A Multidisciplinary Approach for the Personalised Non-Operative Management of Elderly and Frail Rectal Cancer Patients Unable to Undergo TME Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Current Treatment of Elderly and Frail Rectal Cancer Patients
1.1.1. Considerations on the Surgical Treatment of Elderly Rectal Cancer Patients
1.1.2. Epidemiology of the Non-Surgical Treatment of the Elderly and Frail
1.1.3. The Fate of Elderly and Frail Rectal Cancer Patients Refrained from Treatment
1.2. The Need for a Personalised Non-Operative Treatment Approach
2. The Multidisciplinary Patient Approach
2.1. Treatment Outcomes in the Elderly and Frail
2.2. Geriatric Assessment
Comprehensive Geriatric Assessment (CGA)
2.3. Multidisciplinary Evaluation
2.4. Prehabilitation
3. Non-Operative Treatment Options
3.1. Systemic Chemotherapy
3.2. External Beam Radiotherapy (EBRT)
3.3. Dose Escalation of Radiotherapy
3.3.1. Contact X-ray Brachytherapy
3.3.2. High-Dose Rate Endorectal Brachytherapy
3.4. Local Excision
4. Response Evaluation and Follow-Up
4.1. Response Evaluation
4.2. Follow-Up
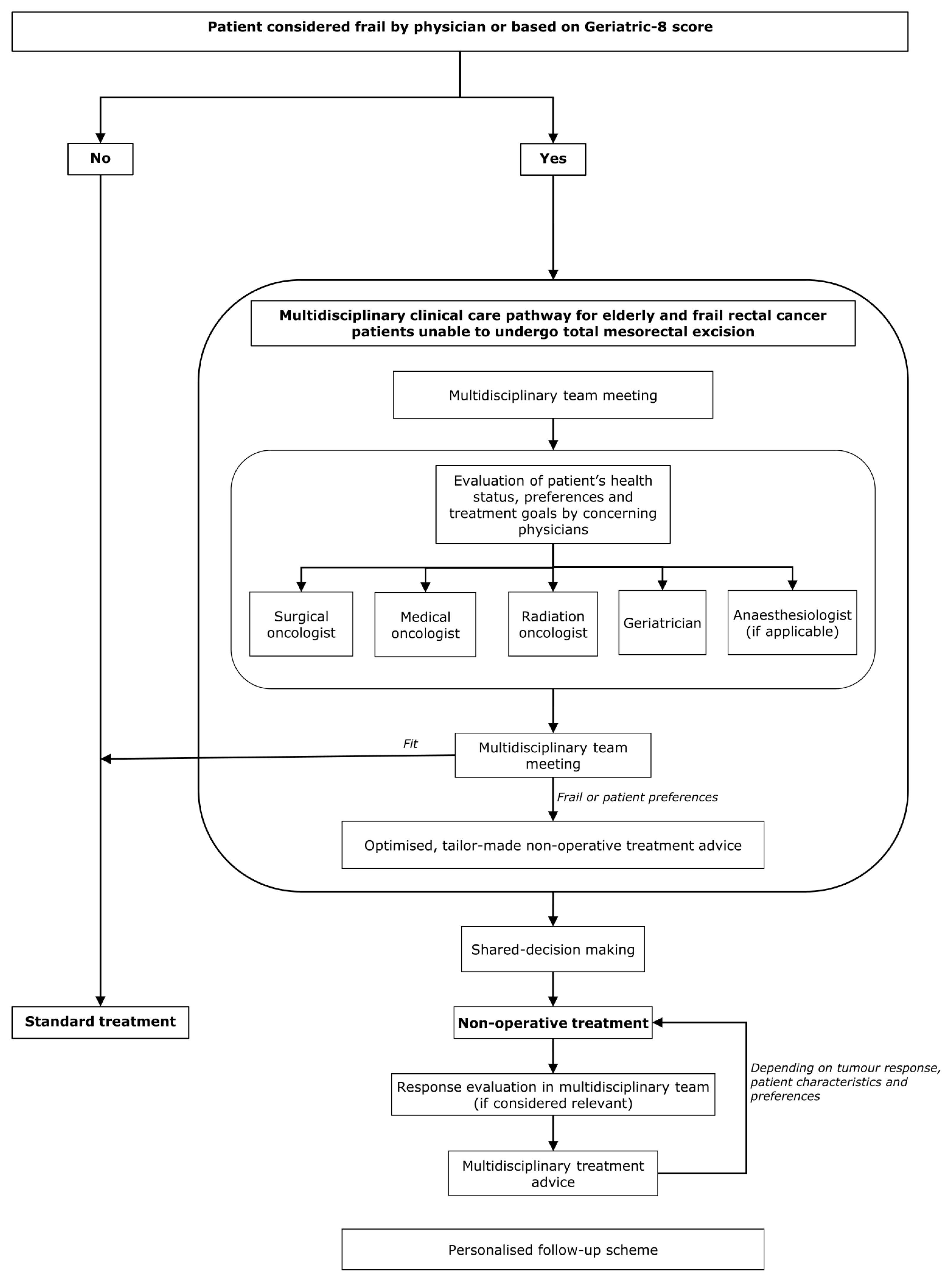
5. Multidisciplinary Clinical Care Pathway
5.1. A Practical Suggestion of a Multidisciplinary Care Pathway
5.2. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van der Vlies, E.; Vernooij, L.M.; van Erning, F.N.; Vink, G.R.; Bos, W.J.W.; Portielje, J.E.A.; Noordzij, P.G.; Los, M. Survival of Surgical and Non-Surgical Older Patients with Non-Metastatic Colorectal Cancer: A Population-Based Study in the Netherlands. Eur. J. Surg. Oncol. 2021, 47, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Benitez Majano, S.; Di Girolamo, C.; Rachet, B.; Maringe, C.; Guren, M.G.; Glimelius, B.; Iversen, L.H.; Schnell, E.A.; Lundqvist, K.; Christensen, J.; et al. Surgical Treatment and Survival from Colorectal Cancer in Denmark, England, Norway, and Sweden: A Population-Based Study. Lancet Oncol. 2019, 20, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Schiphorst, A.; Verweij, N.M.; Pronk, A.; Hamaker, M.E. Age-Related Guideline Adherence and Outcome in Low Rectal Cancer. Dis. Colon Rectum 2014, 57, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Montroni, I.; Ugolini, G.; Saur, N.M.; Spinelli, A.; Rostoft, S.; Millan, M.; Wolthuis, A.; Daniels, I.R.; Hompes, R.; Penna, M.; et al. Personalized Management of Elderly Patients with Rectal Cancer: Expert Recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commissi. Eur. J. Surg. Oncol. 2018, 44, 1685–1702. [Google Scholar] [CrossRef]
- Bujko, K.; Glynne-Jones, R.; Papamichael, D.; Rutten, H.J.T. Optimal Management of Localized Rectal Cancer in Older Patients. J. Geriatr. Oncol. 2018, 9, 696–704. [Google Scholar] [CrossRef]
- Wang, S.J.; Hathout, L.; Malhotra, U.; Maloney-Patel, N.; Kilic, S.; Poplin, E.; Jabbour, S.K. Decision-Making Strategy for Rectal Cancer Management Using Radiation Therapy for Elderly or Comorbid Patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Bethune, R.; Sbaih, M.; Brosnan, C.; Arulampalam, T. What Happens When We Do Not Operate? Survival Following Conservative Bowel Cancer Management. Ann. R. Coll. Surg. Engl. 2016, 98, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Halim, M.; Wu, H.; Poustie, M.; Beveridge, A.; Scott, N.; Mitchell, P.J. Survival after Non-Resection of Colorectal Cancer: The Argument for Including Non-Operatives in Consultant Outcome Reporting in the UK. Ann. R. Coll. Surg. Engl. 2019, 101, 126–132. [Google Scholar] [CrossRef]
- Ronnekleiv-Kelly, S.M.; Kennedy, G.D. Management of Stage IV Rectal Cancer: Palliative Options. World J. Gastroenterol. 2011, 17, 835–847. [Google Scholar] [CrossRef]
- Marijnen, C.A.M. Organ Preservation in Rectal Cancer: Have All Questions Been Answered? Lancet Oncol. 2015, 16, e13–e22. [Google Scholar] [CrossRef]
- Roeder, F.; Meldolesi, E.; Gerum, S.; Valentini, V.; Rödel, C. Recent Advances in (Chemo-)Radiation Therapy for Rectal Cancer: A Comprehensive Review. Radiat. Oncol. 2020, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, D.; Audisio, R.A.; Glimelius, B.; de Gramont, A.; Glynne-Jones, R.; Haller, D.; Köhne, C.H.; Rostoft, S.; Lemmens, V.; Mitry, E.; et al. Treatment of Colorectal Cancer in Older Patients: International Society of Geriatric Oncology (SIOG) Consensus Recommendations 2013. Ann. Oncol. 2015, 26, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Hagemans, J.A.W.; Rothbarth, J.; Kirkels, W.J.; Boormans, J.L.; van Meerten, E.; Nuyttens, J.J.M.E.; Madsen, E.V.E.; Verhoef, C.; Burger, J.W.A. Total Pelvic Exenteration for Locally Advanced and Locally Recurrent Rectal Cancer in the Elderly. Eur. J. Surg. Oncol. 2018, 44, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.M.; Heil, T.C.; Olde Rikkert, M.G.M.; Lemmens, V.E.P.P.; Rutten, H.J.T.; de Wilt, J.H.W.; van Erning, F.N. The Gap in Postoperative Outcome between Older and Younger Patients with Stage I–III Colorectal Cancer Has Been Bridged; Results from the Netherlands Cancer Registry. Eur. J. Cancer 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Ketelaers, S.H.J.; Orsini, R.G.; Burger, J.W.A.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T. Significant Improvement in Postoperative and 1-Year Mortality after Colorectal Cancer Surgery in Recent Years. Eur. J. Surg. Oncol. 2019, 45, 2052–2058. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of Life versus Length of Life Considerations in Cancer Patients: A Systematic Literature Review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
- Millan, M.; Merino, S.; Caro, A.; Feliu, F.; Escuder, J.; Francesch, T. Treatment of Colorectal Cancer in the Elderly. World J. Gastrointest. Oncol. 2015, 7, 204–220. [Google Scholar] [CrossRef]
- Hofman, C.S.; Makai, P.; Boter, H.; Buurman, B.M.; De Craen, A.J.; Olde Rikkert, M.G.M.; Donders, R.; Melis, R.J.F. The Influence of Age on Health Valuations: The Older Olds Prefer Functional Independence While the Younger Olds Prefer Less Morbidity. Clin. Interv. Aging 2015, 10, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Hathout, L.; Maloney-Patel, N.; Malhotra, U.; Wang, S.; Chokavatia, S.; Dalal, I.; Poplin, E.; Jabbour, S.K. Management of Locally Advanced Rectal Cancer in the Elderly: A Critical Review and Algorithm. J. Gastrointest. Oncol. 2018, 9, 363–376. [Google Scholar] [CrossRef]
- Croese, A.D.; Lonie, J.M.; Trollope, A.F.; Vangaveti, V.N.; Ho, Y.-H. A Meta-Analysis of the Prevalence of Low Anterior Resection Syndrome and Systematic Review of Risk Factors. Int. J. Surg. 2018, 56, 234–241. [Google Scholar] [CrossRef]
- Ketelaers, S.H.J.; van Heinsbergen, M.; Orsini, R.G.; Vogelaar, F.J.; Konsten, J.L.M.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T.; Burger, J.W.A.; Bloemen, J.G. Functional Bowel Complaints and the Impact on Quality of Life after Colorectal Cancer Surgery in the Elderly. Front. Oncol. 2021, 12, 832377. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.M.; Maas, C.P.; Marijnen, C.A.M.; Wiggers, T.; Rutten, H.J.; Klein Kranenbarg, E.; Van De Velde, C.J.H. Urinary Dysfunction after Rectal Cancer Treatment Is Mainly Caused by Surgery. Br. J. Surg. 2008, 95, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Bock, D.; Asplund, D.; Ohlsson, B.; Rosenberg, J.; Angenete, E. Urinary Dysfunction in Patients with Rectal Cancer: A Prospective Cohort Study. Color. Dis. 2020, 22, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Traa, M.J.; De, V.J.; Roukema, J.A.; Den Oudsten, B.L. Sexual (Dys)Function and the Quality of Sexual Life in Patients with Colorectal Cancer: A Systematic Review. Ann. Oncol. 2012, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hendren, S.K.; O’Connor, B.I.; Liu, M.; Asano, T.; Cohen, Z.; Swallow, C.J.; MacRae, H.M.; Gryfe, R.; McLeod, R.S. Prevalence of Male and Female Sexual Dysfunction Is High Following Surgery for Rectal Cancer. Ann. Surg. 2005, 242, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Stockton, D.; Elder, A.; Wilson, R.G.; Dunlop, M.G. Assessment of Outcomes after Colorectal Cancer Resection in the Elderly as a Rationale for Screening and Early Detection. Br. J. Surg. 2004, 91, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Buurman, B.M.; Parlevliet, J.L.; Van Deelen, B.A.; De Haan, R.J.; De Rooij, S.E. A Randomised Clinical Trial on a Comprehensive Geriatric Assessment and Intensive Home Follow-up after Hospital Discharge: The Transitional Care Bridge. BMC Health Serv. Res. 2010, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Amemiya, T.; Oda, K.; Ando, M.; Kawamura, T.; Kitagawa, Y.; Okawa, Y.; Yasui, A.; Ike, H.; Shimada, H.; Kuroiwa, K.; et al. Activities of Daily Living and Quality of Life of Elderly Patients after Elective Surgery for Gastric and Colorectal Cancers. Ann. Surg. 2007, 246, 222–228. [Google Scholar] [CrossRef]
- Samuelsson, K.S.; Egenvall, M.; Klarin, I.; Lökk, J.; Gunnarsson, U. Preoperative Geriatric Assessment and Follow-up of Patients Older than 75 Years Undergoing Elective Surgery for Suspected Colorectal Cancer. J. Geriatr. Oncol. 2019, 10, 709–715. [Google Scholar] [CrossRef]
- Berian, J.R.; Mohanty, S.; Ko, C.Y.; Rosenthal, R.A.; Robinson, T.N. Association of Loss of Independence with Readmission and Death after Discharge in Older Patients after Surgical Procedures. JAMA Surg. 2016, 151, e161689. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, G.C.; Correia, A.J.; Thuluvath, P.J. The Impact of Cirrhosis and Portal Hypertension on Mortality Following Colorectal Surgery: A Nationwide, Population-Based Study. Dis. Colon Rectum 2009, 52, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Montomoli, J.; Erichsen, R.; Christiansen, C.F.; Ulrichsen, S.P.; Pedersen, L.; Nilsson, T.; Sørensen, H.T. Liver Disease and 30-Day Mortality after Colorectal Cancer Surgery: A Danish Population-Based Cohort Study. BMC Gastroenterol. 2013, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steppan, J.; Diaz-Rodriguez, N.; Barodka, V.M.; Nyhan, D.; Pullins, E.; Housten, T.; Damico, R.L.; Mathai, S.C.; Hassoun, P.M.; Berkowitz, D.E.; et al. Focused Review of Perioperative Care of Patients with Pulmonary Hypertension and Proposal of a Perioperative Pathway. Cureus 2018, 10, e2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketelaers, S.H.J.; Voogt, E.L.K.; Simkens, G.A.; Bloemen, J.G.; Nieuwenhuijzen, G.A.P.; de Hingh, I.H.J.; Rutten, H.J.T.; Burger, J.W.A.; Orsini, R.G. Age-Related Differences in Morbidity and Mortality after Surgery for Primary Clinical T4 and Locally Recurrent Rectal Cancer. Color. Dis. 2021, 23, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Law, W.L.; Chan, W.F.; Lee, Y.M.; Chu, K.W. Non-Curative Surgery for Colorectal Cancer: Critical Appraisal of Outcomes. Int. J. Colorectal Dis. 2004, 19, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Costi, R.; Leonardi, F.; Zanoni, D.; Violi, V.; Roncoroni, L. Palliative Care and End-Stage Colorectal Cancer Management: The Surgeon Meets the Oncologist. World J. Gastroenterol. 2014, 20, 7602–7621. [Google Scholar] [CrossRef] [PubMed]
- Flyum, I.R.; Mahic, S.; Grov, E.K.; Joranger, P. Health-Related Quality of Life in Patients with Colorectal Cancer in the Palliative Phase: A Systematic Review and Meta-Analysis. BMC Palliat. Care 2021, 20, 144. [Google Scholar] [CrossRef]
- Manceau, G.; Mege, D.; Bridoux, V.; Lakkis, Z.; Venara, A.; Voron, T.; Sielezneff, I.; Karoui, M. Emergency Surgery for Obstructive Colon Cancer in Elderly Patients: Results of a Multicentric Cohort of the French National Surgical Association. Dis. Colon Rectum 2019, 62, 941–951. [Google Scholar] [CrossRef]
- Sun Myint, A.; Smith, F.M.; Gollins, S.; Wong, H.; Rao, C.; Whitmarsh, K.; Sripadam, R.; Rooney, P.; Hershman, M.; Pritchard, D.M. Dose Escalation Using Contact X-ray Brachytherapy After External Beam Radiotherapy as Nonsurgical Treatment Option for Rectal Cancer: Outcomes From a Single-Center Experience. Int. J. Radiat. Oncol. 2018, 100, 565–573. [Google Scholar] [CrossRef]
- Gérard, J.-P.; Barbet, N.; Gal, J.; Dejean, C.; Evesque, L.; Doyen, J.; Coquard, R.; Gugenheim, J.; Benizri, E.; Schiappa, R.; et al. Planned Organ Preservation for Early T2-3 Rectal Adenocarcinoma: A French, Multicentre Study. Eur. J. Cancer 2019, 108, 1–16. [Google Scholar] [CrossRef]
- Custers, P.; Geubels, B.; IL, H.; FP, P.; Engelhardt, E.; Beets, G.; Marijnen, C.; van Leerdam, M.; van Triest, B. Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up. Cancers 2021, 13, 6333. [Google Scholar] [CrossRef] [PubMed]
- Rijkmans, E.C.; Cats, A.; Nout, R.A.; van den Bongard, D.H.J.G.; Ketelaars, M.; Buijsen, J.; Rozema, T.; Franssen, J.H.; Velema, L.A.; van Triest, B.; et al. Endorectal Brachytherapy Boost After External Beam Radiation Therapy in Elderly or Medically Inoperable Patients With Rectal Cancer: Primary Outcomes of the Phase 1 HERBERT Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Verseveld, M.; de Graaf, E.J.R.; Verhoef, C.; van Meerten, E.; Punt, C.J.A.; de Hingh, I.H.J.T.; Nagtegaal, I.D.; Nuyttens, J.J.M.E.; Marijnen, C.A.M.; de Wilt, J.H.W. Chemoradiation Therapy for Rectal Cancer in the Distal Rectum Followed by Organ-Sparing Transanal Endoscopic Microsurgery (CARTS Study). Br. J. Surg. 2015, 102, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; Bradley, E.H.; Towle, V.R.; Allore, H. Understanding the Treatment Preferences of Seriously Ill Patients. N. Engl. J. Med. 2002, 346, 1061–1066. [Google Scholar] [CrossRef]
- Saur, N.M.; Montroni, I.; Shahrokni, A.; Kunitake, H.; Potenti, F.M.; Goodacre, R.C.; Davis, B.R.; Carli, F. Care of the Geriatric Colorectal Surgical Patient and Framework for Creating a Geriatric Program: A Compendium from the 2019 American Society of Colon and Rectal Surgeons Annual Meeting. Dis. Colon Rectum 2020, 63, 1489–1495. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S. Low Anterior Resection Syndrome Score: Development and Validation of a Symptom-Based Scoring System for Bowel Dysfunction after Low Anterior Resection for Rectal Cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Vaizey, C.J.; Carapeti, E.; Cahill, J.A.; Kamm, M.A. Prospective Comparison of Faecal Incontinence Grading Systems. Gut 1999, 44, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Jorge, J.M.N.; Wexner, S.D. Etiology and Management of Fecal Incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef]
- van Andel, G.; Bottomley, A.; Fosså, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An International Field Study of the EORTC QLQ-PR25: A Questionnaire for Assessing the Health-Related Quality of Life of Patients with Prostate Cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef]
- Barry, M.J.; Fowler, F.J.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Rosen, R.C.; Cappelleri, J.C.; Gendrano, N. The International Index of Erectile Function (IIEF): A State-of-the-Science Review. Int. J. Impot. Res. 2002, 14, 226–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suskind, A.M.; Finlayson, E. Focus on Surgical Outcomes That Matter to Older Patients. JAMA Surg. 2016, 151, e161701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, L.Z.; Stuck, A.E.; Siu, A.L.; Wieland, D. Impacts of Geriatric Evaluation and Management Programs on Defined Outcomes: Overview of the Evidence. J. Am. Geriatr. Soc. 1991, 39, 8S–16S. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for Clinical Practice and Public Health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Stolz, E.; Oude Voshaar, R.C.; Deeg, D.J.H.; Huisman, M.; Jeuring, H.W. Trends in Frailty and Its Association With Mortality: Results From the Longitudinal Aging Study Amsterdam, 1995-2016. Am. J. Epidemiol. 2021, 190, 1316–1323. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and Cancer: Implications for Oncology Surgery, Medical Oncology, and Radiation Oncology. CA. Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef] [Green Version]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of Multimorbidity and Implications for Health Care, Research, and Medical Education: A Cross-Sectional Study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Lemmens, V.E.; Janssen-Heijnen, M.L.; Houterman, S.; Verheij, K.D.; Martijn, H.; van de Poll-Franse, L.; Coebergh, J.W. Which Comorbid Conditions Predict Complications after Surgery for Colorectal Cancer? World J. Surg. 2007, 31, 192–199. [Google Scholar] [CrossRef]
- Saur, N.M.; Montroni, I. The Opposite of Undertreating Is Frailty Screening. Eur. J. Surg. Oncol. 2019, 45, 1127–1128. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Li, D.; Yuan, Y.; Lau, Y.M.; Hurria, A. Functional versus Chronological Age: Geriatric Assessments to Guide Decision Making in Older Patients with Cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef]
- Hamaker, M.E.; te Molder, M.; Thielen, N.; van Munster, B.C.; Schiphorst, A.H.; van Huis, L.H. The Effect of a Geriatric Evaluation on Treatment Decisions and Outcome for Older Cancer Patients—A Systematic Review. J. Geriatr. Oncol. 2018, 9, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R.H.; Conroy, S.P.; Kircher, T.; Somme, D.; Saltvedt, I.; et al. Comprehensive Geriatric Assessment for Older Adults Admitted to Hospital. Cochrane Database Syst. Rev. 2017, 9, CD006211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [Green Version]
- Oken, M.M.; Creech, R.H.; Davis, T.E. Toxicology and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. Cancer Clin. Trials 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Karnofsky, D.; Burchenal, J. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In Evaluation of Chemotherapeutic agents; CM, M., Ed.; Columbia University Press: New York, NY, USA, 1949; pp. 191–205. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Wang, Y.C. Four-Meter Gait Speed: Normative Values and Reliability Determined for Adults Participating in the NIH Toolbox Study. Arch. Phys. Med. Rehabil. 2019, 100, 509–513. [Google Scholar] [CrossRef]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.J.; de Craen, A.J.M. Handgrip Strength as a Predictor of Functional, Psychological and Social Health. A Prospective Population-Based Study among the Oldest Old. Age Ageing 2010, 39, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Tuijl, J.P.; Scholte, E.M.; De Craen, A.J.M.; Van Der Mast, R.C. Screening for Cognitive Impairment in Older General Hospital Patients: Comparison of the Six-Item Cognitive Impairment Test with the Mini-Mental State Examination. Int. J. Geriatr. Psychiatry 2012, 27, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a Screen for Dementia: Validation in a Population-Based Sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, J.; Schmand, B.; Tulner, L.; Walstra, G.; Jonker, C. Visual Association Test to Detect Early Dementia of the Alzheimer Type. J. Neurol. Neurosurg. Psychiatry 2002, 73, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royall, D.R.; Cordes, J.A.; Polk, M. CLOX: An Executive Clock Drawing Task. J. Neurol. Neurosurg. Psychiatry 1998, 64, 588–594. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roquéi Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Detection of Alzheimer’s Disease and Other Dementias in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2015, 2015, CD010783. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The Patient Health Questionnaire-2: Validity of a Two-Item Depression Screener. Med. Care 2003, 41, 1284–1292. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. 9/Geriatric Depression Scale (Gds) Recent Evidence and Development of a Shorter Version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the Elderly at Risk for Malnutrition the Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- van der Vlies, E.; Smits, A.B.; Los, M.; van Hengel, M.; Bos, W.J.W.; Dijksman, L.M.; van Dongen, E.P.A.; Noordzij, P.G. Implementation of a Preoperative Multidisciplinary Team Approach for Frail Colorectal Cancer Patients: Influence on Patient Selection, Prehabilitation and Outcome. J. Geriatr. Oncol. 2020, 11, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Sylla, P.; Baiocchi, G.; Adamina, M.; Agnoletti, V.; Agresta, F.; Ansaloni, L.; Arezzo, A.; Avenia, N.; Biffl, W.; et al. Multidisciplinary Management of Elderly Patients with Rectal Cancer: Recommendations from the SICG (Italian Society of Geriatric Surgery), SIFIPAC (Italian Society of Surgical Pathophysiology), SICE (Italian Society of Endoscopic Surgery and New Technolog. World J. Emerg. Surg. 2021, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Bunn, F.; Goodman, C.; Russell, B.; Wilson, P.; Manthorpe, J.; Rait, G.; Hodkinson, I.; Durand, M.A. Supporting Shared Decision Making for Older People with Multiple Health and Social Care Needs: A Realist Synthesis. BMC Geriatr. 2018, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Terret, C.; Zulian, G.B.; Naiem, A.; Albrand, G. Multidisciplinary Approach to the Geriatric Oncology Patient. J. Clin. Oncol. 2007, 25, 1876–1881. [Google Scholar] [CrossRef]
- Monfardini, S.; Giordano, G.; Sandri, R.; Gnocchi, P.L.; Galetti, G. Bringing Geriatrics into Oncology or Also Oncology into Geriatrics? Ann. Oncol. 2012, 23, 801. [Google Scholar] [CrossRef]
- Van Rooijen, S.J.; Molenaar, C.J.L.; Fokkenrood, H.J.P.; Roumen, R.M.H.; Slooter, G.D. Prehabilitation versus No Prehabilitation to Improve Functional Capacity, Reduce Postoperative Complications and Improve Quality of Life in Colorectal Cancer Surgery. Cochrane Database Syst. Rev. 2019, 2019, CD013259. [Google Scholar] [CrossRef]
- Cameron, I.D.; Fairhall, N.; Langron, C.; Lockwood, K.; Monaghan, N.; Aggar, C.; Sherrington, C.; Lord, S.R.; Kurrle, S.E. A Multifactorial Interdisciplinary Intervention Reduces Frailty in Older People: Randomized Trial. BMC Med. 2013, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Minnella, E.M.; Awasthi, R.; Gillis, C.; Fiore, J.F.; Liberman, A.S.; Charlebois, P.; Stein, B.; Bousquet-Dion, G.; Feldman, L.S.; Carli, F. Patients with Poor Baseline Walking Capacity Are Most Likely to Improve Their Functional Status with Multimodal Prehabilitation. Surgery 2016, 160, 1070–1079. [Google Scholar] [CrossRef]
- Carli, F.; Bessissow, A.; Awasthi, R.; Liberman, S. Prehabilitation: Finally Utilizing Frailty Screening Data. Eur. J. Surg. Oncol. 2020, 46, 321–325. [Google Scholar] [CrossRef]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Effect of Prehabilitation on Objectively Measured Physical Fitness after Neoadjuvant Treatment in Preoperative Rectal Cancer Patients: A Blinded Interventional Pilot Study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Moug, S.J.; Mutrie, N.; Barry, S.J.E.; Mackay, G.; Steele, R.J.C.; Boachie, C.; Buchan, C.; Anderson, A.S. Prehabilitation Is Feasible in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy and May Minimize Physical Deterioration: Results from the REx Trial. Color. Dis. 2019, 21, 548–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossa, F.; Chesney, T.; Acuna, S.A.; Baxter, N.N. A Watch-and-Wait Approach for Locally Advanced Rectal Cancer after a Clinical Complete Response Following Neoadjuvant Chemoradiation: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation Dose-Response Model for Locally Advanced Rectal Cancer after Preoperative Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-Term Outcomes of Clinical Complete Responders after Neoadjuvant Treatment for Rectal Cancer in the International Watch & Wait Database (IWWD): An International Multicentre Registry Study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Haak, H.E.; Maas, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Beets, G.L.; Melenhorst, J.; van der Sande, M.E.; van Westreenen, H.L.; Talsma, A.K.; Breukink, S.O.; et al. Is Watch and Wait a Safe and Effective Way to Treat Rectal Cancer in Older Patients? Eur. J. Surg. Oncol. 2020, 46, 358–362. [Google Scholar] [CrossRef]
- Myint, A.S.; Gérard, J.P. Role of Radiotherapy in the Treatment of Rectal Cancer in Older Patients. Eur. J. Surg. Oncol. 2020, 46, 349–357. [Google Scholar] [CrossRef]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-Analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef]
- Perez, R.O.; Habr-Gama, A.; São Julião, G.P.; Proscurshim, I.; Fernandez, L.M.; de Azevedo, R.U.; Vailati, B.B.; Fernandes, F.A.; Gama-Rodrigues, J. Transanal Endoscopic Microsurgery (TEM) Following Neoadjuvant Chemoradiation for Rectal Cancer: Outcomes of Salvage Resection for Local Recurrence. Ann. Surg. Oncol. 2016, 23, 1143–1148. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, R.; García-Aguilar, J. Non Surgical Treatment in Patients With Advanced Rectal Cancer. Cir. Esp. 2021, 99, 401–403. [Google Scholar] [CrossRef]
- Hardiman, K.M.; Antunez, A.G.; Kanters, A.; Schuman, A.D.; Regenbogen, S.E. Clinical and Pathological Outcomes of Induction Chemotherapy before Neoadjuvant Radiotherapy in Locally-Advanced Rectal Cancer. J. Surg. Oncol. 2019, 120, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Masi, G.; Vivaldi, C.; Fornaro, L.; Lonardi, S.; Buccianti, P.; Sainato, A.; Marcucci, L.; Martignetti, A.; Luca Urso, E.D.; Castagna, M.; et al. Total Neoadjuvant Approach with FOLFOXIRI plus Bevacizumab Followed by Chemoradiotherapy plus Bevacizumab in Locally Advanced Rectal Cancer: The TRUST Trial. Eur. J. Cancer 2019, 110, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Chow, O.S.; Smith, D.D.; Marcet, J.E.; Cataldo, P.A.; Varma, M.G.; Kumar, A.S.; Oommen, S.; Coutsoftides, T.; Hunt, S.R.; et al. Effect of Adding MFOLFOX6 after Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Multicentre, Phase 2 Trial. Lancet. Oncol. 2015, 16, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Golo, D.; But-Hadzic, J.; Anderluh, F.; Brecelj, E.; Edhemovic, I.; Jeromen, A.; Omejc, M.; Oblak, I.; Secerov-Ermenc, A.; Velenik, V. Induction Chemotherapy, Chemoradiotherapy and Consolidation Chemotherapy in Preoperative Treatment of Rectal Cancer—Long-Term Results of Phase II OIGIT-01 Trial. Radiol. Oncol. 2018, 52, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Maréchal, R.; Vos, B.; Polus, M.; Delaunoit, T.; Peeters, M.; Demetter, P.; Hendlisz, A.; Demols, A.; Franchimont, D.; Verset, G.; et al. Short Course Chemotherapy Followed by Concomitant Chemoradiotherapy and Surgery in Locally Advanced Rectal Cancer: A Randomized Multicentric Phase II Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1525–1530. [Google Scholar] [CrossRef]
- Calvo, F.A.; Serrano, F.J.; Diaz-González, J.A.; Gomez-Espi, M.; Lozano, E.; Garcia, R.; de la Mata, D.; Arranz, J.A.; García-Alfonso, P.; Pérez-Manga, G.; et al. Improved Incidence of PT0 Downstaged Surgical Specimens in Locally Advanced Rectal Cancer (LARC) Treated with Induction Oxaliplatin plus 5-Fluorouracil and Preoperative Chemoradiation. Ann. Oncol. 2006, 17, 1103–1110. [Google Scholar] [CrossRef]
- Markovina, S.; Youssef, F.; Roy, A.; Aggarwal, S.; Khwaja, S.; DeWees, T.; Tan, B.; Hunt, S.; Myerson, R.J.; Chang, D.T.; et al. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 417–426. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Tabah-Fisch, I.; Bleiberg, H.; De Gramont, A.; Tournigand, C.; Andre, T.; Rothenberg, M.L.; Green, E.; Sargent, D.J. Pooled Analysis of Safety and Efficacy of Oxaliplatin plus Fluorouracil/Leucovorin Administered Bimonthly in Elderly Patients with Colorectal Cancer. J. Clin. Oncol. 2006, 24, 4085–4091. [Google Scholar] [CrossRef]
- Dattani, M.; Heald, R.J.; Goussous, G.; Broadhurst, J.; São Julião, G.P.; Habr-Gama, A.; Oliva Perez, R.; Moran, B.J. Oncological and Survival Outcomes in Watch and Wait Patients with a Clinical Complete Response after Neoadjuvant Chemoradiotherapy for Rectal Cancer a Systematic Review and Pooled Analysis. Ann. Surg. 2018, 268, 955–967. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-Term Outcome in Patients with a Pathological Complete Response after Chemoradiation for Rectal Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Hoendervangers, S.; Couwenberg, A.M.; Intven, M.P.W.; van Grevenstein, W.M.U.; Verkooijen, H.M. Comparison of Pathological Complete Response Rates after Neoadjuvant Short-Course Radiotherapy or Chemoradiation Followed by Delayed Surgery in Locally Advanced Rectal Cancer. Eur. J. Surg. Oncol. 2018, 44, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Verhoeven, R.H.A.; Elferink, M.A.G.; Poortmans, P.M.P.; Nagtegaal, I.D.; de Wilt, J.H.W. Tumor Response after Long Interval Comparing 5x5Gy Radiation Therapy with Chemoradiation Therapy in Rectal Cancer Patients. Eur. J. Surg. Oncol. 2018, 44, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, D.; Lörinc, E.; Holm, T.; Iversen, H.; Cedermark, B.; Glimelius, B.; Martling, A. Tumour Regression in the Randomized Stockholm III Trial of Radiotherapy Regimens for Rectal Cancer. Br. J. Surg. 2015, 102, 972–978. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, A.J.M.; Al-Najami, I.; Abbott, N.L.; Appelt, A.; Baatrup, G.; Bach, S.; Bhangu, A.; Garm Spindler, K.L.; Gray, R.; Handley, K.; et al. Can We Save the Rectum by Watchful Waiting or Trans Anal Microsurgery Following (Chemo) Radiotherapy versus Total Mesorectal Excision for Early REctal Cancer (STAR-TREC Study)?: Protocol for a Multicentre, Randomised Feasibility Study. BMJ Open 2017, 7, e019474. [Google Scholar] [CrossRef] [Green Version]
- François, E.; Azria, D.; Gourgou-Bourgade, S.; Jarlier, M.; Martel-Laffay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; Seitz, J.F.; Conroy, T.; et al. Results in the Elderly with Locally Advanced Rectal Cancer from the ACCORD12/PRODIGE 2 Phase III Trial: Tolerance and Efficacy. Radiother. Oncol. 2014, 110, 144–149. [Google Scholar] [CrossRef]
- François, E.; Pernot, M.; Ronchin, P.; Nouhaud, E.; Martel Lafay, I.; Pascal, A.; Clavere, P.; Vendrely, V.; Boige, V.; Thamphya, B.; et al. NACRE: A Randomized Study Comparing Short Course Radiotherapy with Radiochemotherapy for Locally Advanced Rectal Cancers in the Elderly—Preliminary Results. J. Clin. Oncol. 2021, 39, 4. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-Term Results of a Randomized Trial Comparing Preoperative Short-Course Radiotherapy with Preoperative Conventionally Fractionated Chemoradiation for Rectal Cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- Chen, C.; Sun, P.; Rong, J.; Weng, H.W.; Dai, Q.S.; Ye, S. Short Course Radiation in the Treatment of Localized Rectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 10953. [Google Scholar] [CrossRef] [Green Version]
- Francois, Y.; Nemoz, C.J.; Baulieux, J.; Vignal, J.; Grandjean, J.P.; Partensky, C.; Souquet, J.C.; Adeleine, P.; Gerard, J.P. Influence of the Interval between Preoperative Radiation Therapy and Surgery on Downstaging and on the Rate of Sphincter-Sparing Surgery for Rectal Cancer: The Lyon R90-01 Randomized Trial. J. Clin. Oncol. 1999, 17, 2396. [Google Scholar] [CrossRef]
- Gerard, J.-P.; Chapet, O.; Nemoz, C.; Hartweig, J.; Romestaing, P.; Coquard, R.; Barbet, N.; Maingon, P.; Mahe, M.; Baulieux, J.; et al. Improved Sphincter Preservation in Low Rectal Cancer With High-Dose Preoperative Radiotherapy: The Lyon R96-02 Randomized Trial. J. Clin. Oncol. 2004, 22, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Sun Myint, A.; Smith, F.M.; Gollins, S.W.; Wong, H.; Rao, C.; Whitmarsh, K.; Sripadam, R.; Rooney, P.; Hershman, M.J.; Fekete, Z.; et al. Dose Escalation Using Contact X-ray Brachytherapy (Papillon) for Rectal Cancer: Does It Improve the Chance of Organ Preservation? Br. J. Radiol. 2017, 90, 20170175. [Google Scholar] [CrossRef] [PubMed]
- Sun Myint, A.; Stewart, A.; Mills, J.; Sripadam, R.; Whitmarsh, K.; Roy, R.; Franklin, A.; Dhadda, A. Treatment: The Role of Contact X-ray Brachytherapy (Papillon) in the Management of Early Rectal Cancer. Color. Dis. 2019, 21 (Suppl. S1), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Van Limbergen, E.J.; Gerard, J.P.; Appelt, A.L.; Verhaegen, F.; Berbee, M.; Vuong, T.; Brooker, C.; Rockall, T.; Sun Myint, A. GEC ESTRO ACROP consensus recommendations for contact brachytherapy for rectal cancer. Clin. Transl. Radiat. Oncol. 2022, 33, 15–22. [Google Scholar] [CrossRef]
- Benezery, K.; Montagne, L.; Evesque, L.; Schiappa, R.; Hannoun-Levi, J.-M.; Francois, E.; Thamphya, B.; Gerard, J.-P. Clinical Response Assessment after Contact X-ray Brachytherapy and Chemoradiotherapy for Organ Preservation in Rectal Cancer T2-T3 M0: The Time/Dose Factor Influence. Clin. Transl. Radiat. Oncol. 2020, 24, 92–98. [Google Scholar] [CrossRef]
- Grotenhuis, B.A.; van Triest, B.; Beets, G.L.; Marijnen, C.A.M.; Peters, F.P.; Burger, J.W.A.; Rutten, H.J.T.; Cnossen, J.S. Organ Preservation in Patients with a Good Clinical Response after Neoadjuvant (Chemo)Radiation for Rectal Cancer: Optimization of Treatment Strategies and Defining the Role of Additional Contact X-ray Brachytherapy versus Extending the Waiting Interval A. Available online: https://opaxx.nl/.cm4all/uproc.php/0/C1.OnderzoeksprotocolOPAXXversie2.0dd19-05-2021M20PAX.pdf?cdp=a&_=17b7322f520 (accessed on 9 April 2022).
- Verrijssen, A.S.; Opbroek, T.; Bellezzo, M.; Fonseca, G.P.; Verhaegen, F.; Gerard, J.P.; Sun Myint, A.; Van Limbergen, E.J.; Berbee, M. A Systematic Review Comparing Radiation Toxicity after Various Endorectal Techniques. Brachytherapy 2019, 18, 71–86.e5. [Google Scholar] [CrossRef]
- Dhadda, A.S.; Martin, A.; Killeen, S.; Hunter, I.A. Organ Preservation Using Contact Radiotherapy for Early Rectal Cancer: Outcomes of Patients Treated at a Single Centre in the UK. Clin. Oncol. 2017, 29, 198–204. [Google Scholar] [CrossRef]
- Vuong, T.; Richard, C.; Niazi, T.; Liberman, S.; Letellier, F.; Morin, N.; Hu, K.; Anderson, D.; Devic, S. High Dose Rate Endorectal Brachytherapy for Patients with Curable Rectal Cancer. Semin. Colon Rectal Surg. 2010, 21, 115–119. [Google Scholar] [CrossRef]
- Vuong, T.; Belliveau, P.J.; Michel, R.P.; Moftah, B.A.; Parent, J.; Trudel, J.L.; Reinhold, C.; Souhami, L.; Påhlman, L.; Vuong, T.; et al. Conformal Preoperative Endorectal Brachytherapy Treatment for Locally Advanced Rectal Cancer: Early Results of a Phase I/II Study. Dis. Colon Rectum 2002, 45, 1486–1493. [Google Scholar] [CrossRef]
- Garant, A.; Magnan, S.; Devic, S.; Martin, A.G.; Boutros, M.; Vasilevsky, C.A.; Ferland, S.; Bujold, A.; DesGroseilliers, S.; Sebajang, H.; et al. Image Guided Adaptive Endorectal Brachytherapy in the Nonoperative Management of Patients With Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1005–1011. [Google Scholar] [CrossRef]
- Jakobsen, A.; Ploen, J.; Vuong, T.; Appelt, A.; Lindebjerg, J.; Rafaelsen, S.R. Dose-Effect Relationship in Chemoradiotherapy for Locally Advanced Rectal Cancer: A Randomized Trial Comparing Two Radiation Doses. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.R.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-Dose Chemoradiotherapy and Watchful Waiting for Distal Rectal Cancer: A Prospective Observational Study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Federation of Medical Specialists. Dutch National Guidelines Colorectal Cancer. 2020. Available online: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_-_crc.html (accessed on 1 April 2022).
- Garcia-Aguilar, J.; Mellgren, A.; Sirivongs, P.; Buie, D.; Madoff, R.D.; Rothenberger, D.A. Local Excision of Rectal Cancer without Adjuvant Therapy: A Word of Caution. Ann. Surg. 2000, 231, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Borschitz, T.; Wachtlin, D.; Möhler, M.; Schmidberger, H.; Junginger, T. Neoadjuvant Chemoradiation and Local Excision for T2-3 Rectal Cancer. Ann. Surg. Oncol. 2008, 15, 712–720. [Google Scholar] [CrossRef]
- Pucciarelli, S.; De Paoli, A.; Guerrieri, M.; La Torre, G.; Maretto, I.; De Marchi, F.; Mantello, G.; Gambacorta, M.A.; Canzonieri, V.; Nitti, D.; et al. Local Excision after Preoperative Chemoradiotherapy for Rectal Cancer: Results of a Multicenter Phase II Clinical Trial. Dis. Colon Rectum 2013, 56, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Lezoche, E.; Baldarelli, M.; Lezoche, G.; Paganini, A.M.; Gesuita, R.; Guerrieri, M. Randomized Clinical Trial of Endoluminal Locoregional Resection versus Laparoscopic Total Mesorectal Excision for T2 Rectal Cancer after Neoadjuvant Therapy. Br. J. Surg. 2012, 99, 1211–1218. [Google Scholar] [CrossRef]
- Stijns, R.C.H.; De Graaf, E.J.R.; Punt, C.J.A.; Nagtegaal, I.D.; Nuyttens, J.J.M.E.; Van Meerten, E.; Tanis, P.J.; De Hingh, I.H.J.T.; Van Der Schelling, G.P.; Acherman, Y.; et al. Long-Term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019, 154, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Shi, Q.; Thomas, C.R.; Chan, E.; Cataldo, P.; Marcet, J.; Medich, D.; Pigazzi, A.; Oommen, S.; Posner, M.C. A Phase II Trial of Neoadjuvant Chemoradiation and Local Excision for T2N0 Rectal Cancer: Preliminary Results of the ACOSOG Z6041 Trial. Ann. Surg. Oncol. 2012, 19, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Borstlap, W.A.A.; Coeymans, T.J.; Tanis, P.J.; Marijnen, C.A.M.; Cunningham, C.; Bemelman, W.A.; Tuynman, J.B. Meta-Analysis of Oncological Outcomes after Local Excision of PT1-2 Rectal Cancer Requiring Adjuvant (Chemo)Radiotherapy or Completion Surgery. Br. J. Surg. 2016, 103, 1105–1116. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.J.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.A.; Sosef, M.; Hulsewé, K.W.E.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef] [Green Version]
- Dresen, R.C.; Beets, G.L.; Rutten, H.J.T.; Engelen, S.M.E.; Lahaye, M.J.; Vliegen, R.F.A.; De Bruïne, A.P.; Kessels, A.G.H.; Lammering, G.; Beets-Tan, R.G.H. Locally Advanced Rectal Cancer: MR Imaging for Restaging after Neoadjuvant Radiation Therapy with Concomitant Chemotherapy Part I. Are We Able to Predict Tumor Confined to the Rectal Wall? Radiology 2009, 252, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Sgroi, G.; Sarti, E.; Barni, S. Increasing the Interval between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-Analysis of Published Studies. Ann. Surg. 2016, 263, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Hupkens, B.J.P.; Maas, M.; Martens, M.H.; van der Sande, M.E.; Lambregts, D.M.J.; Breukink, S.O.; Melenhorst, J.; Houwers, J.B.; Hoff, C.; Sosef, M.N.; et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann. Surg. Oncol. 2018, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; São Julião, G.P.; Fernandez, L.M.; Vailati, B.B.; Andrade, A.; Araújo, S.E.A.; Gama-Rodrigues, J.; Perez, R.O. Achieving a Complete Clinical Response After Neoadjuvant Chemoradiation That Does Not Require Surgical Resection: It May Take Longer Than You Think! Dis. Colon Rectum 2019, 62, 802–808. [Google Scholar] [CrossRef]
- Huibertse, L.J.; van Eenbergen, M.; de Rooij, B.H.; Bastiaens, M.T.; Fossion, L.M.C.L.; de la Fuente, R.B.; Kil, P.J.M.; Koldewijn, E.L.; Meier, A.H.P.; Mommers, R.J.M.; et al. Cancer Survivors’ Preference for Follow-up Care Providers: A Cross-Sectional Study from the Population-Based PROFILES-Registry. Acta Oncol. 2017, 56, 278–287. [Google Scholar] [CrossRef] [Green Version]
- van Heinsbergen, M.; Maas, H.; Bessems, S.; Vogelaar, J.; Nijhuis, P.; Keijzer-Bors, L.; van Liempd, A.; Janssen-Heijnen, M. Follow-up after Surgical Treatment in Older Patients with Colorectal Cancer: The Evaluation of Emerging Health Problems and Quality of Life after Implementation of a Standardized Shared-Care Model. J. Geriatr. Oncol. 2019, 10, 126–131. [Google Scholar] [CrossRef]
- Cohen, H.J. A Model for the Shared Care of Elderly Patients with Cancer. J. Am. Geriatr. Soc. 2009, 57, S300–S302. [Google Scholar] [CrossRef]
- Owusu, C.; Studenski, S.A. Shared Care in Geriatric Oncology: Primary Care Providers’ and Medical/Oncologist’s Perspectives. J. Am. Geriatr. Soc. 2009, 57, S239–S242. [Google Scholar] [CrossRef]
- Ekdahl, A.W.; Alwin, J.; Eckerblad, J.; Husberg, M.; Jaarsma, T.; Mazya, A.L.; Milberg, A.; Krevers, B.; Unosson, M.; Wiklund, R.; et al. Long-Term Evaluation of the Ambulatory Geriatric Assessment: A Frailty Intervention Trial (AGe-FIT): Clinical Outcomes and Total Costs After 36 Months. J. Am. Med. Dir. Assoc. 2016, 17, 263–268. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and Moderators of Exercise on Quality of Life and Physical Function in Patients with Cancer: An Individual Patient Data Meta-Analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Spence, R.R.; Heesch, K.C.; Brown, W.J. Colorectal Cancer Survivors’ Exercise Experiences and Preferences: Qualitative Findings from an Exercise Rehabilitation Programme Immediately after Chemotherapy. Eur. J. Cancer Care 2011, 20, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balducci, L.; Fossa, S.D. Rehabilitation of Older Cancer Patients. Acta Oncol. 2013, 52, 233–238. [Google Scholar] [CrossRef] [PubMed]
| Geriatric Domain | Examples of Scoring Tools |
|---|---|
| Age | - |
| Functional status | Eastern Cooperative Oncology Group (ECOG) Performance status [65] Karnofsky Performance status [66] |
| Level of independence | Katz scale of Activities of Daily Living (ADL) [67] Lawton and Brody scale of Instrumental Activities of Daily Living (IADL) [68] |
| Comorbidity | Charlson Comorbidity Index (CCI) [69] |
| Medication use | Number and type of medication use |
| Physical function and mobility | 4-Meter Gait Speed [70] Handgrip strength [71] Timed Up and Go (TUG) [72] |
| Cognitive function | Six-item Cognitive Impairment Test (6-CIT) [73] Mini-Cog [74] Visual Association Test (VAT) [75] Clock Drawing Test (CDT) [76] Mini Mental State Examination (MMSE) [77] |
| Emotional function | Patient Health Questionnaire (PHQ-2) [78] Geriatric Depression Scale-15 (GDS-15) [79] Hospital Anxiety and Depression Scale (HADS) [80] |
| Nutritional status | Mini-Nutritional Assessment Short Form (MNA-SF) [81] Body Mass Index (BMI) |
| Social status | Living arrangements (independent, institutionalised, hospitalised) Availability of an informal and formal caregivers (number of days with home care) |
| Geriatric risk factors or syndromes | Risk to fall/fall history Risk of delirium Vision or hearing difficulties Pain Urinary and/or faecal incontinence |
| Treatment goals and preferences | e.g., Minimising/improving local complaints related to the tumour Maintaining/improving quality of life Maintaining/improving functional status Prolonging survival |
| Variable Group | Variables | |
|---|---|---|
| Patient characteristics | Age Sex Weight, length, BMI Living situation | Medical history ECOG Performance status Comorbidities |
| Primary diagnosis | Clinical complaints Tumour location Tumour size | TNM stage Histology |
| Geriatric assessment | Geriatric scoring tools (e.g., Katz-ADL, Lawton and Brody-IADL, MNA-SF, 6-CIT, Mini-Cog, 4-Meter Gait Speed) Clinical Frailty Score Treatment goals and preferences | |
| Multidisciplinary evaluation | Considerations of the multidisciplinary team | Treatment advice |
| Treatment | Treatment modalities Treatment schedules | Compliance rates Adverse effects/complications |
| Response evaluation | Tumour response Tumour characteristics | Multidisciplinary advice on response evaluation |
| Follow-up | Clinical complaints Local control rates Relapsing disease (local/distant) | Survival outcomes Date of death (if applicable) Cause of death (if applicable) |
| Quality of life and functional outcomes 2 | EORTC 1 QLQ-C30 EORTC QLQ-CR29 EQ-5D-5L | Katz-ADL Lawton and Brody IADL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketelaers, S.H.J.; Jacobs, A.; Verrijssen, A.-S.E.; Cnossen, J.S.; van Hellemond, I.E.G.; Creemers, G.-J.M.; Schreuder, R.-M.; Scholten, H.J.; Tolenaar, J.L.; Bloemen, J.G.; et al. A Multidisciplinary Approach for the Personalised Non-Operative Management of Elderly and Frail Rectal Cancer Patients Unable to Undergo TME Surgery. Cancers 2022, 14, 2368. https://doi.org/10.3390/cancers14102368
Ketelaers SHJ, Jacobs A, Verrijssen A-SE, Cnossen JS, van Hellemond IEG, Creemers G-JM, Schreuder R-M, Scholten HJ, Tolenaar JL, Bloemen JG, et al. A Multidisciplinary Approach for the Personalised Non-Operative Management of Elderly and Frail Rectal Cancer Patients Unable to Undergo TME Surgery. Cancers. 2022; 14(10):2368. https://doi.org/10.3390/cancers14102368
Chicago/Turabian StyleKetelaers, Stijn H. J., Anne Jacobs, An-Sofie E. Verrijssen, Jeltsje S. Cnossen, Irene E. G. van Hellemond, Geert-Jan M. Creemers, Ramon-Michel Schreuder, Harm J. Scholten, Jip L. Tolenaar, Johanne G. Bloemen, and et al. 2022. "A Multidisciplinary Approach for the Personalised Non-Operative Management of Elderly and Frail Rectal Cancer Patients Unable to Undergo TME Surgery" Cancers 14, no. 10: 2368. https://doi.org/10.3390/cancers14102368
APA StyleKetelaers, S. H. J., Jacobs, A., Verrijssen, A.-S. E., Cnossen, J. S., van Hellemond, I. E. G., Creemers, G.-J. M., Schreuder, R.-M., Scholten, H. J., Tolenaar, J. L., Bloemen, J. G., Rutten, H. J. T., & Burger, J. W. A. (2022). A Multidisciplinary Approach for the Personalised Non-Operative Management of Elderly and Frail Rectal Cancer Patients Unable to Undergo TME Surgery. Cancers, 14(10), 2368. https://doi.org/10.3390/cancers14102368






