In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results and Discussion
3.1. Basil and Inflammation Response
3.2. Human Studies on Immunomodulation and Potential Risks on Overdose Effects
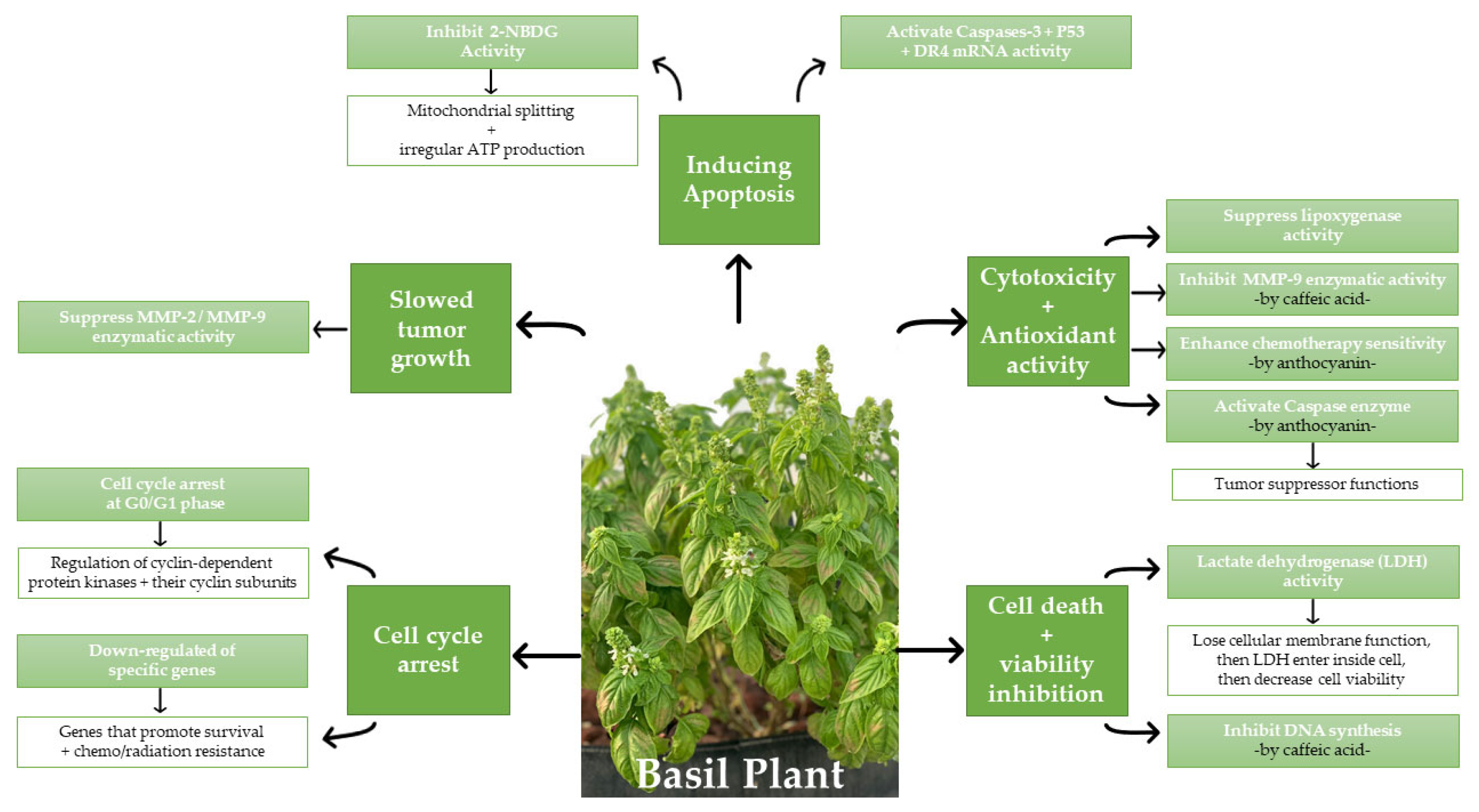
3.3. Cell Death and Viability Inhibition
3.4. Cytotoxicity and Antioxidant Activity
3.5. Inducing Apoptosis
3.6. Slowed Tumor Growth
3.7. Cell Cycle Arrest
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural products to prevent drug resistance in cancer chemotherapy: A review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, M.R.; Jang, J.H.; Na, H.J.; Lee, S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed. Pharmacother. 2018, 103, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Matowa, P.R.; Gundidza, M.; Gwanzura, L.; Nhachi, C.F.B. A survey of ethnomedicinal plants used to treat cancer by traditional medicine practitioners in Zimbabwe. BMC Complement. Med. Ther. 2020, 20, 278. [Google Scholar] [CrossRef]
- Mandal, A.K.; Poudel, M.; Neupane, N.P.; Verma, A.; Mandal, A.K.; Neupane, N.P.; Verma, A.; Poudel, M. Phytochemistry, Pharmacology, and Applications of (Tulsi). In Edible Plants in Health and Diseases; Springer: Singapore, 2022; pp. 135–174. [Google Scholar] [CrossRef]
- Hamedi, A.; Bayat, M.; Asemani, Y.; Amirghofran, Z. A review of potential anti-cancer properties of some selected medicinal plants grown in Iran. J. Herb. Med. 2022, 33, 2210–8033. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, J.-X.; Yang, S.-F.; Yang, C.-K.; Chen, T.-H.; Hsiao, Y.-H. Anticancer Effects and Molecular Mechanisms of Apigenin in Cervical Cancer Cells. Cancers 2022, 14, 1824. [Google Scholar] [CrossRef]
- Jermini, M.; Dubois, J.; Rodondi, P.Y.; Zaman, K.; Buclin, T.; Csajka, C.; Orcurto, A.; Rothuizen, L.E. Complementary medicine use during cancer treatment and potential herb-drug interactions from a cross-sectional study in an academic centre. Sci. Rep. 2019, 9, 5078. [Google Scholar] [CrossRef]
- Zagoto, M.; Cardia, G.F.E.; da Rocha, E.M.T.; Mourão, K.S.M.; Janeiro, V.; Cuman, R.K.N.; Pinto, A.A.; Contiero, R.L.; Freitas, P.S.L. de Biological activities of basil essential oil: A review of the current evidence. Res. Soc. Dev. 2021, 10, e363101220409. [Google Scholar] [CrossRef]
- Guardado Yordi, E.; Matos, M.J.; Buso, P.; Martínez, A.P.; Asanza, M.; Scalvenzi, L.; Manfredini, S.; Tacchini, M.; Radice, M.; Sacchetti, G. A comprehensive ethnobotanical profile of Ocimum campechianum (Lamiaceae): From traditional medicine to phytochemical and pharmacological evidences. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–17. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Al-Subhi, L.; Waly, M.I. Two cultivars of Ocimum basilicum leaves extracts attenuate streptozotocin-mediated oxidative stress in diabetic rats. Pak. J. Biol. Sci. 2020, 23, 1010–1017. [Google Scholar] [CrossRef]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical Characterization and Bioactive Properties of Cinnamon Basil (Ocimum basilicum cv. ‘Cinnamon’) and Lemon Basil (Ocimum × citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices—Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [Green Version]
- Tangpao, T.; Charoimek, N.; Teerakitchotikan, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Chaiyaso, T.; Ruksiriwanich, W.; et al. Volatile Organic Compounds from Basil Essential Oils: Plant Taxonomy, Biological Activities, and Their Applications in Tropical Fruit Productions. Horticulturae 2022, 8, 144. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Lal, R.K.; Gupta, P.; Mishra, A.; Chanotiya, C.S. Evaluation of yield and agronomic components by triallel cross and selection of high essential oil yielding hybrids in Basil. Ind. Crops Prod. 2022, 177, 114486. [Google Scholar] [CrossRef]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Raz, T.; Tait, L.; Shekhar, M.P.V.; Li, H.; Balan, V.; Makker, H.; Fridman, R.; Maddipati, K.; Raz, A. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol. Ther. 2013, 14, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Taie, H.A.A.; Salama, Z.A.-E.R.; Radwan, S. Potential Activity of Basil Plants as a Source of Antioxidants and Anticancer Agents as Affected by Organic and Bio-organic Fertilization. Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 119–127. [Google Scholar] [CrossRef]
- Mahmoud, G.I. Biological effects, antioxidant and anticancer activities of marigold and basil essential oils. J. Med. Plants Res. 2013, 7, 561–572. [Google Scholar] [CrossRef]
- Gajendiran, A.; Thangaraman, V.; Thangamani, S.; Ravi, D.; Abraham, J. Antimicrobial, antioxidant and anticancer screening of Ocimum basilicum seeds. Bull. Pharm. Res. 2016, 6, 114–119. [Google Scholar]
- Alkhateeb, M.A.; Al-Otaibi, W.R.; AlGabbani, Q.; Alsakran, A.A.; Alnafjan, A.A.; Alotaibi, A.M.; Al-Qahtani, W.S. Low-Temperature Extracts of Purple Blossoms of Basil (Ocimum basilicum L.) Intervened Mitochondrial Translocation Contributes Prompted Apoptosis in Human Breast Cancer Cells. Biol. Res. 2021, 54, 2. [Google Scholar] [CrossRef]
- Aburjai, T.A.; Mansi, K.; Azzam, H.; Alqudah, D.A.; Alshaer, W.; Abuirjei, M. Chemical Compositions and Anticancer Potential of Essential Oil from Greenhouse-Cultivated Ocimum basilicum Leaves. Indian J. Pharm. Sci. 2020, 82, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Indrayudha, P.; Hapsari, H.S. Cytotoxic activity of combined ethanolic extract of Cinnamomum burmannii and Ocimum tenuiflorum Linn against T47D cancer cells. Arch. Venez. Farmacol. Ter. 2021, 40, 114–120. [Google Scholar] [CrossRef]
- Doguer, C.; Yıkmış, S.; Levent, O.; Turkol, M. Anticancer effects of enrichment in the bioactive components of the functional beverage of Turkish gastronomy by supplementation with purple basil (Ocimum basilicum L.) and the ultrasound treatment. J. Food Process. Preserv. 2021, 45, e15436. [Google Scholar] [CrossRef]
- Manikandan, D.B.; Sridhar, A.; Krishnasamy Sekar, R.; Perumalsamy, B.; Veeran, S.; Arumugam, M.; Karuppaiah, P.; Ramasamy, T. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng. 2021, 9, 104845. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, P.; Fakhrnezhad, F.R.; Zarringhalami, R.; Orhan, I.E. Cytotoxicity of Ocimum basilicum and Impatiens walleriana Extracts on AGS and SKOV-3 Cancer Cell Lines by Flow Cytometry Analysis. Int. J. Cancer Manag. 2021, 14, e102610. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, O.; Shukla, A.; Singh Rajpurohit, C.; Vasudev, P.G.; Luqman, S.; Kumar Srivastava, S.; Bhushan Pant, A.; Khan, F. Structure-Activity Relationship Studies on Holy Basil (Ocimum sanctum L.) Based Flavonoid Orientin and Its Analogue for Cytotoxic Activity in Liver Cancer Cell Line HepG2. Comb. Chem. High Throughput Screen. 2016, 19, 656–666. [Google Scholar] [CrossRef]
- Zarlaha, A.; Kourkoumelis, N.; Stanojkovic, T.P.; Kovala-Demertzi, D. Cytotoxic activity of essential oil and extracts of Ocimum basilicum against human carcinoma cells. Molecular docking study of isoeugenol as a potent cox and lox inhibitor. Dig. J. Nanomater. Biostructures 2014, 9, 907–917. [Google Scholar]
- Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Złotek, U.; Szychowski, K.A.; Świeca, M. Potential in vitro antioxidant, anti-inflammatory, antidiabetic, and anticancer effect of arachidonic acid-elicited basil leaves. J. Funct. Foods 2017, 36, 290–299. [Google Scholar] [CrossRef]
- Alanazi, M. Natural Plants “Ocimum (Basil) and Achillea” as Anticancer in Human Cell. Ph.D. Thesis, Tennessee State University, Nashville, TN, USA, 2016. [Google Scholar]
- Ashley, N.; Weil, Z.; Nelson, R. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Zhang, J.; Veeramachaneni, N. Targeting interleukin-1β and inflammation in lung cancer. Biomark. Res. 2022, 10, 5. [Google Scholar] [CrossRef]
- Sibi, G. Rabina Inhibition of pro-inflammatory mediators and cytokines by Chlorella vulgaris extracts. Pharmacogn. Res. 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güez, C.M.; de Souza, R.O.; Fischer, P.; de Moura Leão, M.F.; Duarte, J.A.; Boligon, A.A.; Athayde, M.L.; Zuravski, L.; de Oliveira, L.F.S.; Machado, M.M. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 2017, 53, e15098. [Google Scholar] [CrossRef] [Green Version]
- Eftekhar, N.; Moghimi, A.; Mohammadian Roshan, N.; Saadat, S.; Boskabady, M.H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement. Altern. Med. 2019, 19, 349. [Google Scholar] [CrossRef] [PubMed]
- Szymacnowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef]
- Lantto, T.; Colucci, M.; Závadov, V.; Hiltunen, R. Cytotoxicity of curcumin, resveratrol and plant extracts from basil, juniper, laurel and parsley in SH-SY5Y and CV1-P cells. Food Chem. 2009, 117, 405–411. [Google Scholar] [CrossRef]
- Gudkov, A.; Gurova, K.; Komarova, E.; Elena, A. Inflammation and p53: A tale of two stresses. Genes Cancer 2011, 2, 503–516. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Saeed, F.; Imran, A.; Khan, M.R.; Khan, M.A.; Ahmed, S.; Rauf, A. Immunomodulatory perspectives of potential biological spices with special reference to cancer and diabetes. Food Agric. Immunol. 2017, 28, 543–572. [Google Scholar] [CrossRef]
- Shimizu, T.; Torres, M.; Chakraborty, S.; Souchek, J. Holy Basil leaf extract decreases tumorigenicity and metastasis of aggressive human pancreatic cancer cells in vitro and in vivo: Potential role in therapy. Cancer Lett. 2013, 336, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Miele, M.; Milt, J.; Dondero, R.; Ciarallo, G.; Mazzei, M. Methyleugenol in Ocimum basilicum L. Cv. Genovese Gigante. J. Agric. Food Chem. 2001, 49, 517–521. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; Hassan, M.E.; Hassan, R.R.; Elshafey, O.I.; Hamza, Z.K.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Nanoencapsulation of basil essential oil alleviates the oxidative stress, genotoxicity and DNA damage in rats exposed to biosynthesized iron nanoparticles. Heliyon 2021, 7, e07537. [Google Scholar] [CrossRef]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1,2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules 2019, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
| Paper | Type of Study and Level of Evidence | Compound/Extract | Sample | Posology/Treatment | Main Results |
|---|---|---|---|---|---|
| Nangia-Makker et al. [25] | Animal study (level 6) | Ocimum gratissimum (holy basil) aqueous extract | MCF10ADCIS.com cells injected in female nude mice | 4 mg/mL lyophilized Ocimum gratissimum, hydrophobic or hydrophilic fractions | Slowed down MCF10ADCIS.com tumor growth and progression |
| Taie et al. [26] | Animal study (level 6) | Ocimum basilicum (sweet basil) leaves’ ethanolic extract | Ehrlich ascites carcinoma cell line injected in female Swiss albino mice 22–25 g; 8–10 weeks old | Ethanolic extracts with 1250, 1500, 1750, or 2000 ppm, with oil extracted from them (0.04, 0.06, 0.08, and 0.10 mg) | Ocimum ethanolic extract and oil with various concentrations changed the viability of Ehrlich ascites carcinoma cells in comparison with untreated cells |
| Mahmoud [27] | Animal study (level 6) | Ocimum basilicum (basil estragole chemotype) + fresh Tagetes minuta flowers (marigold) (100 g of each) were hydro-distilled | Ehrlich ascites carcinoma cell line (EACC) injected in 48 female Swiss albino mice weighting 20–25 g; 7–8 weeks old | Several volumes of marigold and basil essential oils to finalize with 25, 50, 75, 100, and 200 µg/mL concentrations | Essential oils significantly prevented tumor development (i.e., decreased total EACC number and increased the percentage of dead cells). The pre-initiation treatment was most effective compared to the initiation and post-initiation treatments, with marigold being more effective (i.e., higher percentage of dead cells) |
| Paper | Type of Study and Level of Evidence | Compound/Extract | Sample | Posology/Treatment | Main Results |
|---|---|---|---|---|---|
| Gajendiran et al. [28] | Laboratory study (level 6) | Ocimum basilicum (basil seeds) extracted in petroleum ether and methanol separately | Human osteosarcoma cell line (MG63) | Ocimum basilicum seed extraction (12.5, 25, 50, 100, 200 µg/m) | Increased cell line deterioration and death with the increase in concentration of Ocimum basilicum seed extract. |
| Alkhateeb et al. [29] | Laboratory study (level 6) | Ocimum basilicum (dark purple blossoms of basil) aqueous extract at low temperature (0 °C) | Human MCF7 breast cancer cell line | Ocimum basilicum blossoms aqueous extract (0, 50, 150, and 250 μg/mL for 24 and 48 h) | Greater anticancer and antioxidant activities of Ocimum basilicum blossoms aqueous extract at low temperature in comparison to boiled water solvent (high temperature) and alcoholic extracts. |
| Aburjai et al. [30] | Laboratory study (level 6) | Ocimum basilicum “Cinnamon” leaves’ essential oil extracted by hydrodistillation | Three different cancer cell lines: MDA–MB–231 (triple-negative breast cancer cell line), MCF7 (breast cancer), and U–87 MG (glioblastoma) | Weight by weight (w/w%) of the dry Ocimum basilicum “Cinnamon” leaves was 0.50 % (w/w) | The Ocimum basilicum essential oils linalool, eugenol, and eucalyptol showed effective anticancer activity against several types of cancer cells. |
| Indrayudha and Hapsari [31] | Laboratory study (level 6) | Combination of Cinnamomum burmannii with Ocimum tenuiflorum ethanolic extract | T47D cancer cells | Cinnamomum burmannii ethanolic extract (500 µg/mL) + Ocimum tenuiflorum ethanolic extract (500 and 50 µg/mL) | Combined Cinnamomum and Ocimum ethanolic extracts produced a cooperative impact against T47D cancer cells compared to each extract alone. |
| Doguer et al. [32] | Laboratory study (level 6) | Purple basil (PB) dried leaves’ aqueous extract added to sirkencubin syrup (SC) | Human colon carcinoma cells (Caco-2) | Various concentrations (15–40 µL) of purple basil sirkencubin) in 100 µL of fresh medium (total of 150–400 μL/mL) | Half-maximal inhibitory concentration (IC50) values of SC and PBS were 288.1 and 239.8, respectively. PBS showed better results in terms of anticancer activity against (Caco-2). |
| Manikandan et al. [33] | Laboratory study (level 6) | Ocimum americanum aqueous leaf extract used to fabricate silver nanoparticles (AgNPs) | A549 lung cancer cells | Mixture of 5 g of leaf powder and 50 mL of sterile distilled water; 2 mL of the mixture treated with 100 mL of 1 mM AgNO3 solution | AgNPs fabricated with Ocimum americanum aqueous leaf extract, possessed strong cytotoxic anticancer activity against the A549 lung cancer cell line by inducing apoptosis. |
| Elansary & Mahmoud [34] | Laboratory study (level 6) | Ocimum basilicum (purple ruffle), O. basilicum (dark opal), O. basilicum. (Genovese), O. basilicum (anise), O. basilicum (bush green), and O. basilicum L. (OBL) | Line HeLa, MCF–7, Jurkat, HT–29, T24, MIA PaCa-2 cancer cells and one normal human cell line HEK–293 | Different concentrations of the six international basil cultivars | Compounds present in O. basilicum species—such as rosmarinic acid, chicoric acid, and caftaric acid—varied in their anticancer activities. OBL displayed the highest antioxidant and anti-proliferative activities compared to others. |
| Mahmoud [27] | Laboratory study (level 6) | Ocimum basilicum (basil estragole chemotype) + fresh Tagetes minuta flowers (marigold) (100 g of each) were hydrodistilled | Human promyelocytic leukemia cell lines (HL–60 and NB4) and experimental animal model cancer cells (Ehrlich ascites carcinoma cells, EACC). | Several volumes of marigold and basil essential oils finalized to 25, 50, 75, 100, and 200 µg/mL concentrations | Dead cells increased with increasing concentrations of both estragole and marigold. Basil estragole was more effective on HL–60 than NB4 cell lines. Marigold was more effective on NB4 than HL–60 cell lines. The anticancer activity of marigold was higher than that of estragole against EACC. |
| Hanachi et al. [35] | Laboratory study (level 6) | Ocimum basilicum and Impatiens walleriana leaves extracts | Human gastric adenocarcinoma (AGS) and human ovarian carcinoma (SKOV–3) cancer cell lines | 0.5 mg/mL to 5 mg/mL concentrations | Toxicity of O. basilicum on SKOV3 cell lines was higher compared to I. walleriana, while I. walleriana was more toxic towards AGS compared to O. basilicum. The cytotoxic effect may be attributed to the anthocyanin and flavonoid derivatives present in the extracts. |
| Sharma et al. [36] | Laboratory study (level 6) | Extraction of Ocimum basilicum L. (O. tenuiflorum) orientin and its analogue | Human liver cancer cell line HepG2 | 100 μg/mL (202.389 μM) concentration for 96 h | Cell death was only 41%, thereby indicating its ineffectiveness (in purified form) as an anticarcinogenic agent, with low cytotoxicity activity on the HepG2 liver cancer cell line. |
| Zarlaha et al. [37] | Laboratory study (level 6) | Ocimum basilicum ethanolic extract and essential oil | Human cervix adenocarcinoma HeLa cells, human melanoma FemX cells, human chronic myelogenous leukemia K562 cells, and human ovarian SKOV3 cells | Ranging from 12.5 to 200 μg/mL for 72 h (200, 100, 50, 25, and 12.5 μM) | The phytochemicals rosmarinic and caffeic acids, along with the essential oils eugenol, isoeugenol, and linalool, showed significant anticancer activity on the four cell lines—especially the SKOV3 cell line. |
| Abbasi et al. [38] | Laboratory study (level 6) | Ocimum basilicum (purple basil) callus extract used to produce silver nanoparticles (BC–AgNPs), and silver nanoparticles using anthocyanin extract derived from the same plant (AE–AgNPs) | HepG2 liver carcinoma cells | AgNO3 (1 mM) was added to BC (15 g callus) and AE (anthocyanin) extracts at different ratios (1:1, 1:2, 1:5, and 1:10) | AE–AgNPs showed significant anticancer activity against the HepG2 cell line (75% mortality at 200 µg/mL) compared to BC–AgNPs (approximately 27% mortality at 200 µg/mL). |
| Złotek et al. [39] | Laboratory study (level 6) | Ocimum basilicum (lettuce leaf basil) ethanolic extracts of lyophilized basil leaves | Human squamous carcinoma cell line SCC–15 (ATCC CRL1623) | Different concentrations of the extract (0.125, 0.250, 0.500, and 1.000 mg/mL) | Elicitation of basil with arachidonic acid induced overproduction of phenolic compounds that resulted in a dose-dependent decline in cell metabolism, with greater anticancer activity at higher concentrations. |
| Alanazi [40] | Laboratory study (level 6) | Ocimum spp. basil leaves and Achillea spp. extract | Human lung carcinoma cell line (A549), human prostate cancer cell line (PC3), and cervical cancer cell line (HeLa) | Basil extract concentrations (333, 166.5, and 83.3 mg/mL) for A549 and PC-3 cells, and lower concentrations (41.6, 20.8, and 10.4 mg/mL) for HeLa cells. | Basil extract worked significantly against HeLa cells, but less so against PC–3 cells. Achillea extract worked significantly against the PC–3 cell line. |
| Paper | Type of Study and Level of Evidence | Compound/Extract | Sample | Posology/Treatment | Main Results |
|---|---|---|---|---|---|
| Prasad [28] | Randomized, placebo-controlled clinical trial (level 1) | Ocimum tenuiflorum (tulsi) ethanolic extract (0.5%) of leaves | Healthy adults (30) aged 18–30 years | 1000 mg/day for 2 weeks | Increased physical activity, while lowering increments of lactic acid. Decreased fatigue and CK levels. |
| Mondal et al. [38] | Randomized, double-blind, placebo-controlled crossover (level 1) | Ocimum tenuiflorum (tulsi) ethanolic extract of leaves | Healthy adults (22) aged 22–37 years | 300 mg/day for 4 weeks | Increase in cytokine levels, especially (interferon-ϒ and interleukin-4). |
| Sharma [39] | Open clinical trial (level 1) | Ocimum tenuiflorum (tulsi) aqueous extract of leaves tablets | Adults with asthma (20) | 500 mg × 3/day for a week | Within 3 days of relaxation, showed better vital capacity. |
| Rajalakshmi et al. [40] | Clinical trial (level 1) | Ocimum tenuiflorum (tulsi) aqueous extract of fresh leaves | Adults with viral hepatitis (20) aged 10–60 years | 10 g/day, for 2–3 weeks, depending on the severity of the case | Enhancement of all symptoms within 2 weeks. |
| Das et al. [41] | Randomized, parallel-controlled clinical trial (level 1) | Ocimum tenuiflorum (tulsi) aqueous extract of fresh leaves | Adults with viral encephalitis (14) | 2.5 g × 4/day for 4 weeks | In comparison with steroids, the survival rate was boosted when the examined extract was applied. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers 2022, 14, 2375. https://doi.org/10.3390/cancers14102375
Perna S, Alawadhi H, Riva A, Allegrini P, Petrangolini G, Gasparri C, Alalwan TA, Rondanelli M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers. 2022; 14(10):2375. https://doi.org/10.3390/cancers14102375
Chicago/Turabian StylePerna, Simone, Hajar Alawadhi, Antonella Riva, Pietro Allegrini, Giovanna Petrangolini, Clara Gasparri, Tariq A. Alalwan, and Mariangela Rondanelli. 2022. "In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects" Cancers 14, no. 10: 2375. https://doi.org/10.3390/cancers14102375
APA StylePerna, S., Alawadhi, H., Riva, A., Allegrini, P., Petrangolini, G., Gasparri, C., Alalwan, T. A., & Rondanelli, M. (2022). In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers, 14(10), 2375. https://doi.org/10.3390/cancers14102375







