Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
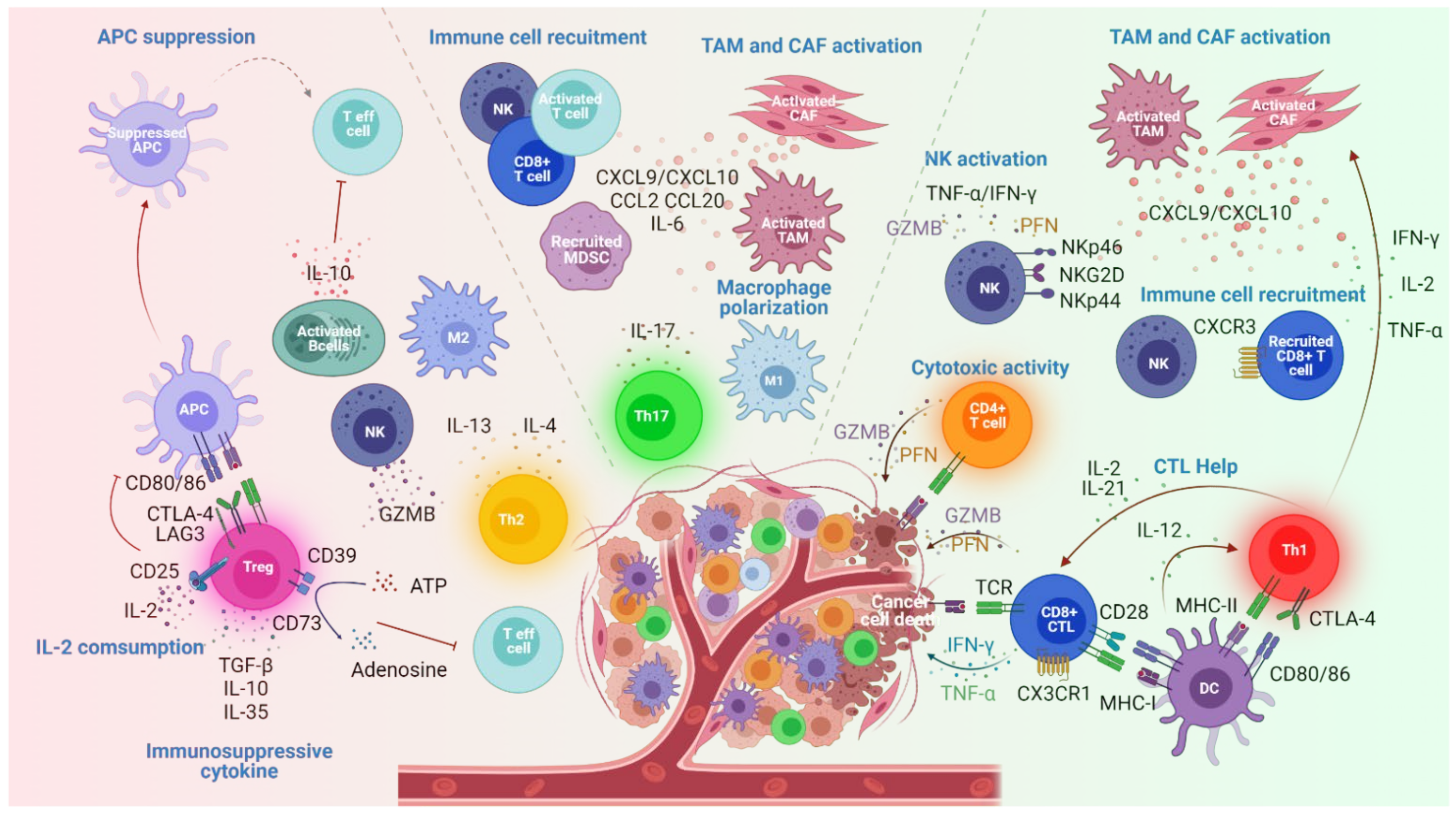
2. Effector CD4+ T Cell Subsets in Cancers
2.1. Th1 Cell Subsets
2.2. Th2 Cell Subsets
2.3. Th9 Cell Subsets
2.4. Th17 Cell Subsets
2.5. Treg Cell Subsets
2.6. CD4+ CTL
2.7. Tfh Cell Subsets
3. CD4+ T Cell Help for Cancer Immunotherapies
3.1. Cancer Vaccines
3.2. Immune Checkpoint Inhibitors
3.3. Adoptive Cell Transfer
3.3.1. TIL Infusions
3.3.2. CAR-T Cell Therapy
4. Prospects of Combining Therapies for CD4+ T Cell Harnessing
5. Conclusions
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Pisibon, C.; Ouertani, A.; Bertolotto, C.; Ballotti, R.; Cheli, Y. Immune Checkpoints in Cancers: From Signaling to the Clinic. Cancers 2021, 13, 4573. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Albukhari, A.; Alsaadi, A.; Artibani, M.; Brenton, J.D.; Curbishley, S.M.; Dong, T.; Dustin, M.L.; Hu, Z.; McGranahan, N.; et al. Promises and Challenges of Adoptive T-Cell Therapies for Solid Tumours. Br. J. Cancer 2021, 124, 1759–1776. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Zanetti, M. Tapping CD4 T Cells for Cancer Immunotherapy: The Choice of Personalized Genomics. J. Immunol. 2015, 194, 2049–2056. [Google Scholar] [CrossRef]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-Specific MHC-II Expression Represents a Tumour-Autonomous Phenotype and Predicts Response to Anti-PD-1/PD-L1 Therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Johnson, D.B.; Bordeaux, J.; Kim, J.Y.; Vaupel, C.; Rimm, D.L.; Ho, T.H.; Joseph, R.W.; Daud, A.I.; Conry, R.M.; Gaughan, E.M.; et al. Quantitative Spatial Profiling of PD-1/PD-L1 Interaction and HLA-DR/IDO-1 Predicts Improved Outcomes of Anti-PD-1 Therapies in Metastatic Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5250–5260. [Google Scholar] [CrossRef]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC Proteins Confer Differential Sensitivity to CTLA-4 and PD-1 Blockade in Untreated Metastatic Melanoma. Sci. Transl. Med. 2018, 10, aar3342. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Redd, R.A.; Cader, F.Z.; Pak, C.J.; Abdelrahman, S.; Ouyang, J.; Sasse, S.; Younes, A.; Fanale, M.; Santoro, A.; et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 942–950. [Google Scholar] [CrossRef]
- Park, I.A.; Hwang, S.-H.; Song, I.H.; Heo, S.-H.; Kim, Y.-A.; Bang, W.S.; Park, H.S.; Lee, M.; Gong, G.; Lee, H.J. Expression of the MHC Class II in Triple-Negative Breast Cancer Is Associated with Tumor-Infiltrating Lymphocytes and Interferon Signaling. PloS One 2017, 12, e0182786. [Google Scholar] [CrossRef]
- Cachot, A.; Bilous, M.; Liu, Y.-C.; Li, X.; Saillard, M.; Cenerenti, M.; Rockinger, G.A.; Wyss, T.; Guillaume, P.; Schmidt, J.; et al. Tumor-Specific Cytolytic CD4 T Cells Mediate Immunity against Human Cancer. Sci. Adv. 2021, 7, abe3348. [Google Scholar] [CrossRef]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-Reactive CD4(+) T Cells Develop Cytotoxic Activity and Eradicate Large Established Melanoma after Transfer into Lymphopenic Hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef]
- Takeuchi, A.; Badr, M.E.S.G.; Miyauchi, K.; Ishihara, C.; Onishi, R.; Guo, Z.; Sasaki, Y.; Ike, H.; Takumi, A.; Tsuji, N.M.; et al. CRTAM Determines the CD4+ Cytotoxic T Lymphocyte Lineage. J. Exp. Med. 2016, 213, 123–138. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Bos, R.; Sherman, L.A. CD4+ T-Cell Help in the Tumor Milieu Is Required for Recruitment and Cytolytic Function of CD8+ T Lymphocytes. Cancer Res. 2010, 70, 8368–8377. [Google Scholar] [CrossRef]
- Kambayashi, T.; Laufer, T.M. Atypical MHC Class II-Expressing Antigen-Presenting Cells: Can Anything Replace a Dendritic Cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef]
- Oldford, S.A.; Robb, J.D.; Codner, D.; Gadag, V.; Watson, P.H.; Drover, S. Tumor Cell Expression of HLA-DM Associates with a Th1 Profile and Predicts Improved Survival in Breast Carcinoma Patients. Int. Immunol. 2006, 18, 1591–1602. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N.; Faille, A.; Loiseau, P.; Poirier, O.; Charron, D.; Calvo, F. Effect of Gamma Interferon on HLA Class-I and -II Transcription and Protein Expression in Human Breast Adenocarcinoma Cell Lines. Int. J. Cancer 1990, 45, 1169–1176. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and Lymphocytes Prevent Primary Tumour Development and Shape Tumour Immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef]
- Tan, K.W.; Evrard, M.; Tham, M.; Hong, M.; Huang, C.; Kato, M.; Prevost-Blondel, A.; Donnadieu, E.; Ng, L.G.; Abastado, J.-P. Tumor Stroma and Chemokines Control T-Cell Migration into Melanoma Following Temozolomide Treatment. Oncoimmunology 2015, 4, e978709. [Google Scholar] [CrossRef]
- Lorvik, K.B.; Hammarström, C.; Fauskanger, M.; Haabeth, O.A.W.; Zangani, M.; Haraldsen, G.; Bogen, B.; Corthay, A. Adoptive Transfer of Tumor-Specific Th2 Cells Eradicates Tumors by Triggering an In Situ Inflammatory Immune Response. Cancer Res. 2016, 76, 6864–6876. [Google Scholar] [CrossRef]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T Helper Type 2 Cell Infiltrate Correlates with Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef]
- Kitajima, M.; Ito, T.; Tumes, D.J.; Endo, Y.; Onodera, A.; Hashimoto, K.; Motohashi, S.; Yamashita, M.; Nishimura, T.; Ziegler, S.F.; et al. Memory Type 2 Helper T Cells Induce Long-Lasting Antitumor Immunity by Activating Natural Killer Cells. Cancer Res. 2011, 71, 4790–4798. [Google Scholar] [CrossRef][Green Version]
- Kusuda, T.; Shigemasa, K.; Arihiro, K.; Fujii, T.; Nagai, N.; Ohama, K. Relative Expression Levels of Th1 and Th2 Cytokine MRNA Are Independent Prognostic Factors in Patients with Ovarian Cancer. Oncol. Rep. 2005, 13, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.E.; Hassan, M.; Burton, B.R.; Britton, G.; Hill, E.V.; Verhagen, J.; Wraith, D.C. IL-4 Enhances IL-10 Production in Th1 Cells: Implications for Th1 and Th2 Regulation. Sci. Rep. 2017, 7, 11315. [Google Scholar] [CrossRef] [PubMed]
- Lazarski, C.A.; Ford, J.; Katzman, S.D.; Rosenberg, A.F.; Fowell, D.J. IL-4 Attenuates Th1-Associated Chemokine Expression and Th1 Trafficking to Inflamed Tissues and Limits Pathogen Clearance. PloS One 2013, 8, e71949. [Google Scholar] [CrossRef] [PubMed]
- Végran, F.; Apetoh, L.; Ghiringhelli, F. Th9 Cells: A Novel CD4 T-Cell Subset in the Immune War against Cancer. Cancer Res. 2015, 75, 475–479. [Google Scholar] [CrossRef]
- Sek, K.; Chan, C.W.; Beavis, P.A.; Darcy, P.K. Adoptive Transfer of Tumor-Specific Th9 Cells Eradicates Heterogeneous Antigen-Expressing Tumor Cells. Cancer Cell 2021, 39, 1564–1566. [Google Scholar] [CrossRef]
- Salazar, Y.; Zheng, X.; Brunn, D.; Raifer, H.; Picard, F.; Zhang, Y.; Winter, H.; Guenther, S.; Weigert, A.; Weigmann, B.; et al. Microenvironmental Th9 and Th17 Lymphocytes Induce Metastatic Spreading in Lung Cancer. J. Clin. Investig. 2020, 130, 3560–3575. [Google Scholar] [CrossRef]
- Lu, L.; Pan, K.; Zheng, H.-X.; Li, J.-J.; Qiu, H.-J.; Zhao, J.-J.; Weng, D.-S.; Pan, Q.-Z.; Wang, D.-D.; Jiang, S.-S.; et al. IL-17A Promotes Immune Cell Recruitment in Human Esophageal Cancers and the Infiltrating Dendritic Cells Represent a Positive Prognostic Marker for Patient Survival. J. Immunother. Hagerstown Md 1997 2013, 36, 451–458. [Google Scholar] [CrossRef]
- Al Omar, S.; Flanagan, B.F.; Almehmadi, M.; Christmas, S.E. The Effects of IL-17 upon Human Natural Killer Cells. Cytokine 2013, 62, 123–130. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Szeliga, W.; Vatan, L.; Zou, W. Endogenous IL-17 Contributes to Reduced Tumor Growth and Metastasis. Blood 2009, 114, 357–359. [Google Scholar] [CrossRef]
- Jang, J.-E.; Hajdu, C.H.; Liot, C.; Miller, G.; Dustin, M.L.; Bar-Sagi, D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-Tumor Immunity in Pancreatic Cancer. Cell Rep. 2017, 20, 558–571. [Google Scholar] [CrossRef]
- Li, J.; Lau, G.K.-K.; Chen, L.; Dong, S.; Lan, H.-Y.; Huang, X.-R.; Li, Y.; Luk, J.M.; Yuan, Y.-F.; Guan, X. Interleukin 17A Promotes Hepatocellular Carcinoma Metastasis via NF-KB Induced Matrix Metalloproteinases 2 and 9 Expression. PloS One 2011, 6, e21816. [Google Scholar] [CrossRef]
- He, D.; Li, H.; Yusuf, N.; Elmets, C.A.; Li, J.; Mountz, J.; Xu, H. IL-17 Promotes Tumor Development through the Induction of Tumor Promoting Microenvironments at Tumor Sites and Myeloid Derived Suppressor Cells. J. Immunol. 2010, 184, 2281–2288. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 Is Associated with Poor Prognosis and Promotes Angiogenesis via Stimulating VEGF Production of Cancer Cells in Colorectal Carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 Promotes Angiogenesis by Stimulating VEGF Production of Cancer Cells via the STAT3/GIV Signaling Pathway in Non-Small-Cell Lung Cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautès-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 Inhibits Tumor Cell Growth by Means of a T-Cell-Dependent Mechanism. Blood 2002, 99, 2114–2121. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and Its Role in Regulating Anti-Tumor Immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An Essential Role for the IL-2 Receptor in Treg Cell Function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage Tracking Reveals Dynamic Relationships of T Cells in Colorectal Cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.-C.S.; Rancan, C.; et al. Intratumoral CD4+ T Cells Mediate Anti-Tumor Cytotoxicity in Human Bladder Cancer. Cell 2020, 181, 1612–1625.e13. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-Cell Analyses Reveal Key Immune Cell Subsets Associated with Response to PD-L1 Blockade in Triple-Negative Breast Cancer. Cancer Cell 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Song, W.; Craft, J. T Follicular Helper Cell Heterogeneity: Time, Space, and Function. Immunol. Rev. 2019, 288, 85–96. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Mann, G.J.; Pupo, G.M.; Campain, A.E.; Carter, C.D.; Schramm, S.-J.; Pianova, S.; Gerega, S.K.; De Silva, C.; Lai, K.; Wilmott, J.S.; et al. BRAF Mutation, NRAS Mutation, and the Absence of an Immune-Related Expressed Gene Profile Predict Poor Outcome in Patients with Stage III Melanoma. J. Investig. Dermatol. 2013, 133, 509–517. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2,000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Ascierto, M.L.; Kmieciak, M.; Idowu, M.O.; Manjili, R.; Zhao, Y.; Grimes, M.; Dumur, C.; Wang, E.; Ramakrishnan, V.; Wang, X.-Y.; et al. A Signature of Immune Function Genes Associated with Recurrence-Free Survival in Breast Cancer Patients. Breast Cancer Res. Treat. 2012, 131, 871–880. [Google Scholar] [CrossRef]
- Leffers, N.; Fehrmann, R.S.N.; Gooden, M.J.M.; Schulze, U.R.J.; Ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; Daemen, T.; de Jong, S.; Nijman, H.W.; et al. Identification of Genes and Pathways Associated with Cytotoxic T Lymphocyte Infiltration of Serous Ovarian Cancer. Br. J. Cancer 2010, 103, 685–692. [Google Scholar] [CrossRef]
- Camus, M.; Tosolini, M.; Mlecnik, B.; Pagès, F.; Kirilovsky, A.; Berger, A.; Costes, A.; Bindea, G.; Charoentong, P.; Bruneval, P.; et al. Coordination of Intratumoral Immune Reaction and Human Colorectal Cancer Recurrence. Cancer Res. 2009, 69, 2685–2693. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Mlecnik, B.; Tosolini, M.; Charoentong, P.; Kirilovsky, A.; Bindea, G.; Berger, A.; Camus, M.; Gillard, M.; Bruneval, P.; Fridman, W.-H.; et al. Biomolecular Network Reconstruction Identifies T-Cell Homing Factors Associated with Survival in Colorectal Cancer. Gastroenterology 2010, 138, 1429–1440. [Google Scholar] [CrossRef]
- Pagès, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients with Early-Stage Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef]
- Laheurte, C.; Dosset, M.; Vernerey, D.; Boullerot, L.; Gaugler, B.; Gravelin, E.; Kaulek, V.; Jacquin, M.; Cuche, L.; Eberst, G.; et al. Distinct Prognostic Value of Circulating Anti-Telomerase CD4+ Th1 Immunity and Exhausted PD-1+/TIM-3+ T Cells in Lung Cancer. Br. J. Cancer 2019, 121, 405–416. [Google Scholar] [CrossRef]
- Chraa, D.; Naim, A.; Olive, D.; Badou, A. T Lymphocyte Subsets in Cancer Immunity: Friends or Foes. J. Leukoc. Biol. 2019, 105, 243–255. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Su, Q.; Huang, H.; Zhou, P.; Luan, J.; Liu, J.; Wang, J.; Chen, X. Expression of Th1- Th2- and Th17-Associated Cytokines in Laryngeal Carcinoma. Oncol. Lett. 2016, 12, 1941–1948. [Google Scholar] [CrossRef]
- Dengel, L.T.; Norrod, A.G.; Gregory, B.L.; Clancy-Thompson, E.; Burdick, M.D.; Strieter, R.M.; Slingluff, C.L.; Mullins, D.W. Interferons Induce CXCR3-Cognate Chemokine Production by Human Metastatic Melanoma. J. Immunother. 2010, 33, 965–974. [Google Scholar] [CrossRef]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset That Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e4. [Google Scholar] [CrossRef]
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic Impact of Tumour-Infiltrating Th2 and Regulatory T Cells in Classical Hodgkin Lymphoma. Hematol. Oncol. 2009, 27, 31–39. [Google Scholar] [CrossRef]
- Yoon, N.K.; Maresh, E.L.; Shen, D.; Elshimali, Y.; Apple, S.; Horvath, S.; Mah, V.; Bose, S.; Chia, D.; Chang, H.R.; et al. Higher Levels of GATA3 Predict Better Survival in Women with Breast Cancer. Hum. Pathol. 2010, 41, 1794–1801. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming Growth Factor-β “reprograms” the Differentiation of T Helper 2 Cells and Promotes an Interleukin 9–Producing Subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef]
- Schmitt, E.; Klein, M.; Bopp, T. Th9 Cells, New Players in Adaptive Immunity. Trends Immunol. 2014, 35, 61–68. [Google Scholar] [CrossRef]
- Purwar, R.; Schlapbach, C.; Xiao, S.; Kang, H.S.; Elyaman, W.; Jiang, X.; Jetten, A.M.; Khoury, S.J.; Fuhlbrigge, R.C.; Kuchroo, V.K.; et al. Robust Tumor Immunity to Melanoma Mediated by Interleukin-9–Producing T Cells. Nat. Med. 2012, 18, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hong, S.; Li, H.; Park, J.; Hong, B.; Wang, L.; Zheng, Y.; Liu, Z.; Xu, J.; He, J.; et al. Th9 Cells Promote Antitumor Immune Responses in Vivo. J. Clin. Investig. 2012, 122, 4160–4171. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Q.; Xue, G.; Bi, E.; Ma, X.; Wang, A.; Qian, J.; Dong, C.; Yi, Q. Th9 Cells Represent a Unique Subset of CD4+ T Cells Endowed with the Ability to Eradicate Advanced Tumors. Cancer Cell 2018, 33, 1048–1060.e7. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Bijman, M.; Molin, D.; Cormont, F.; Uyttenhove, C.; van Snick, J.; Sundström, C.; Enblad, G.; Nilsson, G. Increased Serum Levels of Interleukin-9 Correlate to Negative Prognostic Factors in Hodgkin’s Lymphoma. Leukemia 2003, 17, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and Clinical Significance of Th17 Cells in the Tumor Microenvironment and Peripheral Blood of Pancreatic Cancer Patients. Int. J. Mol. Sci. 2011, 12, 7424–7437. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Yan, J.; Xu, J.; Pang, X.-H.; Chen, M.-S.; Li, L.; Wu, C.; Li, S.-P.; Zheng, L. Increased Intratumoral IL-17-Producing Cells Correlate with Poor Survival in Hepatocellular Carcinoma Patients. J. Hepatol. 2009, 50, 980–989. [Google Scholar] [CrossRef]
- Ye, Z.-J.; Zhou, Q.; Gu, Y.-Y.; Qin, S.-M.; Ma, W.-L.; Xin, J.-B.; Tao, X.-N.; Shi, H.-Z. Generation and Differentiation of IL-17-Producing CD4+ T Cells in Malignant Pleural Effusion. J. Immunol. 2010, 185, 6348–6354. [Google Scholar] [CrossRef]
- Lv, L.; Pan, K.; Li, X.; She, K.; Zhao, J.; Wang, W.; Chen, J.; Chen, Y.; Yun, J.; Xia, J. The Accumulation and Prognosis Value of Tumor Infiltrating IL-17 Producing Cells in Esophageal Squamous Cell Carcinoma. PloS One 2011, 6, e18219. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.; Liang, X.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.; Wang, Q.; Li, Y.; Chen, S.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60. [Google Scholar] [CrossRef]
- Alves, J.J.P.; De Medeiros Fernandes, T.A.A.; De Araújo, J.M.G.; Cobucci, R.N.O.; Lanza, D.C.F.; Bezerra, F.L.; Andrade, V.S.; Fernandes, J.V. Th17 Response in Patients with Cervical Cancer. Oncol. Lett. 2018, 16, 6215–6227. [Google Scholar] [CrossRef]
- Fabre, J.A.S.; Giustinniani, J.; Garbar, C.; Merrouche, Y.; Antonicelli, F.; Bensussan, A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018, 19, E3880. [Google Scholar] [CrossRef]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 Produced by Tumor Microenvironment Promotes Self-Renewal of CD133+ Cancer Stem-like Cells in Ovarian Cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Chen, C.; Ge, D.; Qu, Y.; Chen, R.; Fan, Y.-M.; Li, N.; Tang, W.W.; Zhang, W.; et al. Hyperinsulinemia Enhances Interleukin-17-Induced Inflammation to Promote Prostate Cancer Development in Obese Mice through Inhibiting Glycogen Synthase Kinase 3-Mediated Phosphorylation and Degradation of Interleukin-17 Receptor. Oncotarget 2016, 7, 13651–13666. [Google Scholar] [CrossRef]
- Furuta, S.; Jeng, Y.-M.; Zhou, L.; Huang, L.; Kuhn, I.; Bissell, M.J.; Lee, W.-H. IL-25 Causes Apoptosis of IL-25R–Expressing Breast Cancer Cells Without Toxicity to Nonmalignant Cells. Sci. Transl. Med. 2011, 3, 78ra31. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The Two Faces of IL-6 in the Tumor Microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef]
- Barilla, R.M.; Diskin, B.; Caso, R.C.; Lee, K.B.; Mohan, N.; Buttar, C.; Adam, S.; Sekendiz, Z.; Wang, J.; Salas, R.D.; et al. Specialized Dendritic Cells Induce Tumor-Promoting IL-10+IL-17+ FoxP3neg Regulatory CD4+ T Cells in Pancreatic Carcinoma. Nat. Commun. 2019, 10, 1424. [Google Scholar] [CrossRef]
- Jarnicki, A.G.; Lysaght, J.; Todryk, S.; Mills, K.H.G. Suppression of Antitumor Immunity by IL-10 and TGF-Beta-Producing T Cells Infiltrating the Growing Tumor: Influence of Tumor Environment on the Induction of CD4+ and CD8+ Regulatory T Cells. J. Immunol. 2006, 177, 896–904. [Google Scholar] [CrossRef]
- Turnis, M.E.; Sawant, D.V.; Szymczak-Workman, A.L.; Andrews, L.P.; Delgoffe, G.M.; Yano, H.; Beres, A.J.; Vogel, P.; Workman, C.J.; Vignali, D.A.A. Interleukin-35 Limits Anti-Tumor Immunity. Immunity 2016, 44, 316–329. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sakaguchi, S. Regulatory T Cells in Tumor Immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef]
- Levine, A.G.; Arvey, A.; Jin, W.; Rudensky, A.Y. Continuous Requirement for the TCR in Regulatory T Cell Function. Nat. Immunol. 2014, 15, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Bonertz, A.; Weitz, J.; Pietsch, D.-H.K.; Rahbari, N.N.; Schlude, C.; Ge, Y.; Juenger, S.; Vlodavsky, I.; Khazaie, K.; Jaeger, D.; et al. Antigen-Specific Tregs Control T Cell Responses against a Limited Repertoire of Tumor Antigens in Patients with Colorectal Carcinoma. J. Clin. Investig. 2009, 119, 3311–3321. [Google Scholar] [CrossRef]
- François, V.; Ottaviani, S.; Renkvist, N.; Stockis, J.; Schuler, G.; Thielemans, K.; Colau, D.; Marchand, M.; Boon, T.; Lucas, S.; et al. The CD4(+) T-Cell Response of Melanoma Patients to a MAGE-A3 Peptide Vaccine Involves Potential Regulatory T Cells. Cancer Res. 2009, 69, 4335–4345. [Google Scholar] [CrossRef]
- Vence, L.; Palucka, A.K.; Fay, J.W.; Ito, T.; Liu, Y.-J.; Banchereau, J.; Ueno, H. Circulating Tumor Antigen-Specific Regulatory T Cells in Patients with Metastatic Melanoma. Proc. Natl. Acad. Sci. USA 2007, 104, 20884–20889. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lee, D.A.; Peng, G.; Guo, Z.; Li, Y.; Kiniwa, Y.; Shevach, E.M.; Wang, R.F. Tumor-Specific Human CD4+ Regulatory T Cells and Their Ligands: Implications for Immunotherapy. Immunity 2004, 20, 107–118. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Pasetto, A.; Jia, L.; Deniger, D.C.; Stevanović, S.; Robbins, P.F.; Rosenberg, S.A. Tumor-Infiltrating Human CD4+ Regulatory T Cells Display a Distinct TCR Repertoire and Exhibit Tumor and Neoantigen Reactivity. Sci. Immunol. 2019, 4, aao4310. [Google Scholar] [CrossRef]
- Katz, S.C.; Bamboat, Z.M.; Maker, A.V.; Shia, J.; Pillarisetty, V.G.; Yopp, A.C.; Hedvat, C.V.; Gonen, M.; Jarnagin, W.R.; Fong, Y.; et al. Regulatory T Cell Infiltration Predicts Outcome Following Resection of Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2013, 20, 946–955. [Google Scholar] [CrossRef]
- Osman, A.; Yan, B.; Li, Y.; Pavelko, K.D.; Quandt, J.; Saadalla, A.; Singh, M.P.; Kazemian, M.; Gounari, F.; Khazaie, K. TCF-1 Controls Treg Cell Functions That Regulate Inflammation, CD8+ T Cell Cytotoxicity and Severity of Colon Cancer. Nat. Immunol. 2021, 22, 1152–1162. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Frey, D.M.; Droeser, R.A.; Viehl, C.T.; Zlobec, I.; Lugli, A.; Zingg, U.; Oertli, D.; Kettelhack, C.; Terracciano, L.; Tornillo, L. High Frequency of Tumor-Infiltrating FOXP3(+) Regulatory T Cells Predicts Improved Survival in Mismatch Repair-Proficient Colorectal Cancer Patients. Int. J. Cancer 2010, 126, 2635–2643. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.M.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W. Prognostic Significance of Tumor-Infiltrating T-Lymphocytes in Primary and Metastatic Lesions of Advanced Stage Ovarian Cancer. Cancer Immunol. Immunother. CII 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PloS One 2009, 4, e6412. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-Infiltrating FOXP3+ T Regulatory Cells Show Strong Prognostic Significance in Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Wang, S. Tumor-Infiltrating FoxP3+ Tregs Predict Favorable Outcome in Colorectal Cancer Patients: A Meta-Analysis. Oncotarget 2017, 8, 75361–75371. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T Cell Subpopulations Distinctly Control the Prognosis of Colorectal Cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef]
- Billings, P.; Burakoff, S.; Dorf, M.; Benacerraf, B. Cytotoxic T Lymphocytes Specific for I Region Determinants Do Not Require Interactions with H-2K or D Gene Products. J. Exp. Med. 1977, 145, 1387–1392. [Google Scholar] [CrossRef]
- Soghoian, D.Z.; Jessen, H.; Flanders, M.; Sierra-Davidson, K.; Cutler, S.; Pertel, T.; Ranasinghe, S.; Lindqvist, M.; Davis, I.; Lane, K.; et al. HIV-Specific Cytolytic CD4 T Cell Responses during Acute HIV Infection Predict Disease Outcome. Sci. Transl. Med. 2012, 4, 123ra25. [Google Scholar] [CrossRef]
- Stuller, K.A.; Flaño, E. CD4 T Cells Mediate Killing during Persistent Gammaherpesvirus 68 Infection. J. Virol. 2009. [Google Scholar] [CrossRef]
- van Leeuwen, E.M.M.; Remmerswaal, E.B.M.; Vossen, M.T.M.; Rowshani, A.T.; Wertheim-van Dillen, P.M.E.; van Lier, R.A.W.; ten Berge, I.J.M. Emergence of a CD4+CD28- Granzyme B+, Cytomegalovirus-Specific T Cell Subset after Recovery of Primary Cytomegalovirus Infection. J. Immunol. Baltim. Md 1950 2004, 173, 1834–1841. [Google Scholar] [CrossRef]
- Appay, V.; Zaunders, J.J.; Papagno, L.; Sutton, J.; Jaramillo, A.; Waters, A.; Easterbrook, P.; Grey, P.; Smith, D.; McMichael, A.J.; et al. Characterization of CD4(+) CTLs Ex Vivo. J. Immunol. 2002, 168, 5954–5958. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Yurdaydin, C.; Wiegand, J.; Greten, T.; Ciner, A.; Meyer, M.F.; Heiken, H.; Kuhlmann, B.; Kaiser, T.; Bozkaya, H.; et al. Cytotoxic CD4+ T Cells in Viral Hepatitis. J. Viral Hepat. 2006, 13, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, M.; Ohminami, H.; Arai, J.; Kasahara, Y.; Ishida, Y.; Fujita, S. Granule Exocytosis, and Not the Fas/Fas Ligand System, Is the Main Pathway of Cytotoxicity Mediated by Alloantigen-Specific CD4(+) as Well as CD8(+) Cytotoxic T Lymphocytes in Humans. Blood 2000, 95, 2352–2355. [Google Scholar] [CrossRef] [PubMed]
- Lancki, D.W.; Hsieh, C.S.; Fitch, F.W. Mechanisms of Lysis by Cytotoxic T Lymphocyte Clones. Lytic Activity and Gene Expression in Cloned Antigen-Specific CD4+ and CD8+ T Lymphocytes. J. Immunol. Baltim. Md 1950 1991, 146, 3242–3249. [Google Scholar]
- Brown, D.M. Cytolytic CD4 Cells: Direct Mediators in Infectious Disease and Malignancy. Cell. Immunol. 2010, 262, 89–95. [Google Scholar] [CrossRef]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue Adaptation of Regulatory and Intraepithelial CD4+ T Cells Controls Gut Inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef]
- Oh, D.Y.; Fong, L. Cytotoxic CD4+ T Cells in Cancer: Expanding the Immune Effector Toolbox. Immunity 2021, 54, 2701–2711. [Google Scholar] [CrossRef]
- Hirschhorn-Cymerman, D.; Budhu, S.; Kitano, S.; Liu, C.; Zhao, F.; Zhong, H.; Lesokhin, A.M.; Avogadri-Connors, F.; Yuan, J.; Li, Y.; et al. Induction of Tumoricidal Function in CD4+ T Cells Is Associated with Concomitant Memory and Terminally Differentiated Phenotype. J. Exp. Med. 2012, 209, 2113–2126. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global Characterization of T Cells in Non-Small-Cell Lung Cancer by Single-Cell Sequencing. Nat. Med. 2018, 24, 978–985. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary Lymphoid Structures Improve Immunotherapy and Survival in Melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ Follicular Helper T Cell Infiltration Predicts Breast Cancer Survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.; Fagerberg, E.; Chen, P.-M.; Connolly, K.A.; Damo, M.; Cheung, J.F.; Mao, T.; Askari, A.S.; Chen, S.; et al. Neoantigen-Driven B Cell and CD4 T Follicular Helper Cell Collaboration Promotes Anti-Tumor CD8 T Cell Responses. Cell 2021, 184, 6101–6118.e13. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer Immunotherapy: Moving beyond Current Vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Melief, C.J.M.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic Cancer Vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef]
- Ahrends, T.; Spanjaard, A.; Pilzecker, B.; Bąbała, N.; Bovens, A.; Xiao, Y.; Jacobs, H.; Borst, J. CD4+ T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 2017, 47, 848–861.e5. [Google Scholar] [CrossRef]
- Ahrends, T.; Busselaar, J.; Severson, T.M.; Bąbała, N.; de Vries, E.; Bovens, A.; Wessels, L.; van Leeuwen, F.; Borst, J. CD4+ T Cell Help Creates Memory CD8+ T Cells with Innate and Help-Independent Recall Capacities. Nat. Commun. 2019, 10, 5531. [Google Scholar] [CrossRef]
- Ahrends, T.; Bąbała, N.; Xiao, Y.; Yagita, H.; van Eenennaam, H.; Borst, J. CD27 Agonism Plus PD-1 Blockade Recapitulates CD4+ T-Cell Help in Therapeutic Anticancer Vaccination. Cancer Res. 2016, 76, 2921–2931. [Google Scholar] [CrossRef]
- Melssen, M.; Slingluff, C.L. Vaccines Targeting Helper T Cells for Cancer Immunotherapy. Curr. Opin. Immunol. 2017, 47, 85–92. [Google Scholar] [CrossRef]
- Koski, G.K.; Koldovsky, U.; Xu, S.; Mick, R.; Sharma, A.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. A Novel Dendritic Cell-Based Immunization Approach for the Induction of Durable Th1-Polarized Anti-HER-2/Neu Responses in Women with Early Breast Cancer. J. Immunother. 2012, 35, 54–65. [Google Scholar] [CrossRef]
- Sharma, A.; Koldovsky, U.; Xu, S.; Mick, R.; Roses, R.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. HER-2 Pulsed Dendritic Cell Vaccine Can Eliminate HER-2 Expression and Impact Ductal Carcinoma in Situ. Cancer 2012, 118, 4354–4362. [Google Scholar] [CrossRef]
- Lowenfeld, L.; Mick, R.; Datta, J.; Xu, S.; Fitzpatrick, E.; Fisher, C.S.; Fox, K.R.; DeMichele, A.; Zhang, P.J.; Weinstein, S.P.; et al. Dendritic Cell Vaccination Enhances Immune Responses and Induces Regression of HER2pos DCIS Independent of Route: Results of Randomized Selection Design Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2961–2971. [Google Scholar] [CrossRef]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Lowik, M.J.G.; Berends-van der Meer, D.M.A.; Vloon, A.P.G.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 Oncoproteins for Vulvar Intraepithelial Neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.E.; Welters, M.J.P.; Vermeij, R.; Stynenbosch, L.F.M.; Loof, N.M.; Berends-van der Meer, D.M.A.; Löwik, M.J.G.; Hamming, I.L.E.; van Esch, E.M.G.; Hellebrekers, B.W.J.; et al. Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2342–2350. [Google Scholar] [CrossRef]
- Brunsvig, P.F.; Aamdal, S.; Gjertsen, M.K.; Kvalheim, G.; Markowski-Grimsrud, C.J.; Sve, I.; Dyrhaug, M.; Trachsel, S.; Møller, M.; Eriksen, J.A.; et al. Telomerase Peptide Vaccination: A Phase I/II Study in Patients with Non-Small Cell Lung Cancer. Cancer Immunol. Immunother. CII 2006, 55, 1553–1564. [Google Scholar] [CrossRef]
- Brunsvig, P.F.; Kyte, J.A.; Kersten, C.; Sundstrøm, S.; Møller, M.; Nyakas, M.; Hansen, G.L.; Gaudernack, G.; Aamdal, S. Telomerase Peptide Vaccination in NSCLC: A Phase II Trial in Stage III Patients Vaccinated after Chemoradiotherapy and an 8-Year Update on a Phase I/II Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6847–6857. [Google Scholar] [CrossRef]
- Fenoglio, D.; Traverso, P.; Parodi, A.; Tomasello, L.; Negrini, S.; Kalli, F.; Battaglia, F.; Ferrera, F.; Sciallero, S.; Murdaca, G.; et al. A Multi-Peptide, Dual-Adjuvant Telomerase Vaccine (GX301) Is Highly Immunogenic in Patients with Prostate and Renal Cancer. Cancer Immunol. Immunother. CII 2013, 62, 1041–1052. [Google Scholar] [CrossRef]
- Staff, C.; Mozaffari, F.; Frödin, J.-E.; Mellstedt, H.; Liljefors, M. Telomerase (GV1001) Vaccination Together with Gemcitabine in Advanced Pancreatic Cancer Patients. Int. J. Oncol. 2014, 45, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Dosset, M.; Godet, Y.; Vauchy, C.; Beziaud, L.; Lone, Y.C.; Sedlik, C.; Liard, C.; Levionnois, E.; Clerc, B.; Sandoval, F.; et al. Universal Cancer Peptide-Based Therapeutic Vaccine Breaks Tolerance against Telomerase and Eradicates Established Tumor. Clin. Cancer Res. 2012, 18, 6284–6295. [Google Scholar] [CrossRef] [PubMed]
- Adotévi, O.; Dosset, M.; Galaine, J.; Beziaud, L.; Godet, Y.; Borg, C. Targeting Antitumor CD4 Helper T Cells with Universal Tumor-Reactive Helper Peptides Derived from Telomerase for Cancer Vaccine. Hum. Vaccines Immunother. 2013, 9, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Galaine, J.; Kellermann, G.; Guillaume, Y.; Boidot, R.; Picard, E.; Loyon, R.; Queiroz, L.; Boullerot, L.; Beziaud, L.; Jary, M.; et al. Heparan Sulfate Proteoglycans Promote Telomerase Internalization and MHC Class II Presentation on Dendritic Cells. J. Immunol. Baltim. Md 1950 2016, 197, 1597–1608. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Dosset, M.; Castro, A.; Carter, H.; Zanetti, M. Telomerase and CD4 T Cell Immunity in Cancer. Cancers 2020, 12, E1687. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-Small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA Mutanome Vaccines Mobilize Poly-Specific Therapeutic Immunity against Cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer Immunotherapy. A Dendritic Cell Vaccine Increases the Breadth and Diversity of Melanoma Neoantigen-Specific T Cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen Vaccine Generates Intratumoral T Cell Responses in Phase Ib Glioblastoma Trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Racle, J.; Michaux, J.; Rockinger, G.A.; Arnaud, M.; Bobisse, S.; Chong, C.; Guillaume, P.; Coukos, G.; Harari, A.; Jandus, C.; et al. Robust Prediction of HLA Class II Epitopes by Deep Motif Deconvolution of Immunopeptidomes. Nat. Biotechnol. 2019, 37, 1283–1286. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A Cancer Entity with a Shared Sensitivity to the PD-1/PD-L1 Pathway Blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-Mediated Inhibition of Metastases after Treatment with Local Radiation and CTLA-4 Blockade in a Mouse Model of Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Paradis, T.J.; Floyd, E.; Burkwit, J.; Cole, S.H.; Brunson, B.; Elliott, E.; Gilman, S.; Gladue, R.P. The Anti-Tumor Activity of Anti-CTLA-4 Is Mediated through Its Induction of IFN Gamma. Cancer Immunol. Immunother. CII 2001, 50, 125–133. [Google Scholar] [CrossRef]
- Shrikant, P.; Khoruts, A.; Mescher, M.F. CTLA-4 Blockade Reverses CD8+ T Cell Tolerance to Tumor by a CD4+ T Cell- and IL-2-Dependent Mechanism. Immunity 1999, 11, 483–493. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-Dependent Depletion of Tumor-Infiltrating Regulatory T Cells Co-Defines the Efficacy of Anti-CTLA-4 Therapy against Melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti–CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers—Response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3469–3470. [Google Scholar] [CrossRef]
- Wei, S.C.; Anang, N.-A.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination Anti–CTLA-4 plus Anti–PD-1 Checkpoint Blockade Utilizes Cellular Mechanisms Partially Distinct from Monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709. [Google Scholar] [CrossRef]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 2019, 179, 1177–1190.e13. [Google Scholar] [CrossRef]
- Moreno, B.H.; Zaretsky, J.M.; Garcia-Diaz, A.; Tsoi, J.; Parisi, G.; Robert, L.; Meeth, K.; Ndoye, A.; Bosenberg, M.; Weeraratna, A.T.; et al. Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells. Cancer Immunol. Res. 2016, 4, 845–857. [Google Scholar] [CrossRef]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional Systemic CD4 Immunity Is Required for Clinical Responses to PD-L1/PD-1 Blockade Therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef]
- Arakawa, A.; Vollmer, S.; Tietze, J.; Galinski, A.; Heppt, M.V.; Bürdek, M.; Berking, C.; Prinz, J.C. Clonality of CD4+ Blood T Cells Predicts Longer Survival With CTLA4 or PD-1 Checkpoint Inhibition in Advanced Melanoma. Front. Immunol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502.e15. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, G.J.; Havel, L.S.; Crowley, M.J.; Ban, Y.; Lee, S.B.; Thalappillil, J.S.; Narula, N.; Bhinder, B.; Elemento, O.; Wong, S.T.; et al. Immune Reprogramming via PD-1 Inhibition Enhances Early-Stage Lung Cancer Survival. JCI Insight 2018, 3, 6839. [Google Scholar] [CrossRef] [PubMed]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-Cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef]
- Brown, D.M.; Lampe, A.T.; Workman, A.M. The Differentiation and Protective Function of Cytolytic CD4 T Cells in Influenza Infection. Front. Immunol. 2016, 7, 93. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Perdomo-Celis, F.; Acevedo-Saenz, L.; Rugeles, M.T.; Velilla, P.A. Cytotoxic CD4+ T-Cells during HIV Infection: Targets or Weapons? J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 119, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual Expression of the Transcription Factors Runx3 and ThPOK Regulates Intestinal CD4+ T Cell Immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, J.; Togashi, Y.; Sugawara, T.; Itami, M.; Yamauchi, N.; Yuda, J.; Sugano, M.; Ohara, Y.; Minami, Y.; Nakamae, H.; et al. The Critical Role of CD4+ T Cells in PD-1 Blockade against MHC-II-Expressing Tumors Such as Classic Hodgkin Lymphoma. Blood Adv. 2020, 4, 4069–4082. [Google Scholar] [CrossRef]
- Dudley, M.E.; Yang, J.C.; Sherry, R.; Hughes, M.S.; Royal, R.; Kammula, U.; Robbins, P.F.; Huang, J.; Citrin, D.E.; Leitman, S.F.; et al. Adoptive Cell Therapy for Patients with Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5233–5239. [Google Scholar] [CrossRef]
- Verdegaal, E.M.E.; de Miranda, N.F.C.C.; Visser, M.; Harryvan, T.; van Buuren, M.M.; Andersen, R.S.; Hadrup, S.R.; van der Minne, C.E.; Schotte, R.; Spits, H.; et al. Neoantigen Landscape Dynamics during Human Melanoma-T Cell Interactions. Nature 2016, 536, 91–95. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.-C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T Cell Help in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T. A Progress Report on the Treatment of 157 Patients with Advanced Cancer Using Lymphokine-Activated Killer Cells and Interleukin-2 or High-Dose Interleukin-2 Alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. A Preliminary Report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef]
- Moeller, M.; Haynes, N.M.; Kershaw, M.H.; Jackson, J.T.; Teng, M.W.L.; Street, S.E.; Cerutti, L.; Jane, S.M.; Trapani, J.A.; Smyth, M.J.; et al. Adoptive Transfer of Gene-Engineered CD4+ Helper T Cells Induces Potent Primary and Secondary Tumor Rejection. Blood 2005, 106, 2995–3003. [Google Scholar] [CrossRef]
- Wang, L.-X.; Shu, S.; Disis, M.L.; Plautz, G.E. Adoptive Transfer of Tumor-Primed, in Vitro-Activated, CD4+ T Effector Cells (TEs) Combined with CD8+ TEs Provides Intratumoral TE Proliferation and Synergistic Antitumor Response. Blood 2007, 109, 4865–4876. [Google Scholar] [CrossRef]
- Hunder, N.N.; Wallen, H.; Cao, J.; Hendricks, D.W.; Reilly, J.Z.; Rodmyre, R.; Jungbluth, A.; Gnjatic, S.; Thompson, J.A.; Yee, C. Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1. N. Engl. J. Med. 2008, 358, 2698–2703. [Google Scholar] [CrossRef]
- Li, K.; Donaldson, B.; Young, V.; Ward, V.; Jackson, C.; Baird, M.; Young, S. Adoptive Cell Therapy with CD4+ T Helper 1 Cells and CD8+ Cytotoxic T Cells Enhances Complete Rejection of an Established Tumour, Leading to Generation of Endogenous Memory Responses to Non-Targeted Tumour Epitopes. Clin. Transl. Immunol. 2017, 6, e160. [Google Scholar] [CrossRef]
- Veatch, J.R.; Lee, S.M.; Fitzgibbon, M.; Chow, I.-T.; Jesernig, B.; Schmitt, T.; Kong, Y.Y.; Kargl, J.; Houghton, A.M.; Thompson, J.A.; et al. Tumor-Infiltrating BRAFV600E-Specific CD4+ T Cells Correlated with Complete Clinical Response in Melanoma. J. Clin. Investig. 2018, 128, 1563–1568. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Santegoets, S.J.; Sturm, G.; Ehsan, I.; van Egmond, S.L.; Finotello, F.; Trajanoski, Z.; Welters, M.J.P.; van Poelgeest, M.I.E.; van der Burg, S.H. CD39 Identifies the CD4 + Tumor-Specific T-Cell Population in Human Cancer. Cancer Immunol. Res. 2020, 8, 1311–1321. [Google Scholar] [CrossRef]
- Balança, C.-C.; Salvioni, A.; Scarlata, C.-M.; Michelas, M.; Martinez-Gomez, C.; Gomez-Roca, C.; Sarradin, V.; Tosolini, M.; Valle, C.; Pont, F.; et al. PD-1 Blockade Restores Helper Activity of Tumor-Infiltrating, Exhausted PD-1hiCD39+ CD4 T Cells. JCI Insight 2021, 6, 2513. [Google Scholar] [CrossRef]
- Gliwiński, M.; Piotrowska, M.; Iwaszkiewicz-Grześ, D.; Urban-Wójciuk, Z.; Trzonkowski, P. Therapy with CD4+CD25+ T Regulatory Cells – Should We Be Afraid of Cancer? Contemp. Oncol. 2019, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Klatzmann, D.; Abbas, A.K. The Promise of Low-Dose Interleukin-2 Therapy for Autoimmune and Inflammatory Diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Saibil, S.D.; Sotov, V.; Le, M.X.; Khoja, L.; Ghazarian, D.; Bonilla, L.; Majeed, H.; Hogg, D.; Joshua, A.M.; et al. Phase II Clinical Trial of Adoptive Cell Therapy for Patients with Metastatic Melanoma with Autologous Tumor-Infiltrating Lymphocytes and Low-Dose Interleukin-2. Cancer Immunol. Immunother. CII 2019, 68, 773–785. [Google Scholar] [CrossRef]
- Caserta, S.; Alessi, P.; Basso, V.; Mondino, A. IL-7 Is Superior to IL-2 for Ex Vivo Expansion of Tumour-Specific CD4(+) T Cells. Eur. J. Immunol. 2010, 40, 470–479. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Sportès, C.; Ahmadzadeh, M.; Fry, T.J.; Ngo, L.T.; Schwarz, S.L.; Stetler-Stevenson, M.; Morton, K.E.; Mavroukakis, S.A.; Morre, M.; et al. IL-7 Administration to Humans Leads to Expansion of CD8+ and CD4+ Cells but a Relative Decrease of CD4+ T-Regulatory Cells. J. Immunother. Hagerstown Md 1997 2006, 29, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-C.; Habtetsion, T.; Cao, Y.; Li, T.; Liu, C.; Kuczma, M.; Chen, T.; Hao, Z.; Bryan, L.; Munn, D.H.; et al. Adjuvant IL-7 Potentiates Adoptive T Cell Therapy by Amplifying and Sustaining Polyfunctional Antitumor CD4+ T Cells. Sci. Rep. 2017, 7, 12168. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer Regression and Autoimmunity in Patients after Clonal Repopulation with Antitumor Lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Rossi, J.; Paczkowski, P.; Shen, Y.-W.; Morse, K.; Flynn, B.; Kaiser, A.; Ng, C.; Gallatin, K.; Cain, T.; Fan, R.; et al. Preinfusion Polyfunctional Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated with Clinical Outcomes in NHL. Blood 2018, 132, 804–814. [Google Scholar] [CrossRef]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical Development of CAR T Cells-Challenges and Opportunities in Translating Innovative Treatment Concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric Antigen Receptor-Modified T Cells Derived from Defined CD8+ and CD4+ Subsets Confer Superior Antitumor Reactivity in Vivo. Leukemia 2016, 30, 492–500. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T Cells of Defined CD4+:CD8+ Composition in Adult B Cell ALL Patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Abramson, J.S.; Gordon, L.I.; Palomba, M.L.; Lunning, M.A.; Arnason, J.E.; Forero-Torres, A.; Wang, M.; Maloney, D.G.; Sehgal, A.; Andreadis, C.; et al. Updated Safety and Long Term Clinical Outcomes in TRANSCEND NHL 001, Pivotal Trial of Lisocabtagene Maraleucel (JCAR017) in R/R Aggressive NHL. J. Clin. Oncol. 2018, 36, 7505. [Google Scholar] [CrossRef]
- Boulch, M.; Cazaux, M.; Loe-Mie, Y.; Thibaut, R.; Corre, B.; Lemaître, F.; Grandjean, C.L.; Garcia, Z.; Bousso, P. A Cross-Talk between CAR T Cell Subsets and the Tumor Microenvironment Is Essential for Sustained Cytotoxic Activity. Sci. Immunol. 2021, 6, 4344. [Google Scholar] [CrossRef]
- Yang, Y.; Kohler, M.E.; Chien, C.D.; Sauter, C.T.; Jacoby, E.; Yan, C.; Hu, Y.; Wanhainen, K.; Qin, H.; Fry, T.J. TCR Engagement Negatively Affects CD8 but Not CD4 CAR T Cell Expansion and Leukemic Clearance. Sci. Transl. Med. 2017, 9, 1209. [Google Scholar] [CrossRef]
- Agarwal, S.; Hanauer, J.D.S.; Frank, A.M.; Riechert, V.; Thalheimer, F.B.; Buchholz, C.J. In Vivo Generation of CAR T Cells Selectively in Human CD4+ Lymphocytes. Mol. Ther. 2020, 28, 1783–1794. [Google Scholar] [CrossRef]
- Wang, D.; Aguilar, B.; Starr, R.; Alizadeh, D.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; Forman, S.J.; Brown, C.E. Glioblastoma-Targeted CD4+ CAR T Cells Mediate Superior Antitumor Activity. JCI Insight 2018, 3, e99048. [Google Scholar] [CrossRef]
- Rath, J.A.; Bajwa, G.; Carreres, B.; Hoyer, E.; Gruber, I.; Martínez-Paniagua, M.A.; Yu, Y.-R.; Nouraee, N.; Sadeghi, F.; Wu, M.; et al. Single-Cell Transcriptomics Identifies Multiple Pathways Underlying Antitumor Function of TCR- and CD8αβ-Engineered Human CD4+ T Cells. Sci. Adv. 2020, 6, eaaz7809. [Google Scholar] [CrossRef]
- Bailey, S.R.; Nelson, M.H.; Majchrzak, K.; Bowers, J.S.; Wyatt, M.M.; Smith, A.S.; Neal, L.R.; Shirai, K.; Carpenito, C.; June, C.H.; et al. Human CD26high T Cells Elicit Tumor Immunity against Multiple Malignancies via Enhanced Migration and Persistence. Nat. Commun. 2017, 8, 1961. [Google Scholar] [CrossRef]
- Nelson, M.H.; Knochelmann, H.M.; Bailey, S.R.; Huff, L.W.; Bowers, J.S.; Majchrzak-Kuligowska, K.; Wyatt, M.M.; Rubinstein, M.P.; Mehrotra, S.; Nishimura, M.I.; et al. Identification of Human CD4+ T Cell Populations with Distinct Antitumor Activity. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Gacerez, A.T.; Sentman, C.L. T-Bet Promotes Potent Antitumor Activity of CD4+ CAR T Cells. Cancer Gene Ther. 2018, 25, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. IL-12 Secreting Tumor-Targeted Chimeric Antigen Receptor T Cells Eradicate Ovarian Tumors in Vivo. Oncoimmunology 2015, 4, e994446. [Google Scholar] [CrossRef] [PubMed]
- Yeku, O.O.; Purdon, T.J.; Koneru, M.; Spriggs, D.; Brentjens, R.J. Armored CAR T Cells Enhance Antitumor Efficacy and Overcome the Tumor Microenvironment. Sci. Rep. 2017, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors That Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017, 21, 3205–3219. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M. Use of CAR-T Cell Therapy, PD-1 Blockade, and Their Combination for the Treatment of Hematological Malignancies. Clin. Immunol. Orlando Fla 2020, 214, 108382. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Good, C.R.; Aznar, M.A.; Kuramitsu, S.; Samareh, P.; Agarwal, S.; Donahue, G.; Ishiyama, K.; Wellhausen, N.; Rennels, A.K.; Ma, Y.; et al. An NK-like CAR T Cell Transition in CAR T Cell Dysfunction. Cell 2021, 184, 6081–6100.e26. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric Antigen Receptor T-Cell Therapy — Assessment and Management of Toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.-K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef]
- Arnaud, M.; Chiffelle, J.; Genolet, R.; Navarro Rodrigo, B.; Perez, M.A.S.; Huber, F.; Magnin, M.; Nguyen-Ngoc, T.; Guillaume, P.; Baumgaertner, P.; et al. Sensitive Identification of Neoantigens and Cognate TCRs in Human Solid Tumors. Nat. Biotechnol. 2021, 1–5. [Google Scholar] [CrossRef]
- Garnett, C.T.; Palena, C.; Chakraborty, M.; Chakarborty, M.; Tsang, K.-Y.; Schlom, J.; Hodge, J.W. Sublethal Irradiation of Human Tumor Cells Modulates Phenotype Resulting in Enhanced Killing by Cytotoxic T Lymphocytes. Cancer Res. 2004, 64, 7985–7994. [Google Scholar] [CrossRef]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte Composition of Human Breast Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef]
- Matar, P.; Rozados, V.R.; Gervasoni, S.I.; Scharovsky, G.O. Th2/Th1 Switch Induced by a Single Low Dose of Cyclophosphamide in a Rat Metastatic Lymphoma Model. Cancer Immunol. Immunother. CII 2002, 50, 588–596. [Google Scholar] [CrossRef]
- Ding, Z.-C.; Blazar, B.R.; Mellor, A.L.; Munn, D.H.; Zhou, G. Chemotherapy Rescues Tumor-Driven Aberrant CD4+ T-Cell Differentiation and Restores an Activated Polyfunctional Helper Phenotype. Blood 2010, 115, 2397–2406. [Google Scholar] [CrossRef]
- Godet, Y.; Fabre, E.; Dosset, M.; Lamuraglia, M.; Levionnois, E.; Ravel, P.; Benhamouda, N.; Cazes, A.; Le Pimpec-Barthes, F.; Gaugler, B.; et al. Analysis of Spontaneous Tumor-Specific CD4 T-Cell Immunity in Lung Cancer Using Promiscuous HLA-DR Telomerase-Derived Epitopes: Potential Synergistic Effect with Chemotherapy Response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Godet, Y.; Dosset, M.; Borg, C.; Adotevi, O. Is Preexisting Antitumor CD4 T Cell Response Indispensable for the Chemotherapy Induced Immune Regression of Cancer? Oncoimmunology 2012, 1, 1617–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galaine, J.; Turco, C.; Vauchy, C.; Royer, B.; Mercier-Letondal, P.; Queiroz, L.; Loyon, R.; Mouget, V.; Boidot, R.; Laheurte, C.; et al. CD4 T Cells Target Colorectal Cancer Antigens Upregulated by Oxaliplatin. Int. J. Cancer 2019, 145, 3112–3125. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Lauret Marie Joseph, E.; Kirilovsky, A.; Lecoester, B.; El Sissy, C.; Boullerot, L.; Rangan, L.; Marguier, A.; Tochet, F.; Dosset, M.; Boustani, J.; et al. Chemoradiation Triggers Antitumor Th1 and Tissue Resident Memory-Polarized Immune Responses to Improve Immune Checkpoint Inhibitors Therapy. J. Immunother. Cancer 2021, 9, e002256. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, G.; Fridman, M. Abscopal Effect of Radiation in Papillary Adenocarcinoma. Br. J. Radiol. 1973, 46, 220–222. [Google Scholar] [CrossRef]
- Wersäll, P.J.; Blomgren, H.; Pisa, P.; Lax, I.; Kälkner, K.-M.; Svedman, C. Regression of Non-Irradiated Metastases after Extracranial Stereotactic Radiotherapy in Metastatic Renal Cell Carcinoma. Acta Oncol. 2006, 45, 493–497. [Google Scholar] [CrossRef]
- Milhem, C.; Moralès, O.; Ingelaere, C.; Pasquier, D.; Mordon, S.; Mortier, L.; Mirabel, X.; Delhem, N. Combination of High Dose Hypofractionated Radiotherapy with Anti-PD1 Single Dose Immunotherapy Leads to a Th1 Immune Activation Resulting in a Complete Clinical Response in a Melanoma Patient. Int. J. Mol. Sci. 2020, 21, E6772. [Google Scholar] [CrossRef]
- Seung, S.K.; Curti, B.D.; Crittenden, M.; Walker, E.; Coffey, T.; Siebert, J.C.; Miller, W.; Payne, R.; Glenn, L.; Bageac, A.; et al. Phase 1 Study of Stereotactic Body Radiotherapy and Interleukin-2--Tumor and Immunological Responses. Sci. Transl. Med. 2012, 4, 137ra74. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy Induces Responses of Lung Cancer to CTLA-4 Blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Han, H.; Liu, Z.; Huang, S.; Cao, W.; Liu, B.; Qin, Z.; Guo, S.; Zhang, Z.; et al. Survival Outcomes After Adding Stereotactic Body Radiotherapy to Metastatic Renal Cell Carcinoma Patients Treated With Tyrosine Kinase Inhibitors. Am. J. Clin. Oncol. 2020, 43, 58–63. [Google Scholar] [CrossRef]
- Chow, J.; Hoffend, N.C.; Abrams, S.I.; Schwaab, T.; Singh, A.K.; Muhitch, J.B. Radiation Induces Dynamic Changes to the T Cell Repertoire in Renal Cell Carcinoma Patients. Proc. Natl. Acad. Sci. USA 2020, 117, 23721–23729. [Google Scholar] [CrossRef]
- Ji, D.; Song, C.; Li, Y.; Xia, J.; Wu, Y.; Jia, J.; Cui, X.; Yu, S.; Gu, J. Combination of Radiotherapy and Suppression of Tregs Enhances Abscopal Antitumor Effect and Inhibits Metastasis in Rectal Cancer. J. Immunother. Cancer 2020, 8, e000826. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.-P.; Yamazaki, T.; Zhang, T.; Charpentier, M.; Galluzzi, L.; Dephoure, N.; Clement, C.C.; Santambrogio, L.; Zhou, X.K.; et al. Radiotherapy-Exposed CD8+ and CD4+ Neoantigens Enhance Tumor Control. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Renaude, E.; Kroemer, M.; Borg, C.; Peixoto, P.; Hervouet, E.; Loyon, R.; Adotévi, O. Epigenetic Reprogramming of CD4+ Helper T Cells as a Strategy to Improve Anticancer Immunotherapy. Front. Immunol. 2021, 12, 669992. [Google Scholar] [CrossRef]
- Goswami, S.; Apostolou, I.; Zhang, J.; Skepner, J.; Anandhan, S.; Zhang, X.; Xiong, L.; Trojer, P.; Aparicio, A.; Subudhi, S.K.; et al. Modulation of EZH2 Expression in T Cells Improves Efficacy of Anti-CTLA-4 Therapy. J. Clin. Investig. 2018, 128, 3813–3818. [Google Scholar] [CrossRef]
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Cañadas, I.; Roehle, K.; Heckler, M.; et al. EZH2 Inhibition Activates a DsRNA–STING–Interferon Stress Axis That Potentiates Response to PD-1 Checkpoint Blockade in Prostate Cancer. Nat. Cancer 2021, 2, 444–456. [Google Scholar] [CrossRef]
- Llopiz, D.; Ruiz, M.; Villanueva, L.; Iglesias, T.; Silva, L.; Egea, J.; Lasarte, J.J.; Pivette, P.; Trochon-Joseph, V.; Vasseur, B.; et al. Enhanced Anti-Tumor Efficacy of Checkpoint Inhibitors in Combination with the Histone Deacetylase Inhibitor Belinostat in a Murine Hepatocellular Carcinoma Model. Cancer Immunol. Immunother. CII 2019, 68, 379–393. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Park, S.-M.; Ha, H.C.; Lee, A.R.; Won, H.; Cha, H.; Cho, S.; Cho, J.M. HDAC Inhibitor, CG-745, Enhances the Anti-Cancer Effect of Anti-PD-1 Immune Checkpoint Inhibitor by Modulation of the Immune Microenvironment. J. Cancer 2020, 11, 4059–4072. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T Cell Metabolism Drives Immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kamiński, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine Metabolism Is a Central Determinant of Helper T Cell Lineage Fidelity. Cell 2021, 184, 4186–4202. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, R.; Serganova, I.; Cohen, I.J.; Maeda, M.; Shindo, M.; Senbabaoglu, Y.; Watson, M.J.; Leftin, A.; Maniyar, R.; Verma, S.; et al. CTLA-4 Blockade Drives Loss of Treg Stability in Glycolysis-Low Tumours. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- NewLink Genetics Corporation A Phase 1/2 Study of the Concomitant Administration of Indoximod Plus Immune Checkpoint Inhibitors for Adult Patients With Advanced or Metastatic Melanoma; clinicaltrials.gov. 2020. Available online: https://adisinsight.springer.com/trials/700291079 (accessed on 29 November 2021).
- Incyte Corporation A Phase 3 Randomized, Double-Blind Trial of Pembrolizumab (MK-3475) in Combination With Epacadostat (INCB024360) or Placebo in Participants With Cisplatin-Ineligible Urothelial Carcinoma (KEYNOTE-672/ECHO-307); clinicaltrials.gov. 2020. Available online: https://www.clinicaltrials.gov/ProvidedDocs/74/NCT02752074/Prot_SAP_000.pdf (accessed on 29 November 2021).

| Subsets | Transcription Factors | Secretome | Functions | Impact on Tumor Growth | |
|---|---|---|---|---|---|
| Antitumoral | Protumoral | ||||
| Th1 | T-bet+ | IFNγ,IL-2, TNF | -Stimulate CTL and NK cell functions [16,17,18] -Enhance MHC-I and II expression by tumor cells [19,20] -Promote tumor effector T cell infiltration (CXCL9 and CXCL10 secretion by TAM and CAF) [21,22] | Inhibition | |
| Th2 | GATA3+ IRFA4+ | IL-4, IL-5, IL-9,IL-13 | -Induce M2 macrophage infiltration and cancer eradication [23] -NK stimulation [24,25,26] | -Promote B cell activation and IL-10 production [27,28] | Promotion |
| Th9 | IRF4+ PU.1+ | IL-9 | -Promote antigen presentation by DC leading to D8+ T cell activation [29] -Present cytolytic activity [30] | -Promote tumor metastasis [31] | Ambivalent |
| Th17 | IRF4+ RORyt+ | IL-17 IL-21 IL-22 | -Induce apoptosis of cancer cells (IL17 receptor ligation) [32] -Enhance antitumor macrophage polarization and cytotoxic markers expression on NK [33,34] -Recruit and activate T cells, DC and NK cells [33,35] | -Stimulate tumor cell proliferation and cancer invasion and metastasis [36,37] -Promote angiogenesis [38,39] -Recruit MDSC [40] | Ambivalent |
| Treg | FOXP3 | IL10 TGFβ | -Inhibit antitumor immunity through immune suppressive mechanisms [41,42] | Promotion | |
| CD4+CTL | GZM PFN | -Induce tumor cells eradication [12,43,44,45,46,47,48,49] | Inhibition | ||
| GC Tfh cTfh | BCL6+ BCL6- | IL-21 IL-4 IL-21 IL-10 IL-2 | -Interaction with B cells enhance GZMB expression [50] | Inhibition | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Khelil, M.; Godet, Y.; Abdeljaoued, S.; Borg, C.; Adotévi, O.; Loyon, R. Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy. Cancers 2022, 14, 260. https://doi.org/10.3390/cancers14010260
Ben Khelil M, Godet Y, Abdeljaoued S, Borg C, Adotévi O, Loyon R. Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy. Cancers. 2022; 14(1):260. https://doi.org/10.3390/cancers14010260
Chicago/Turabian StyleBen Khelil, Myriam, Yann Godet, Syrine Abdeljaoued, Christophe Borg, Olivier Adotévi, and Romain Loyon. 2022. "Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy" Cancers 14, no. 1: 260. https://doi.org/10.3390/cancers14010260
APA StyleBen Khelil, M., Godet, Y., Abdeljaoued, S., Borg, C., Adotévi, O., & Loyon, R. (2022). Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy. Cancers, 14(1), 260. https://doi.org/10.3390/cancers14010260






