-positive breast cancer or on anthracycline treatment | 6 |
| - Time since cancer diagnosis < 6 months | 4 |
| - CVC | 3 |
| - Advanced stage cancer | 2 |
| Predisposing risk factors | |
| - Cardiovascular risk factors (composed of at least two of the following predictors: personal history of peripheral artery disease, ischemic stroke, coronary artery disease, hypertension, hyperlipidemia, diabetes, obesity) | 5 |
| Recent hospitalization for acute medical illness | 5 |
| Personal history of VTE | 1 |
| Biomarkers | |
| Platelets count > 350 × 109/L | 2 |
| Risk Factor | Score |
|---|---|
| Khorana score > 2 | 1 |
| Previous thromboembolism | 1 |
| Metastatic disease | 1 |
| Vascular/lymphatic macroscopic compression | 1 |
| Total ONKOTEV score | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, F.; Di Lorenzo, G.; Stabile, G.; Mirandola, M.; Restaino, S.; Ianniello, P.; Mirenda, G.; Ricci, G. A Systematic Review of the Guidelines on Venous Thromboembolism Prophylaxis in Gynecologic Oncology. Cancers 2022, 14, 2439. https://doi.org/10.3390/cancers14102439
Romano F, Di Lorenzo G, Stabile G, Mirandola M, Restaino S, Ianniello P, Mirenda G, Ricci G. A Systematic Review of the Guidelines on Venous Thromboembolism Prophylaxis in Gynecologic Oncology. Cancers. 2022; 14(10):2439. https://doi.org/10.3390/cancers14102439
Chicago/Turabian StyleRomano, Federico, Giovanni Di Lorenzo, Guglielmo Stabile, Mariateresa Mirandola, Stefano Restaino, Patrizia Ianniello, Giuseppe Mirenda, and Giuseppe Ricci. 2022. "A Systematic Review of the Guidelines on Venous Thromboembolism Prophylaxis in Gynecologic Oncology" Cancers 14, no. 10: 2439. https://doi.org/10.3390/cancers14102439
APA StyleRomano, F., Di Lorenzo, G., Stabile, G., Mirandola, M., Restaino, S., Ianniello, P., Mirenda, G., & Ricci, G. (2022). A Systematic Review of the Guidelines on Venous Thromboembolism Prophylaxis in Gynecologic Oncology. Cancers, 14(10), 2439. https://doi.org/10.3390/cancers14102439









