Oral Microbiota—A New Frontier in the Pathogenesis and Management of Head and Neck Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Study Registration
2.2. Inclusion and Exclusion Criteria
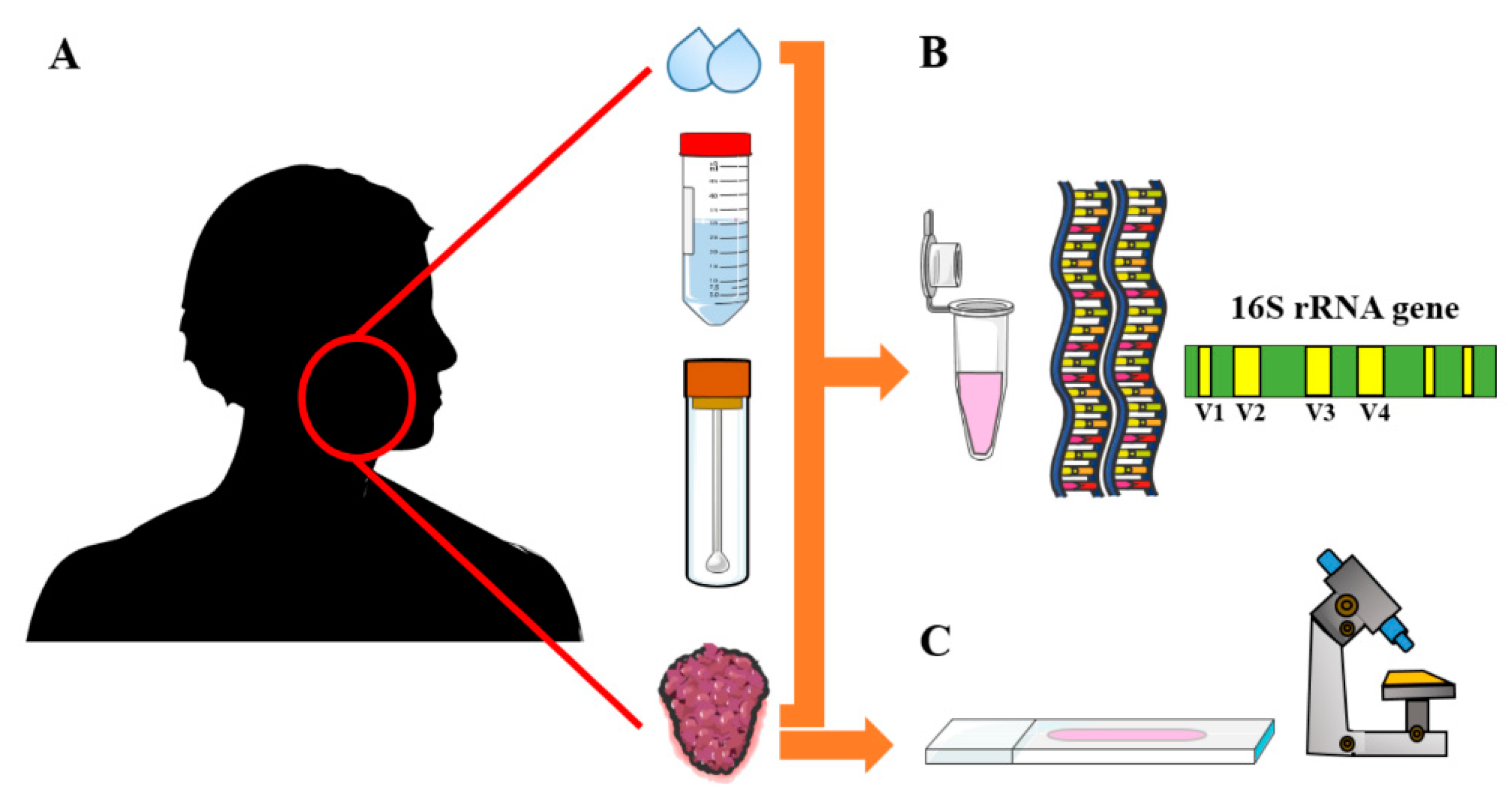
2.3. Search Strategy and Study Screening
2.4. Data Extraction and Study Items
2.5. Assessment of Reporting Quality and the Risk of Bias
3. Results
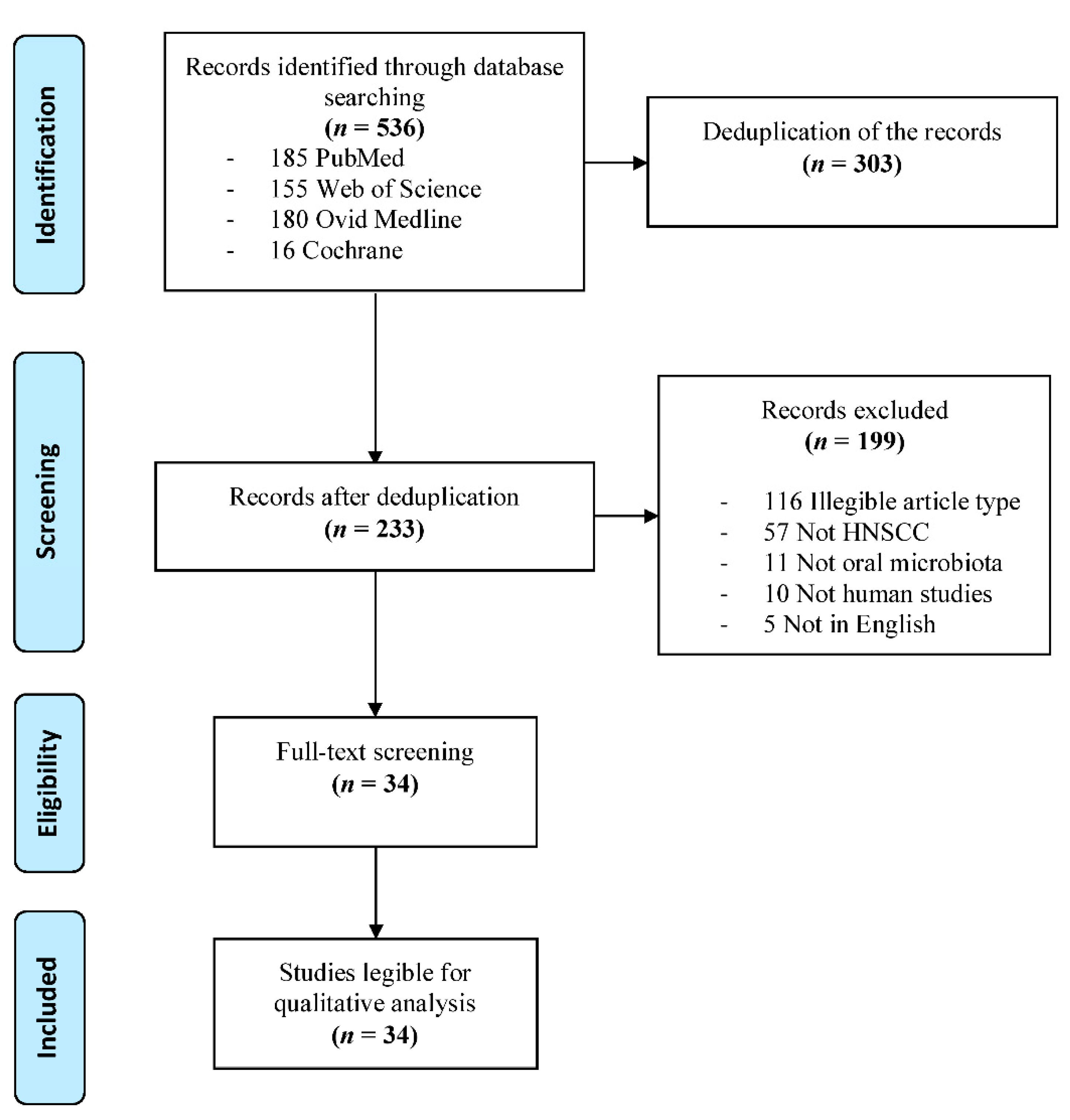
3.1. Study Selection
3.2. Baseline Characteristics of the Studies
3.3. Reporting Quality and the Risk of Bias
3.4. Oral Microbiota and OPMDs
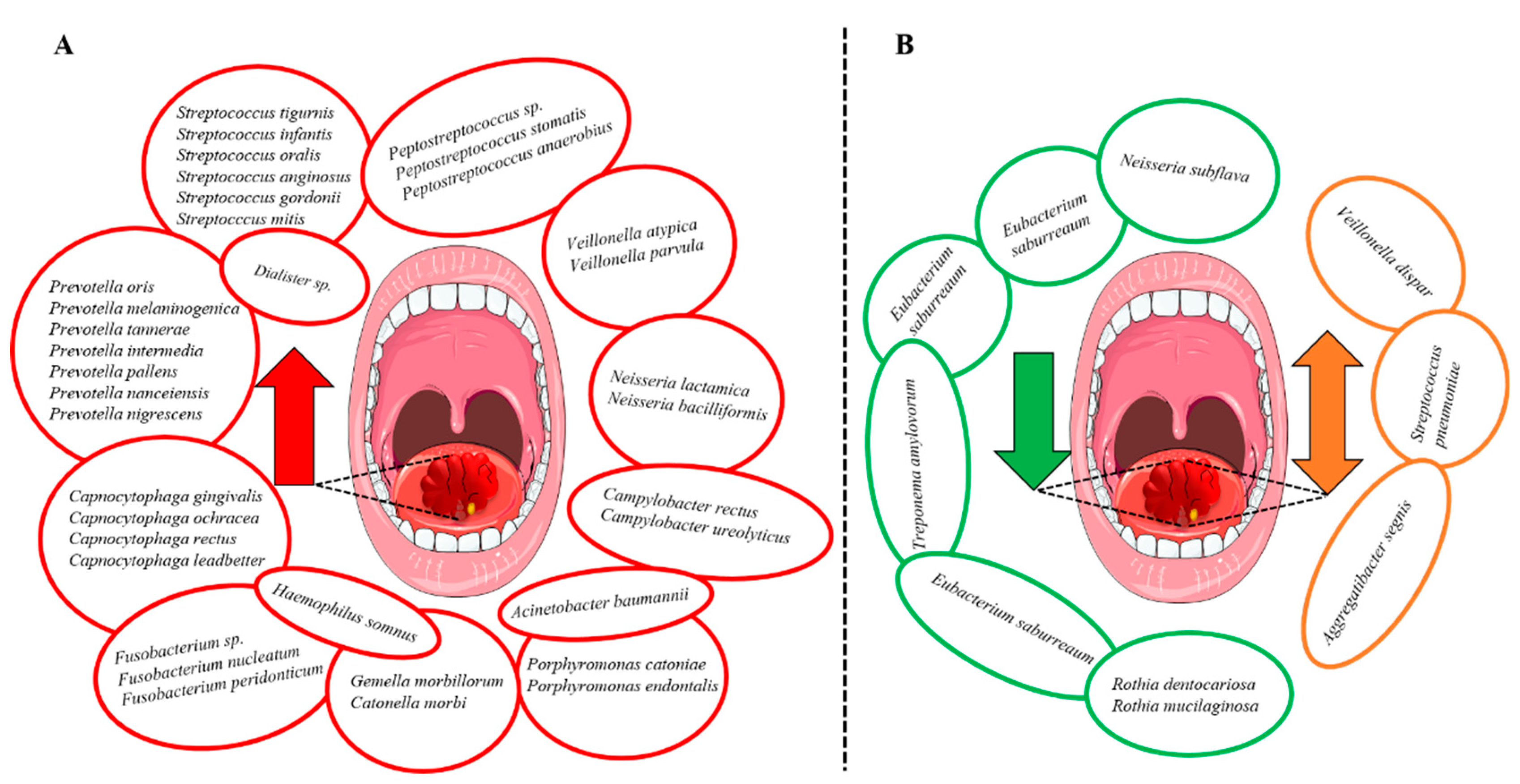
3.5. Oral Microbiota and OSCC
3.6. Oral Microbiota in Other Types of HNSCC
3.7. Oral Dysbiosis and Tumor Progression in HNSCC
3.8. The Prognostic Value of Oral Microbiota in HNSCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Economopoulou, P.; De Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, N.; Williams, M.D. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Q.; Peng, Y.; Wang, D.; Liu, Y. The effect of periodontal bacteria infection on incidence and prognosis of cancer: A systematic review and meta-analysis. Medicine 2020, 99, e19698. [Google Scholar] [CrossRef]
- Teles, F.; Alawi, F.; Castilho, R.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020, 99, 1411–1424. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Zhou, W.; Chu, Y.; Chen, Q.; Zhang, Y.; Li, C.; Chen, H.; Liu, P.; Zhao, Z.; et al. Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression. ACS Nano 2021, 15, 13826–13838. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Hujanen, R.; Almahmoudi, R.; Karinen, S.; Nwaru, B.I.; Salo, T.; Salem, A. Vasculogenic Mimicry: A Promising Prognosticator in Head and Neck Squamous Cell Carcinoma and Esophageal Cancer? A Systematic Review and Meta-Analysis. Cells 2020, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- Mager, D.; Haffajee, A.; Devlin, P.; Norris, C.; Posner, M.R.; Goodson, J. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Pushalkar, S.; Mane, S.P.; Ji, X.; Li, Y.; Evans, C.; Crasta, O.R.; Morse, D.; Meagher, R.; Singh, A.; Saxena, D. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol. Med. Microbiol. 2011, 61, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef] [Green Version]
- Börnigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef]
- Lee, W.-H.; Chen, H.-M.; Yang, S.-F.; Wen-Liang, C.; Peng, C.-Y.; Tzu-Ling, Y.; Tsai, L.-L.; Wu, B.-C.; Hsin, C.-H.; Huang, C.-N.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.-R.; Chang, C.-C.; Lee, W.-T.; Huang, C.-C.; Ou, C.-Y.; Tsai, S.-T.; Chen, K.-C.; Huang, J.-S.; Wong, T.-Y.; Lai, Y.-H.; et al. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogens 2018, 39, 778–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, M.; Al-Hebshi, N.; Perera, I.; Ipe, D.; Ulett, G.; Speicher, D.; Chen, T.; Johnson, N. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-F.; Huang, H.-D.; Fan, W.-L.; Jong, Y.-J.; Chen, M.-K.; Huang, C.-N.; Kuo, Y.-L.; Hung, S.-I.; Su, S.-C. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncol. 2018, 77, 1–8. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int. J. Cancer 2019, 145, 775–784. [Google Scholar] [CrossRef]
- Hashimoto, K.; Shimizu, D.; Hirabayashi, S.; Ueda, S.; Miyabe, S.; Oh-Iwa, I.; Nagao, T.; Shimozato, K.; Nomoto, S. Changes in oral microbial profiles associated with oral squamous cell carcinoma vs leukoplakia. J. Investig. Clin. Dent. 2019, 10, e12445. [Google Scholar] [CrossRef]
- Takahashi, Y.; Park, J.; Hosomi, K.; Yamada, T.; Kobayashi, A.; Yamaguchi, Y.; Iketani, S.; Kunisawa, J.; Mizuguchi, K.; Maeda, N.; et al. Analysis of oral microbiota in Japanese oral cancer patients using 16S rRNA sequencing. J. Oral Biosci. 2019, 61, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Gopinath, D.; Menon, R.K.; Wie, C.C.; Banerjee, M.; Panda, S.; Mandal, D.; Behera, P.K.; Roychoudhury, S.; Kheur, S.; Botelho, M.G.; et al. Salivary bacterial shifts in oral leukoplakia resemble the dysbiotic oral cancer bacteriome. J. Oral Microbiol. 2021, 13, 1857998. [Google Scholar] [CrossRef]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.-H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2020, 82, 1030–1046. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Yuan, R.; Yu, X.; Chen, Z.; Yang, F.; Sun, G.; Dong, Q. Signatures of Mucosal Microbiome in Oral Squamous Cell Carcinoma Identified Using a Random Forest Model. Cancer Manag. Res. 2020, 12, 5353–5363. [Google Scholar] [CrossRef]
- Granato, D.C.; Neves, L.X.; Trino, L.D.; Carnielli, C.M.; Lopes, A.F.; Yokoo, S.; Pauletti, B.A.; Domingues, R.R.; Sá, J.O.; Persinoti, G.; et al. Meta-omics analysis indicates the saliva microbiome and its proteins associated with the prognosis of oral cancer patients. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2021, 1869, 140659. [Google Scholar] [CrossRef]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-C.; Chang, L.-C.; Huang, H.-D.; Peng, C.-Y.; Chuang, C.-Y.; Chen, Y.-T.; Lu, M.-Y.; Chiu, Y.-W.; Chen, P.-Y.; Yang, S.-F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e106297. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.F.; Karuthan, C.; Cheah, Y.K.; Ngeow, W.C.; Rosnah, Z.; Yap, S.F.; Ong, H.K.A. The oral microbiome community variations associated with normal, potentially malignant disorders and malignant lesions of the oral cavity. Malays. J. Pathol. 2017, 39, 1–15. [Google Scholar]
- Robayo, D.A.G.; Erira, H.A.T.; Jaimes, F.O.G.; Torres, A.M.; Galindo, A.I.C. Oropharyngeal Squamous Cell Carcinoma: Human Papilloma Virus Coinfection with Streptococcus anginosus. Braz. Dent. J. 2019, 30, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, J.N.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debelius, J.W.; Huang, T.; Cai, Y.; Ploner, A.; Barrett, D.; Zhou, X.; Xiao, X.; Li, Y.; Liao, J.; Zheng, Y.; et al. Subspecies Niche Specialization in the Oral Microbiome Is Associated with Nasopharyngeal Carcinoma Risk. mSystems 2020, 5, e00065-20. [Google Scholar] [CrossRef]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Radcliff, F.; Taylor, M.W.; Douglas, R.G. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin. Exp. Dent. Res. 2018, 4, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Zuo, H.-J.; Fu, M.R.; Zhao, H.-L.; Du, X.-W.; Hu, Z.-Y.; Zhao, X.-Y.; Ji, X.-Q.; Feng, X.-Q.; Zhumajiang, W.; Zhou, T.-H.; et al. Study on the Salivary Microbial Alteration of Men with Head and Neck Cancer and Its Relationship With Symptoms in Southwest China. Front. Cell. Infect. Microbiol. 2020, 10, 514943. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liška, V.; Bruha, J.; Neary, P.; DeZeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumor Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef]
- Geng, F.; Liu, J.; Guo, Y.; Li, C.; Wang, H.; Zhao, H.; Pan, Y. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Front. Cell. Infect. Microbiol. 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Sembler-Møller, M.L.; Grande, M.A.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Microbial profile comparisons of saliva, pooled and site-specific subgingival samples in periodontitis patients. PLoS ONE 2017, 12, e0182992. [Google Scholar] [CrossRef] [Green Version]
- Gomar-Vercher, S.; Simon-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belstrøm, D.; Holmstrup, P.; Bardow, A.; Kokaras, A.; Fiehn, N.-E.; Paster, B.J. Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. J. Oral Microbiol. 2016, 8, 30112. [Google Scholar] [CrossRef]
- Cheng, A.; Schmidt, B.L. Management of the N0 Neck in Oral Squamous Cell Carcinoma. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef]
- Kumar, A.T.; Knops, A.; Swendseid, B.; Martinez-Outschoom, U.; Harshyne, L.; Philp, N.; Rodeck, U.; Luginbuhl, A.; Cognetti, D.; Johnson, J.; et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Russo, L.L.; Cirillo, N.; Muzio, L.L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef]
| Study | Study Origin | Lesion Type(s) | Lesion Site(s) | Tumor Stage/Grade | Number of Lesion Cases | Age (Years) | Gender M/F | Study Period |
|---|---|---|---|---|---|---|---|---|
| [18] | US | OSCC | Oral cavity | - | 45 | 57.6 (±2.34) (M) | 32/13 | - |
| [44] | US | GSCC | Gingiva | - | 10 | - | - | - |
| [19] | US | OSCC | Floor of the mouth | - | 3 | >50 | 3/0 | - |
| [20] | US | OSCC | Tongue, floor of the mouth | T1–T4b, N0–N2b | 10 | 59 (Med) | †. | - |
| [41] | US | OSCC, OPMDs | Buccal mucosa, tongue, gingiva, alveolar ridge, floor of the mouth, retromolar trigone | Study 1: pT2–pT4, N0–N2b; Study 2: CIS, T1–T4b, N0–N2b | Study 1: 5; Study 2: 16; OPMDs: 8 | Study 1: 69.2; Study 2: 63.37; OPMDs: 58.5 (M) | 18/11 | 2011–2012 |
| [21] | US | OSCC, OPSCC | Oral cavity, oropharynx | - | 121 | 58 (Med) | 94/27 | 2011–2013 |
| [22] | Taiwan | OSCC, OPMDs | Tongue, floor of the mouth, lip, buccal mucosa, alveolar ridge, hard palate | I–IV | OSCC: 125; OPMDs: 124 | OSCC: 53 ± 10; OPMDs: 50 ± 11 (M) | 223/26 | 2014–2015 |
| [42] | Malaysia | OSCC, OPMDs | Oral cavity | - | 18 (9 per group) | OSCC: 60; OPMDs: 54 (M) | 6/12 | - |
| [47] | US | HNSCC | Oral cavity, oropharynx, hypopharynx, larynx | I–IV | 121 | 63 ± 11 (M) | 74/47 | 2003–2014 |
| [23] | China | OSCC | Oral cavity | - | 40 | 62 (Med) | 24/16 | - |
| [48] | US | HNSCC | Oral cavity, pharynx, larynx | - | 129 | Group 1: 71; 2: 62.7 (M) | 100/29 | 1992–2010 |
| [24] | Taiwan | OSCC | Oral cavity | - | 138 | 54.7 ± 1.2; 53.4 ± 1.3 (M) | - | 2010–2013 |
| [45] | Australia | OCC, OPC | Oral cavity, oropharynx | I–IV | 52 | 65 (M) | 46/6 | - |
| [25] | Sri Lanka | OSCC | Tongue, buccal mucosa | Well/moderately diff. | 25 | 61.00 ± 9.5 (M) | 25/0 | - |
| [50] | New Zealand | HNSCC | Oral cavity, left parotid, tonsils | - | 14 | 49–81 (Range) | 11/3 | - |
| [26] | Taiwan | OSCC | Tongue, gingiva, floor of the mouth | I–IV | 197 | 32–87 (Range) | 177/20 | - |
| [27] | Taiwan | OSCC | Buccal mucosa, tongue, lip, gingiva, others | I–IV | 39 | 53.33 ± 10.95 (M) | 39/0 | 2014–2015 |
| [28] | US | OSCC | - | Non-metastatic OSCC | 4 | 40–64 (Range) | 4/0 | - |
| [29] | US | OSCC, OPMDs | Oral cavity | - | OSCC: 18; OPMDs: 8 | OSCC: 59.8 ± 10.9 OPMDs: 66.1 ± 17.9 (M) | 16/10 | - |
| [30] | Japan | OSCC, OPMDs | Tongue, gingiva, buccal mucosa | CIS, I–IV | 12 (6 per group) | OSCC: 50.66; OPMDs: 58.33 (M) | 9/3 | - |
| [43] | Colombia | OPSCC | Oropharynx | - | 26 | 31 ≥ 70 (Range) | 17/9 | 2014–2017 |
| [31] | Japan | OSCC | Oral cavity | T1–4, N0–3 | 60 | 63.7 (M) | 50/10 | 2016–2018 |
| [32] | China | OSCC | Buccal mucosa | I–IV | 50 | 60.7 (M); 61 (Med) | 32/18 | 2018 |
| [49] | Hong Kong | HNSCC | Oral cavity, oropharynx, larynx and others | T1–4, N0–2 | 68 | >60 y = 48, ≤60 y = 20 | 51/17 | 2015–2018 |
| [46] | China | NPC | Nasopharynx | - | 499 | 48.4 (M) | 356/143 | 2010–2014 |
| [33] | India | OSCC, OPMDs | Floor of the mouth, buccal mucosa, tongue, gingiva | Well/moderately/poorly diff.; Lymph node status (+/−) | OSCC: 31; OPMDs: 20 | OSCC: 49.31 ± 13.24 OPMDs: 45.67 ± 6.81 (M) | - | - |
| [34] | Poland | OSCC | Tonsil, throat, floor of the mouth, tongue | - | 18 | - | - | - |
| [35] | China | OSCC | Oral cavity | I–IV | 24 | 61.1 ± 12.4 (M) | 17/7 | - |
| [51] | China | HNSCC | Larynx, hypopharynx, other | I–IV | 56 | 61.5 ± 8.8 (M) | 56/0 | - |
| [36] | Brazil | OSCC | Oral cavity (non-active lesion: L0; active L1) | T1–4; Lymph node status (+/−) | 16 (8 per group) | L0: 55.8; L1: 57.7 (M) | 14/2 | - |
| [37] | France | OSCC | Oral cavity | I–IV | 151 | 57 (Med) | 93/58 | 1990–2006 |
| [38] | India | OSCC | - | T2–4, N0–3; Well/mod diff. | 25 | 55.32 (M) | 16/9 | - |
| [39] | India | OSCC | - | Well diff. | 50 | 52.68 (M) | 32/18 | - |
| [40] | Taiwan | OSCC | Buccal mucosa | I–IV | 116 | 54.81 ± 10.73 | 116/0 | - |
| Study | Method | Sampling Type | Number of Samples | Microbiota Type | Microbiota Characterization |
|---|---|---|---|---|---|
| [18] | DNA-DNA hybridization | Whole unstimulated saliva through expectoration | 274 (229 OSCC-free controls; 45 OSCC) | 40 common oral bacteria were tested | Digoxigenin-labeled DNA using random primer technique was used |
| [44] | IHC | Tissue biopsy, PEFF | 15 (5 normal tissue; 10 GSCC) | P. gingivalis; S. gordonii | Rabbit polyclonal antibodies (1:1000) |
| [19] | 16S rRNA PCR | Stimulated saliva | 5 (2 matched non-OSCC controls; 3 OSCC) | Total bacterial diversity and relative abundance | PCR primers were based on the V4–V5 hypervariable region |
| [20] | 16S rRNA PCR | DNA extraction from tissue biopsy samples | 20 (10 tumor-free tissues from OSCC patients; 10 OSCC) | Total bacterial diversity and relative abundance | PCR primers for V4–V5 hypervariable region; the eubacterial primers: prbac1 and prbac2 |
| [41] | 16S rRNA PCR | Swab samples from normal controls and lesions | 83 (49 normal controls; 34 OSCC/OPMDs) | Total bacterial diversity and relative abundance | 16S rDNA V4 hypervariable region were sequenced using the Illumina MiSeq platform |
| [21] | 16S rRNA PCR | Oral rinse samples | 363 (242 normal controls; 121 OSCC/OPSCC cases) | Total bacterial diversity and relative abundance | The Illumina MiSeq primers targeting the V4 variable region |
| [22] | 16S rRNA PCR | Unstimulated saliva | 376 (127 normal controls; 124 OPMDs; 125 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (F515/ R806) targeting the V4 region of bacterial 16S rDNA |
| [42] | 16S rRNA PCR | Swab samples from normal controls and lesions | 27 (9 normal controls; 9 OPMDs; 9 cancer) | Total bacterial diversity and relative abundance | The primer pair D88/E94 produced near full length of 16S amplicons (targets V6–V9) |
| [47] | 16S rRNA PCR | Paired normal and tumoral resection specimens | 242 (121 tumor-free controls; 121 tumors) | Total bacterial diversity and relative abundance | PCR of the V1–V4 hypervariable regions of the 16S rRNA gene using the M13 primers |
| [23] | 16S rRNA PCR | Swab samples from normal controls and lesions | 80 (40 anatomically matched normal controls; 40 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (515F/926R) targeting the V4–V5 regions using Illumina MiSeq tool |
| [48] | 16S rRNA PCR | Mouth wash samples | 383 (254 matched normal controls; 129 HNSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (347F/803R) targeting the V3–V4 variable regions of the 16S rRNA |
| [24] | 16S rRNA PCR | Unstimulated saliva; peripheral blood (genotyping) | 289 (151 matched controls; 138 OSCC) | 20 species were included for case–control comparison | The PCR primer pair (341F/926R) targeting the V3–V5 regions of the 16S rRNA |
| [45] | 16S rRNA PCR | Oral rinse samples | 83 (20 normal controls; 11 high-risk; 52 tumors) | Total bacterial diversity and relative abundance | The PCR primer pair (515F/806R) targeting the V4 variable region of the 16S rRNA |
| [25] | 16S rRNA PCR | Tissue biopsy samples | 52 (27 oral fibroepithelial polyp as controls; 25 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (27FYMF/519R) targeting the V1-V3 regions of the 16S rRNA |
| [50] | 16S rRNA PCR | Unstimulated whole saliva | 30 (7 healthy controls; 9 dental compromised; 14 HNSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (341F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [26] | 16S rRNA PCR | Oral rinse samples | 248 (51 healthy individuals; 197 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (16SF/16SR) targeting the V3–V4 variable regions of the 16S rRNA |
| [27] | 16S rRNA PCR | Unstimulated saliva samples | 39 (OSCC) | Total bacterial diversity and relative abundance | The PCR primers (F515/R806) targeting the V4 region of the 16S rRNA |
| [28] | RNA amplification | Oral swab samples | 15 (4 OSCC; 11 OSCC-free sites/healthy individuals) | Active communities in tumor/tumor-free areas | Illumina adapter-specific primers were used to amplify the cDNA generated from mRNA |
| [29] | 16S rRNA PCR | Oral rinse samples | 38 (12 thyroid nodules as controls; 18 OSCC; 8 OPMDs) | Total bacterial diversity and relative abundance | The PCR primer pair (347F/803R) targeting the V3–V4 variable regions of the 16S rRNA |
| [30] | 16S rRNA PCR | Unstimulated saliva samples | 16 (4 healthy controls; 6 OSCC; 6 OPMDs) | Total bacterial diversity and relative abundance | The PCR primers (F515/R806) targeting the V4 gene region of the 16S rRNA |
| [43] | 16S rRNA PCR | Cytobrush (control); Tissue biopsy (OPSCC) | 52 (26 OPSCC; 26 controls) | P. melanogenica, F. naviforme, S. anginosus | Species-specific construct was designed that contained analyzed bacteria sequences |
| [31] | 16S rRNA PCR | Stimulated saliva samples | 140 (80 non-cancer controls; 60 OSCC) | Total bacterial diversity and relative abundance | PCR primers were developed for V3–V4 region of the 16S rRNA gene |
| [32] | 16S rRNA PCR | Oral swabs from tumor and normal tissues | 100 (50 from non-tumor sites; 50 tumors) | Total bacterial diversity and relative abundance | The PCR primer pair (338F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [49] | 16S rRNA PCR | Oral rinse samples; Tissue biopsy | 272 (136 non-tumor controls; 136 tumor samples) | Total bacterial diversity and relative abundance | The PCR primer pair (341F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [46] | 16S rRNA PCR | Saliva samples | 994 (495 healthy controls; 499 patients with NPC) | Total bacterial diversity and ASVs prevalence | The PCR primer pair (341F/805R) targeting the V3–V4 variable regions of the 16S rRNA |
| [33] | 16S rRNA PCR | Unstimulated whole mouth fluid | 74 (23 healthy controls; 31 OSCC; 20 OPMDs) | Total bacterial diversity and relative abundance | The PCR primer pair (319F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [34] | 16S rRNA PCR | Saliva samples; Tissue biopsy | 59 (18 non-tumor tissues;18 tumor tissue; 23 OSCC saliva) | Total bacterial diversity and relative abundance | Adaptor-ligated 16S primers targeting the V4 region of the 16S rRNA gene fragment |
| [35] | 16S rRNA PCR | Tissue biopsy samples | 48 (24 paracancerous control tissues; 24 tumor tissues) | Total bacterial diversity and relative abundance | The PCR primer pair (341F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [51] | 16S rRNA PCR | Unstimulated saliva samples | 120 (64 healthy controls; 56 from cancer patients) | Total bacterial diversity and relative abundance | The PCR primer pair (341F/806R) targeting the V3–V4 variable regions of the 16S rRNA |
| [36] | 16S rRNA PCR | Unstimulated saliva samples | 24 (8 healthy controls; 16 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (515F/806R) targeting the V4 region of the 16S rRNA was used |
| [37] | 16S rRNA PCR, IHC | Tissue biopsy samples | 212 (HNSCC) | F. nucleatum; gram-negative bacteria | A unique PCR primer for F. nucleatum; LPS monoclonal Mouse antibody (clone C8) |
| [38] | 16S rRNA PCR | Unstimulated saliva samples | 49 (24 healthy controls; 25 OSCC) | Total bacterial diversity and relative abundance | The PCR primer pair (16SF/16SR) targeting the V3–V4 variable regions of the 16S rRNA |
| [39] | 16S rRNA PCR | Tissue biopsy samples | 100 (50 paracancerous control tissues; 50 tumor tissues) | Total bacterial diversity and relative abundance | A PCR primer pair targeting the V3–V4 variable regions of the 16S rRNA was used |
| [40] | 16S rRNA PCR | Oral swabs from tumor and normal tissues | 232 (116 contralateral normal tissues, 116 tumor tissues) | Total bacterial diversity and relative abundance | The PCR primer pair (515F/806R) targeting the V4 region of the 16S rRNA was used |
| Study | Cancer Type | Statistics | Analysis Target | Analysis Results | Prognostic Effect | Result Interpretation |
|---|---|---|---|---|---|---|
| [29] | OSCC | Kruskal Wallis and Mann–Whitney tests | RA and recurrence | Capnocytophaga was higher in recurrent tumors (median = 1.54 vs. 0.27%); p = 0.0083 | Unfavorable | Capnocytophaga is associated with OSCC recurrence. In contrast, no taxa were associated with the tumor stage, lymph node status, or distant metastasis |
| [43] | OSCC | Kaplan-Meier and Log-Rank tests | HPV+ve patients vs. HPV+ve/S. anginosus+ve patients | No statistically significant differences were observed; p = 0.559 | Potentially unfavorable | HPV+ve/S. anginosus+ve patients tend to exhibit shorter survival outcomes although it was not significant |
| [49] | HNSCC | Uni-/Multi-variate analyses | RA and clinical prognostic factors | F. nucleatum enrichment had better 3-year DSS (86.7% vs. 47.6%, p ≤ 0.001); DFS (85.0% vs. 41.8%, p ≤ 0.001); lower T-stage | Favorable | Fusobacterium is an independent predictor of DSS and may facilitate the use of oral bacteria as biomarkers in patients with HNSCC |
| [36] | OSCC | Kaplan-Meier and Log-Rank tests; Pearson correlation coefficient and cross-tabulation with the chi-square test | RA and clinical prognostic factors | Enrichment of six genera (Stenotrophomonas, Staphylococcus, Selenomonas, Centipeda, Alloscardovia, and Acinetobacter) had shorter OS (p < 0.05); Veillonella and Centipeda were correlated with tumor size and clinical stage | Unfavorable | Oral microbiota and their protein abundance have potential diagnosis and prognosis value for oral cancer patients |
| [37] | OSCC | Kaplan-Meier and Log-Rank tests; Uni-/Multi-variate analyses; Chi-square correlation test | Intratumoral F. nucleatum and clinical prognostic factors | In the merged cohort, F. nucleatum+ve tumors had better OS than negative tumors (HR: 0.51, p = 0.009; 5-year OS 60.5% vs. 37.7%; 10-year OS 47.9% vs. 18.8%) | Favorable | F. nucleatum identified a subgroup of OSCC; it was frequent in older, non-drinking patients; associated with less lymph node invasion and distant relapse. F. nucleatum expression showed favorable OS (independent predictor), RFS and MFS outcomes in the merged cohort |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metsäniitty, M.; Hasnat, S.; Salo, T.; Salem, A. Oral Microbiota—A New Frontier in the Pathogenesis and Management of Head and Neck Cancers. Cancers 2022, 14, 46. https://doi.org/10.3390/cancers14010046
Metsäniitty M, Hasnat S, Salo T, Salem A. Oral Microbiota—A New Frontier in the Pathogenesis and Management of Head and Neck Cancers. Cancers. 2022; 14(1):46. https://doi.org/10.3390/cancers14010046
Chicago/Turabian StyleMetsäniitty, Marjut, Shrabon Hasnat, Tuula Salo, and Abdelhakim Salem. 2022. "Oral Microbiota—A New Frontier in the Pathogenesis and Management of Head and Neck Cancers" Cancers 14, no. 1: 46. https://doi.org/10.3390/cancers14010046
APA StyleMetsäniitty, M., Hasnat, S., Salo, T., & Salem, A. (2022). Oral Microbiota—A New Frontier in the Pathogenesis and Management of Head and Neck Cancers. Cancers, 14(1), 46. https://doi.org/10.3390/cancers14010046







