Simple Summary
Prostate cancer is the second leading cause of cancer deaths in men. Attempts to improve patient outcomes include trials of neoadjuvant androgen deprivation therapy for patients with high-risk disease. Neoadjuvant treatment refers to androgen deprivation therapy that is administered prior to surgery (or radiation therapy). Patients typically respond well to this treatment regimen, showing a decrease in tumour size, but a significant proportion of patients eventually relapse and progress to metastatic disease. The mechanisms driving this resistance to neoadjuvant treatment are currently unknown. This review explores theories of resistance broadly, and their possible applications in the prostate cancer setting. Additionally, this review draws comparisons between breakthrough resistance and neoadjuvant resistance, and lastly investigates the current biomarkers for treatment sensitivity.
Abstract
Prostate cancer (PCa) is a hormone driven cancer, characterised by defects in androgen receptor signalling which drive the disease process. As such, androgen targeted therapies have been the mainstay for PCa treatment for over 70 years. High-risk PCa presents unique therapeutic challenges, namely in minimising the primary tumour, and eliminating any undetected micro metastases. Trials of neoadjuvant androgen deprivation therapy aim to address these challenges. Patients typically respond well to neoadjuvant treatment, showing regression of the primary tumour and negative surgical margins at the time of resection, however the majority of patients relapse and progress to metastatic disease. The mechanisms affording this resistance are largely unknown. This commentary attempts to explore theories of resistance more broadly, namely, clonal evolution, cancer stem cells, cell persistence, and drug tolerance. Moreover, it aims to explore the application of these theories in the PCa setting. This commentary also highlights the distinction between castration resistant PCa, and neoadjuvant resistant disease, and identifies the markers and characteristics of neoadjuvant resistant disease presented by current literature.
1. Introduction
Prostate epithelial cells rely on androgens for growth and survival, and the androgen signalling axis plays a pivotal role in the pathogenesis and progression of prostate cancer (PCa) [1,2]. As such, interfering with androgen receptor signalling has been the cornerstone of treatment approaches to advanced disease for over 70 years [1]. Broadly speaking, these androgen deprivation therapies (ADT) disrupt the androgen receptor (AR) signalling axis either by inhibiting androgen biosynthesis, or inhibiting normal AR function.
PCa can be stratified into low, intermediate, and high-risk categories, which are associated with an increasing risk of failure of local treatments, as well as the development of metastases and death. These categories are assigned by considering tumour invasiveness, serum prostate specific antigen (PSA) levels and tumor grade (the Gleason score) [3]. High-risk disease denotes a locally advanced tumour with PSA > 20 ng/mL and a Gleason score of 8–10 [3]. Two therapeutic challenges arise when treating patients with high-risk PCa: firstly, controlling the primary tumour to prevent growth, and secondly, halting the invasion of adjacent tissues and achieving systemic control of micro metastases. This necessitates a multimodal treatment approach and may include neoadjuvant androgen deprivation therapy (nADT), which attempts to address both challenges [4]. nADT refers to administering ADT prior to surgery (or radiation) instead of after [4]. Clinical outcomes differ for nADT given prior to surgery compared to radiation therapy. When used in combination with external beam radiotherapy, ADT (neo-adjuvant and adjuvant) improves long term oncological outcomes in high and intermediate risk disease, which is not to the case with surgery [5]. For the purpose of this commentary, however, nADT refers to ADT given prior to radical prostatectomy, as individual mechanisms of treatment resistance and identification of specific biomarkers of response are harder to deconvolve when two modalities that affect cell survival are given concomitantly. In surgical patients nADT may be used to reduce the size of locally advanced tumours, facilitating surgical resection. Patients undergoing nADT typically show a reduction in tumour size at the time of surgery, but most patients still develop resistance and the PCa progresses to metastatic disease. Presently, nADT is only administered within the context of clinical trials as this treatment regimen has been unable to produce significant improvements in overall survival (OS) [4].
Understanding how PCa develops resistance to nADT necessitates an appreciation of resistance more broadly. There are a number of theories concerning tumorigenesis and treatment resistance (Figure 1).
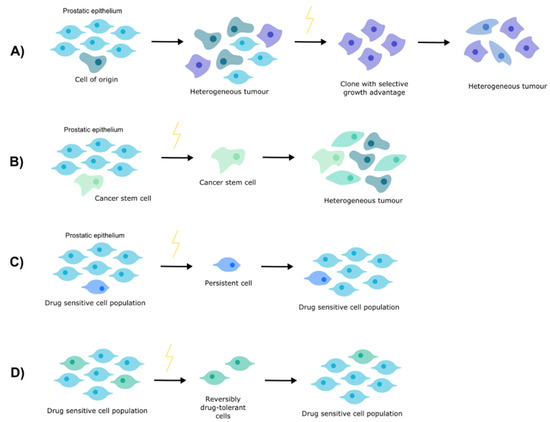
Figure 1.
Theories of resistance. (A) Clonal evolution. (B) Cancer stem cells. (C) Cell persistence. (D) Drug tolerance.
2. Clonal Evolution
In the 1970s, Nowell proposed a model for tumour evolution, whereby a single cell gives rise to mutant clones that expand and mutate in parallel. This process is termed clonal evolution [6].
Nowell posited that neoplasia begins with the expansion of a single cell. This ‘cell of origin’ has escaped normal growth controls and possesses a selective growth advantage over the parent cell population. The genetic instability of this cell predisposes it to producing mutant daughter cells upon replication. If a daughter cell acquires an additional selective growth advantage it will persist and give rise to a subpopulation within the tumour [6]. If different cells display similar growth advantages simultaneously, multiple subpopulations can coexist [7]. The result is a heterogeneous tumour, constantly evolving and consisting of multiple different sub clonal populations at any given point in time [8].
The dynamics of clonal evolution are determined by the mutation and expansion rates of different clonal subpopulations. Mutation rates are variable across distinct genomic regions, mutation types [9], and for epigenetic changes [7]. In response to environmental pressures, natural selection acts on tumours, positively selecting for specific subclones that express favourable genetic or epigenetic changes [6,8,10].
Pharmacological intervention is an example of environmental pressure. Whilst these interventions might eliminate certain clonal subpopulations, they could also serve as positive selective pressures for inherently resistant clones [8]. For instance, clones that possess mutated drug targets or constitutive signalling have a growth advantage over other subpopulations and will thrive in the presence of treatment. An example which highlights the implications of clonal evolution for treatment outcomes is PCa, where clones harbouring mutations in the AR or components of the AR signalling axis are able to resist ADT [11].
3. Cancer Stem Cells
A second model for tumorigenesis, the cancer stem cell (CSC) model, postulates that tumours consist of a subset of cells known as CSCs which are the driving force for tumour development, progression, and resistance [12]. CSCs also supposedly contribute to tumour heterogeneity as they can self-renew and differentiate, giving rise to diverse tumour cell populations, some of which may metastasise or develop resistance [13].
CSCs share similarities with normal stem cells, namely, multipotency and their ability to self-renew [14,15]. They are a dynamic cell type, with variations in CSC phenotype occurring across different cancer subtypes and even within the same cancer over the life of the tumour [16,17]. There are also differences between normal stem cells and CSCs. Whilst growth and differentiation are strictly regulated in normal stem cells, mutations cause CSCs to exhibit uncontrolled growth and differentiation [13,18]. Unlike normal stem cells, CSCs are also tumorigenic, meaning they can form tumours when transplanted into animals [19].
CSCs often have a number of characteristics that afford resistance to treatment, including acquired proliferative dormancy, drug efflux mechanisms, enhanced DNA repair, decreased apoptosis, and favourable interactions with their microenvironment [16]. In physiological contexts, stem cells dormancy is necessary for tissue homeostasis; in pathological contexts, dormancy allows CSCs to evade chemotherapeutics that target rapidly proliferating cells [20]. CSCs can express multi drug resistance transporters which efflux cytotoxic drugs [21]. CSCs also repair DNA damage more efficiently than normal cells, affording them resistance to radiation therapy (RT) [22]. Additionally, CSCs upregulate reactive oxygen species (ROS) scavengers, protecting them from ROS-mediated DNA damage, a mechanism by which RT causes cell death [23]. Apoptosis is also altered in CSCs due to dysregulation of key apoptotic pathway proteins [24]. For example, the mitochondrial Bcl-2 family of proteins are upregulated in a number of cancers, including PCa, where it is associated with resistance to the chemotherapeutic, docetaxel [25]. Like normal stem cells, CSCs rely on their microenvironment, the stem cell niche, to retain their self-renewal capabilities and remain undifferentiated [26]. Components of the stem cell niche include stromal cells, inflammatory cells, and blood vessels which interact with and support CSCs in maintaining their phenotype, enabling the CSCs to enact resistance to treatment and support tumour survival [27].
4. Cell Persistence
In both cancer and infectious disease management, analogous challenges are faced, namely in identifying the mechanisms by which cells persist after treatment and developing therapeutics that can eliminate these persistent cells. Parallels can be drawn from microbiology research to facilitate understanding of cancer cell resistance.
In the context of infectious disease, ‘persister cells’ are cells which evade treatment by antibiotics but are distinct from antibiotic resistant cells. Unlike resistant cells, persister cells lack any drug-resistance mutations [28]. Additionally, persister cells are sensitive to antibiotic treatment, but do not proliferate during treatment. This allows them to resist treatment that targets proliferating cells. Once treatment is withdrawn these cells will spontaneously resume proliferation, giving rise to a population of cells that are equally as sensitive as the original persister to the bactericidal agent [29,30].
The phenomenon of bacterial persistence shares similarities with CSC-mediated relapse. In CSC-mediated resistance, treatment is initially effective and the tumour regresses, but a small population of cancer cells persist and initiate relapse [28]. Like bacterial persisters, these residual cancer cells lack any resistance mutations that could explain their persistence in the presence of treatment. Moreover, when these residual cancer cells are expanded, they give rise to a population of cancer cells that are drug sensitive. It is hypothesized that these persistent cells are CSCs as they express cell-surface antigens consistent with those found on tissue stem cells [28]. If this hypothesis is correct, then it can be assumed that the CSCs employ the various mechanisms outlined earlier such as proliferative dormancy, enhanced DNA repair, and decreased apoptosis to resist treatment.
5. Drug Tolerance
A phenomenon known as re-treatment response is being increasingly observed in cancer [31]. It describes the scenario in which a drug is administered, and a response is observed but is eventually followed by treatment failure. Treatment is then withdrawn and following a ‘drug holiday’ the same drug is re-administered, and the patient displays a second treatment response [32,33]. This suggests that initial resistance to treatment is due to a reversible drug-tolerant state [31].
Sharma et al. investigated this drug-tolerant state in the PC9 pulmonary adenocarcinoma cell line [31]. They found that following high-dose drug treatment a small population of cells that were drug-tolerant persisted. These cells, termed drug-tolerant persisters (DTPs), were non-proliferative and expressed stem-cell markers. In drug treatment conditions approximately 20% of the DTPs underwent a phenotype change, losing their stem-cell markers and resuming normal proliferation. These proliferative cells were termed drug-tolerant expanded persisters (DTEPs). It was also shown that treatment-free passaging of both DTPs and DTEPs resensitised the cells to treatment, highlighting the reversible nature of the drug-tolerant state. They concluded that the in vitro cancer cell population consists of three discrete subpopulations: the parental PC9 cells, DTPs, and DTEPs. These three cell types were genetically identical, yet displayed differing functional phenotypes, suggesting a role of the epigenome in developing and regulating the reversibly drug-tolerant state. Further analyses identified a crucial role of the chromatin state in sustaining drug-tolerant cell populations. KDM5A, a histone demethylase, was recognised as a crucial chromatin-modifying enzyme in the establishment of the drug-tolerant state [31]. A role for KDM5A in drug resistance has also been suggested by others [34,35] and was investigated by Vinogradova et al. who showed that KDM5A messenger ribonucleic acid (mRNA) was increased in drug-tolerant cell models. They also showed that combination therapy with a KDM5A inhibitor (CPI-455) decreased the number of cells that could survive a lethal drug dose in multiple cell culture models [36]. More recently it has been recognized that these epigenetic changes drive loss of epithelial markers with only partial acquisition of mesenchymal markers in drug tolerant cells [37,38]. This drug tolerant plasticity, characterized by an incomplete EMT switch, appears important in acute stress survival, contributing to cell persistence during drug challenges. These findings support the hypothesis that reversible drug tolerance is epigenetically moderated.
The transiently drug-tolerant state of these cells is also analogous to bacterial persister cells. Whilst some studies equate bacterial persister cells with CSCs [28], Sharma et al. contends that bacterial persisters are instead homologous with DTPs, and that DTPs are distinct from CSCs. This is based on the observation that both bacterial persisters and DTPs exhibit phenotypic heterogeneity which is not genetically regulated. When treatment is ceased, both DTPs and bacterial persisters give rise to subpopulations that are as drug-sensitive as the original persistent cells [31]. Sharma et al. also suggests that DTPs are distinct from CSCs as they do not employ drug efflux mechanisms to evade treatment. Presently a clear consensus does not exist, but current literature indicates a likely interconnection between the CSC and the reversibly drug-tolerant cell populations [31,39,40,41].
6. Castration Resistance
Castration resistant prostate cancer (CRPC) refers to cancer which progresses in the androgen-depleted environment created by ADT [42,43]. In the majority of patients, PCa is initially hormone-sensitive and patients respond well to ADT, showing significant tumour regression, a fall in serum prostate specific antigen levels, and improvements in quality of life [44]. However, some cancer cells persist in the androgen-depleted environment, resulting in androgen-independent, or castration-resistant cancer [42,45].
A number of mechanisms of castration resistance have been identified (Table 1), including amplification and/or mutation of the AR, constitutively active AR splice variants, increased intracrine androgen synthesis, altered expression or activity of AR coactivators or corepressors, and increased androgen biosynthesis by conversion of non-testicular androgens in peripheral tissue to potent androgens [46,47]. These mechanisms enable sustained signalling through the AR in the androgen-depleted environment.

Table 1.
Summary of the Unique Mechanisms and Characteristics of Disease.
A second hypothesis detailing the mechanism of CRPC suggests that a subpopulation of neuroendocrine (NE) cells within the cancer cell population act as autocrine-paracrine signallers to encourage a more aggressive cancer phenotype [48,49,50]. These NE cells originally arise from adenocarcinoma and differentiate, producing secretory products which promote tumour cell proliferation, prevent apoptosis, and encourage angiogenesis in the androgen-depleted environment [51]. NE PCa has low or no expression of the AR and androgen-regulated genes (ARGs). This impacts treatment options, specifically the efficacy of ADT [48,51,52]. The NE differentiation is more prominent following ADT [51] and represents a possible mechanism by which previously responsive, hormone-sensitive cancer develops resistance to ADT [45,48,53].
7. Neoadjuvant Androgen Deprivation Therapy Resistance
Patients treated with nADT typically show a reduction in tumour volume and significant decreases in extra-prostatic disease [54,55,56,57] but complete pathological responses are rare and ultimately there is little to no improvement in OS [4]. Clinical trials assessing the effects of nADT have shown that this treatment regimen can produce appreciable improvements in the burden of local disease at the time of surgery. Importantly, ADT has been shown to decrease the rates of extracapsular extension, which describes the presence of neoplastic cells in the periprostatic tissue. ADT also increases negative surgical margins and lowers the frequency of lymph node metastases after radical prostatectomy (RP). Despite these favourable outcomes, trials of nADT have been unable to show a translation of these positive effects to improvements in progression-free survival or OS [54,55,56,57,58,59,60,61,62,63,64,65,66].
Identification of the characteristics of nADT resistant disease, specifically those differentiating it from CRPC (Table 1), are of significance as they serve as potential biomarkers for treatment response.
Wang et al. suggested that a greater insight into predictive morphological parameters and molecular markers of nADT-resistant tumours could aid in identifying subsets of patients likely to develop resistance to nADT. Core needle biopsies revealed that tumour cribriform growth pattern, macro-nucleoli, ductal adenocarcinoma differentiation, and PTEN loss in the untreated tumour were associated with treatment resistance [66]. This suggests that patients possessing these cellular and morphological characteristics may be unsuitable candidates for nADT.
Unlike CRPC, the mechanisms causing patient relapse and driving resistance to treatment in the neoadjuvant setting are yet to be determined, representing a gap in current literature and a possible therapeutic target for combination therapy.
Whilst the mechanisms of nADT resistance are not clearly defined, a number of characteristics of resistant disease have been identified. McKay et al. examined outcomes of a randomized phase II trial of neoadjuvant enzalutamide (a second generation antiandrogen) or leuprolide (a gonadotropin releasing hormone agonist), with or without abiraterone (an inhibitor of androgen biosynthesis). They showed that ERG-positive and PTEN-loss tumours harboured greater residual disease. Interestingly, ERG-positive, and PTEN-loss tumours were associated with reduced AR expression, suggesting androgen-independent survival mechanisms in these tumours. These findings also suggest an inherent insensitivity to ADT which could explain the extensive residual tumours observed in these patients [67].
Sowalsky et al. examined residual PCa foci in RP samples from patients treated with nADT. Transcriptome profiling of residual tumours revealed that AR signalling was reduced but continual—a commonality between nADT-resistant and castration-resistant disease [68]. Whole exome sequencing (WES) revealed a genomic loss in the tumour suppressor gene RB1. Moreover, a significant negative correlation was found between RB1 mRNA levels and proliferation index, which suggests that downregulation of RB1 could be driving proliferation. In cases where multiple tumour foci were micro dissected, WES revealed that the foci shared a common clonal origin; however, each focus had multiple unique oncogenic alterations. This suggests that a subpopulation of cells harbouring oncogenic alterations commonly observed in CRPC were present within the primary tumour, and were positively selected for by nADT [69].
Sowalsky et al. highlighted several distinctions between CRPC and nADT-resistant PCa. RB1 loss is typically associated with NE differentiation in CRPC; however, Sowalsky et al. did not find a significant correlation between RB1 loss and NE markers such as chromogranin A, chromogranin B, or synaptophysin in residual tumours. AR activity in residual tumour foci was not positively correlated with aldo-keto reductase family 1 member c3 expression, an enzyme-encoding gene whose expression drives intratumoral androgen synthesis in CRPC. Additionally, no increase in the expression of AR splice variant AR-V7 was observed in neoadjuvant resistant tumour foci, contrary to CRPC where increased AR-V7 is correlated with resistance to ADT [69,70].
8. Biomarkers of Resistance
For the first time, Cmero et al. [71] identified loss of SNAI2 as a marker for AR sensitivity. Specifically, SNAI2 deficient tumour cells display increased sensitivity to AR signalling inhibition.
Snai2 is a member of the Snail superfamily which are transcription factors implicated in EMT. Snail family members modulate EMT by binding the gene promoters of epithelial factors to repress their expression [72,73]. They also simultaneously promote the expression of mesenchymal factors [74,75] and other EMT-promoting transcription factors [46,75,76]. Upregulation of mesenchymal markers promotes migratory and invasive characteristics in the epithelial cells and causes significant phenotypic alterations [77]. Aberrant expression of members of the Snail superfamily has a myriad of pathological consequences. Their expression can promote changes in cell morphology, loss of normal cell–cell contacts, acquisition of invasive growth properties, and resistance to DNA-damage-indued cell death [78].
In the PCa setting, in vitro studies have shown that Snai2 overexpression is associated with increased proliferation, invasiveness [79,80], and resistance to treatments such as chemotherapy [81,82], radiation therapy [83,84], and tyrosine kinase inhibitors [85,86]. Additionally, Cmero et al. showed that Snai2 expression is upregulated in patients receiving acute ADT [71], and moreover, upregulation of Snai2 is associated with poor prognosis in a number of cancer contexts [87,88,89,90]. Given that Snai2 is important in the cell plasticity associated with ‘drug-tolerant persister’ cells, this suggests that loss of this plasticity renders prostate epithelial cells lethally vulnerable to the stress associated with acute androgen receptor signalling inhibition.
9. Combination Therapy to Overcome Resistance
Drug resistance is a major barrier to cancer treatment. One strategy that has been employed to overcome resistance is combination therapy. Accumulating evidence over the last 5 years indicates that upfront combination therapies in metastatic hormone response prostate cancer delays the onset of castration-resistance and results in greater gains in overall survival compared to traditional treatment sequencing. Agents successfully used in combination include docetaxel, a taxane based chemotherapy which because of its distinct mechanism of cytotoxic action synergises with ADT to maximise cell kill [91,92]. However, similar survival gains have been seen with novel androgen receptor signalling inhibitors such as abiraterone [93] and enzamlutamide [94], presumably as conventional ADT does not result in full AR signalling axis suppression. In the neo-adjvuant setting use of these agents also results in improved response rates [95,96] but whether this translates into improved overall survival is as yet unclear, with a number of phase III studies underway. A complementary approach is to combine ADT with agents that might disrupt pathways that are important in prostate cancer survival. Examples include ADT in combination with IMC-A12 (a monoclonal antibody against IGF-1R, trial ID: NCT00769795); ipatasertib (a small molecule AKT inhibitor; trial ID: NCT04737109), and erdafitinib (a tyrosine kinase inhibitor of FGFR1-4, trial ID: ACTRN12618001061224). The results of these studies are awaited.
10. Conclusions
The molecular mechanisms driving the persistence of prostate tumour cells during acute AR signalling inhibition appear distinct from those responsible for breakthrough, progressive castration resistant disease. Initial evidence suggests that tumour cells with low AR signalling dependence may be inherently resistant, while cells lacking the machinery required for an adaptive acute stress response are intrinsically sensitive. As such an increasing number of molecular markers are associated with treatment response, but these require larger validation studies to assess utility in the clinical setting.
Author Contributions
Conceptualization, N.M.C. and C.M.H.; writing—original draft preparation, M.P.; writing—review and editing, M.P., N.M.C., C.M.H. and B.K.C.; supervision, C.M.H. and N.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
Support was provided through the PRECEPT program grant, co-funded by Movember and the Australian Federal Government (PI NMC). N.M.C. was supported by a Movember – Distinguished Gentleman’s Ride Clinician Scientist Award through the Prostate Cancer Foundation of Australia’s Research Program.
Acknowledgments
N.M.C. was supported by a Movember—Distinguished Gentleman’s Ride Clinician Scientist Award through the Prostate Cancer Foundation of Australia’s Research Program, and more recently by the PRECEPT program is funded by the Prostate Cancer Research Alliance, a joint initiative between Movember and the Australian Government to improve outcomes for men with prostate cancer in the next 5 to 7 years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Lyou, Y.; Dorff, T.B. Hormonal manipulation in androgen signaling: A narrative review on using novel androgen therapy agents to optimize clinical outcomes and minimize side effects for prostate cancer patients. Transl. Androl. Urol. 2021, 10, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer Foundation. Risk Groups. 2020. Available online: https://www.pcf.org/about-prostate-cancer/diagnosis-staging-prostate-cancer/risk-groups/ (accessed on 12 September 2020).
- McKay, R.R.; Choueiri, T.K.; Taplin, M.E. Rationale for and review of neoadjuvant therapy prior to radical prostatectomy for patients with high-risk prostate cancer. Drugs 2013, 73, 1417–1430. [Google Scholar] [CrossRef]
- Ashrafi, A.N.; Yip, W.; Aron, M. Neoadjuvant Therapy in High-Risk Prostate Cancer. Indian J. Urol. 2020, 36, 251–261. [Google Scholar] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hu, Z.; Curtis, C. Big Bang Tumor Growth and Clonal Evolution. Cold Spring Harb. Perspect. Med. 2018, 8, a028381. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Schwartz, M.; Zlotorynski, E.; Kerem, B. The molecular basis of common and rare fragile sites. Cancer Lett. 2006, 232, 13–26. [Google Scholar] [CrossRef]
- Varley, K.E.; Mutch, D.G.; Edmonston, T.B.; Goodfellow, P.J.; Mitra, R.D. Intra-tumor heterogeneity of MLH1 promoter methylation revealed by deep single molecule bisulfite sequencing. Nucleic Acids Res. 2009, 37, 4603–4612. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Dehm, S.M. Clonal origin and spread of metastatic prostate cancer. Endocr. Relat. Cancer 2016, 23, R207–R217. [Google Scholar] [CrossRef]
- Campbell, L.L.; Polyak, K. Breast tumor heterogeneity: Cancer stem cells or clonal evolution? Cell Cycle 2007, 6, 2332–2338. [Google Scholar] [CrossRef]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology 2014, 28, 1101–1107. [Google Scholar]
- Beck, B.; Blanpain, C. Unravelling cancer stem cell potential. Nat. Rev. Cancer 2013, 13, 727–738. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.I. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol. 2018, 53, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer stem cells: An old idea–A paradigm shift. Cancer Res. 2006, 66, 1883–1890. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Kleffel, S.; Schatton, T. Tumor dormancy and cancer stem cells: Two sides of the same coin? In Systems Biology of Tumor Dormancy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 145–179. [Google Scholar]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, I.; Debbage, P.; Kumar, V.; Skvortsov, S. (Eds.) Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Goff, D.J.; Recart, A.C.; Sadarangani, A.; Chun, H.-J.; Barrett, C.L.; Krajewska, M.; Leu, H.; Low-Marchelli, J.; Ma, W.; Shih, A.Y.; et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell 2013, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Domenech, J.; Vidal, S.J.; Rodriguez-Bravo, V.; Castillo-Martin, M.; Quinn, S.A.; Rodriguez-Barrueco, R.; Bonal, D.M.; Charytonowicz, E.; Gladoun, N.; Iglesia-Vicente, J.d.l.; et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch-and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012, 22, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7. [Google Scholar]
- Borovski, T.; De Sousa, E.M.F.; Vermeulen, L.; Medema, J.P. Cancer stem cell niche: The place to be. Cancer Res. 2011, 71, 634–639. [Google Scholar] [CrossRef]
- Glickman, M.S.; Sawyers, C.L. Converting cancer therapies into cures: Lessons from infectious diseases. Cell 2012, 148, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef]
- Kurata, T.; Tamura, K.; Kaneda, H.; Nogami, T.; Uejima, H.; Asai Go, G.; Nakagawa, K.; Fukuoka, M. Effect of re-treatment with gefitinib (‘Iressa’, ZD1839) after acquisition of resistance. Ann. Oncol. 2004, 15, 173–174. [Google Scholar] [CrossRef]
- Yano, S.; Nakataki, E.; Ohtsuka, S.; Inayama, M.; Tomimoto, H.; Edakuni, N.; Kakiuchi, S.; Nishikubo, N.; Muguruma, H.; Sone, S. Retreatment of lung adenocarcinoma patients with gefitinib who had experienced favorable results from their initial treatment with this selective epidermal growth factor receptor inhibitor: A report of three cases. Oncol. Res. 2005, 15, 107–111. [Google Scholar] [CrossRef]
- Roesch, A.; Fukunaga-Kalabis, M.; Schmidt, E.C.; Zabierowski, S.E.; Brafford, P.A.; Vultur, A.; Basu, D.; Gimotty, P.; Vogt, T.; Herlyn, M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 2010, 141, 583–594. [Google Scholar] [CrossRef]
- Roesch, A.; Vultur, A.; Bogeski, I.; Wang, H.; Zimmermann, K.M.; Speicher, D.; Körbel, C.; Laschke, M.W.; Gimotty, P.A.; Philipp, S.E.; et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1Bhigh cells. Cancer Cell 2013, 23, 811–825. [Google Scholar] [CrossRef]
- Vinogradova, M.; Gehling, V.S.; Gustafson, A.; Arora, S.; Tindell, C.A.; Wilson, C.; Williamson, K.E.; Guler, G.D.; Gangurde, P.; Manieri, W.; et al. An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat. Chem. Biol. 2016, 12, 531–538. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R.; Gangurde, P.; et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Kang, S.-K. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007, 16, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, R.H.; Lorenzi, T.; Lorz, A.; Larsen, A.K.; de Almeida, L.N.; Escargueil, A.; Clairambault, E. Emergence of drug tolerance in cancer cell populations: An evolutionary outcome of selection, nongenetic instability, and stress-induced adaptation. Cancer Res. 2015, 75, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, S.; Ng, P.; Bubley, G.J.; Nelson, P.S.; Mostaghel, E.A.; Marck, B.; Matsumoto, A.M.; Simon, N.I.; Wang, H.; et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011, 71, 6503–6513. [Google Scholar] [CrossRef]
- Lou, D.Y.; Fong, L. Neoadjuvant therapy for localized prostate cancer: Examining mechanism of action and efficacy within the tumor. Urol. Oncol. 2016, 34, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Granata, I.; Capaia, M.; Piccirillo, M.; Guarracino, M.R.; Venè, R.; Brizzolara, A.; Petretto, A.; Inglese, E.; Morini, M.; et al. Adaptive phenotype drives resistance to androgen deprivation therapy in prostate cancer. Cell Commun. Signal. 2017, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, P.A. Neuroendocrine cells in tumour growth of the prostate. Endocr. Relat. Cancer 1999, 6, 503–519. [Google Scholar] [CrossRef]
- Miao, L.; Yang, L.; Li, R.; Rodrigues, D.N.; Crespo, M.; Hsieh, J.T.; Tilley, W.D.; Bono, J.d.; Selth, L.A.; Raj, G.A. Disrupting Androgen Receptor Signaling Induces Snail-Mediated Epithelial-Mesenchymal Plasticity in Prostate Cancer. Cancer Res. 2017, 77, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Isaacsson Velho, P.; Fu, W.; Wang, H.; Mirkheshti, N.; Qazi, F.; Lima, F.A.S.; Shaukat, F.; Carducci, M.A.; Denmeade, S.R.; Paller, C.J.; et al. Wnt-pathway Activating Mutations Are Associated with Resistance to First-line Abiraterone and Enzalutamide in Castration-resistant Prostate Cancer. Eur. Urol. 2020, 77, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lucas, J.M.; Lam, H.-M.; Dumpit, R.; Corey, E.; Chéry, L.; et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2015, 21, 4698–4708. [Google Scholar] [CrossRef]
- Aprikian, A.G.; Cordon-Cardo, C.; Fair, W.R.; Reuter, V.E. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 1993, 71, 3952–3965. [Google Scholar] [CrossRef]
- Vashchenko, N.; Abrahamsson, P.A. Neuroendocrine differentiation in prostate cancer: Implications for new treatment modalities. Eur. Urol. 2005, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Amin, M.B.; Beltran, H.; Lotan, T.L.; Mosquera, J.M.; Reuter, V.E.; Robinson, B.D.; Troncoso, P.; Rubin, M.A. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 2014, 38, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Nelson, E.C.; Cambio, A.J.; Yang, J.C.; Ok, J.H.; Lara, P.N., Jr.; Evans, C.P. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic Dis. 2007, 10, 6–14. [Google Scholar] [CrossRef][Green Version]
- Klotz, L.H.; Goldenberg, S.L.; Jewett, M.A.; Fradet, Y.; Nam, R.; Barkin, J.; Chin, J.; Chatterjee, S.; Canadian Uro-Oncology Group. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J. Urol. 2003, 170, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Dalkin, B.L.; Ahmann, F.R.; Nagle, R.; Johnson, C.S. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. J. Urol. 1996, 155, 1357–1360. [Google Scholar] [CrossRef]
- Schulman, C.C.; Debruyne, F.M.; Forster, G.; Selvaggi, F.P.; Zlotta, A.R.; Witjes, W.P. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur. Urol. 2000, 38, 706–713. [Google Scholar] [CrossRef]
- Labrie, F.; Cusan, L.; Gomez, J.L.; Diamond, P.; Suburu, R.; Lemay, M.; Tetu, B.; Fradet, Y.; Bélanger, A.; Candas, B. Neoadjuvant hormonal therapy: The Canadian experience. Urology 1997, 49, 56–64. [Google Scholar] [CrossRef]
- Bandini, M.; Fossati, N.; Gandaglia, G.; Preisser, F.; Dell’Oglio, P.; Zaffuto, E.; Stabile, A.; Gallina, A.; Suardi, N.; Shariat, S.F.; et al. Neoadjuvant and adjuvant treatment in high-risk prostate cancer. Expert Rev. Clin. Pharmacol. 2018, 11, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Aus, G.; Abrahamsson, P.A.; Ahlgren, G.; Hugosson, J.; Lundberg, S.; Schain, M.; Schelin, S.; Pedersen, K. Three-month neoadjuvant hormonal therapy before radical prostatectomy: A 7-year follow-up of a randomized controlled trial. BJU Int. 2002, 90, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Gleave, M.E.; Goldenberg, S.L.; Chin, J.L.; Warner, J.; Saad, F.; Klotz, L.H.; Jewett, M.; Kassabian, V.; Chetner, M.; Dupont, C.; et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. J. Urol. 2001, 166, 500–506. [Google Scholar] [CrossRef]
- Prezioso, D.; Lotti, T.; Polito, M.; Montironi, R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: A randomized study. Urol. Int. 2004, 72, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Selli, C.; Montironi, R.; Bono, A.; Pagano, F.; Zattoni, F.; Manganelli, A.; Selvaggi, F.P.; Comeri, G.; Fiaccavento, G.; Guazzieri, S.; et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J. Clin. Pathol. 2002, 55, 508–513. [Google Scholar] [CrossRef]
- Soloway, M.S.; Pareek, K.; Sharifi, R.; Wajsman, Z.; McLeod, D.; Wood, D.P., Jr.; Puras-Baez, A.; Lupron Depot Neoadjuvant Prostate Cancer Study Group. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J. Urol. 2002, 167, 112–116. [Google Scholar] [CrossRef]
- Van der Kwast, T.H.; Têtu, B.; Candas, B.; Gomez, J.L.; Cusan, L.; Labrie, F. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: Three versus six months of endocrine therapy. Urology 1999, 53, 523–529. [Google Scholar] [CrossRef]
- Yee, D.S.; Lowrance, W.T.; Eastham, J.A.; Maschino, A.C.; Cronin, A.M.; Rabbani, F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010, 105, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, M.; Zhang, J.; Sun, X.; Guo, H.; Pang, Y.; Zhang, Q.; Chen, X.; Zhang, R.; Liu, Z.; et al. Differential response to neoadjuvant hormonal therapy in prostate cancer: Predictive morphological parameters and molecular markers. Prostate 2019, 79, 709–719. [Google Scholar] [CrossRef]
- McKay, R.R.; Ye, H.; Xie, W.; Lis, R.; Calagua, C.; Zhang, Z.; Trinh, Q.-D.; Chang, S.L.; Harshman, L.C.; Ross, A.E.; et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide with or Without Abiraterone. J. Clin. Oncol. 2019, 37, 923–931. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Werner, L.; Mostaghel, E.A.; Lis, R.; Voznesensky, O.; Zhang, Z.; Marck, B.T.; Matsumoto, A.M.; Domachevsky, L.; Zukotynski, K.A.; et al. A Phase II Trial of Abiraterone Combined with Dutasteride for Men with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2017, 23, 935–945. [Google Scholar] [CrossRef]
- Sowalsky, A.G.; Ye, H.; Bhasin, M.; Van Allen, E.M.; Loda, M.; Lis, R.T.; Montaser-Kouhsari, L.; Calagua, C.; Ma, F.; Russo, J.W.; et al. Neoadjuvant-Intensive Androgen Deprivation Therapy Selects for Prostate Tumor Foci with Diverse Subclonal Oncogenic Alterations. Cancer Res. 2018, 78, 4716–4730. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Cmero, M.; Kurganovs, N.J.; Stuchbery, R.; McCoy, P.; Grima, C.; Ngyuen, A.; Chow, K.; Mangiola, S.; Macintyre, G.; Howard, N.; et al. Loss of SNAI2 in Prostate Cancer Correlates with Clinical Response to Androgen Deprivation Therapy. JCO Precis. Oncol. 2021, 5, 1048–1059. [Google Scholar] [CrossRef]
- Deep, G.; Jain, A.K.; Ramteke, A.; Ting, H.; Vijendra, K.C.; Gangar, S.C.; Agarwal, C.; Agarwal, R. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol. Cancer 2014, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.L.; Henderson, V.; Smith, B.N.; McKeithen, D.; Graham, T.; Vo, B.T.; Odero-Marah, V.A. Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells. BMC Cancer 2012, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Smith, B.N.; Odero-Marah, V.A. The role of Snail in prostate cancer. Cell Adh. Migr. 2012, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Takkunen, M.; Grenman, R.; Hukkanen, M.; Korhonen, M.; García de Herreros, A.; Virtanen, I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J. Histochem. Cytochem. 2006, 54, 1263–1275. [Google Scholar] [CrossRef]
- Vega, S.; Morales, A.V.; Ocaña, O.H.; Valdés, F.; Fabregat, I.; Nieto, M.A. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004, 18, 1131–1143. [Google Scholar] [CrossRef]
- Kajita, M.; McClinic, K.N.; Wade, P.A. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol. Cell. Biol. 2004, 24, 7559–7566. [Google Scholar] [CrossRef]
- Esposito, S.; Russo, M.V.; Airoldi, I.; Tupone, M.G.; Sorrentino, C.; Barbarito, G.; Meo, S.D.; Carlo, E.D. SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression. Oncotarget 2015, 6, 17121–17134. [Google Scholar] [CrossRef] [PubMed]
- Emadi Baygi, M.; Soheili, Z.S.; Essmann, F.; Deezagi, A.; Engers, R.; Goering, W.; Schulz, W.A. Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol. 2010, 31, 297–307. [Google Scholar] [CrossRef]
- Kurrey, N.K.; Jalgaonkar, S.P.; Joglekar, A.V.; Ghanate, A.D.; Chaskar, P.D.; Doiphode, R.Y.; Bapat, S.A. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009, 27, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Haslehurst, A.M.; Koti, M.; Dharsee, M.; Nuin, P.; Evans, K.; Geraci, J.; Childs, T.; Chen, J.; Li, J.; Weberpals, J.; et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 2012, 12, 91. [Google Scholar] [CrossRef]
- Wu, W.S.; Heinrichs, S.; Xu, D.; Garrison, S.P.; Zambetti, G.P.; Adams, J.M.; Look, A.T. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005, 123, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhou, L.; Wei, C.; Zhao, W.; Yu, D. Slug inhibition increases radiosensitivity of oral squamous cell carcinoma cells by upregulating PUMA. Int. J. Oncol. 2016, 49, 709–719. [Google Scholar] [CrossRef]
- Vitali, R.; Mancini, C.; Cesi, V.; Tanno, B.; Mancuso, M.; Bossi, G.; Zhang, Y.; Martinez, R.V.; Calabretta, B.; Dominici, C.; et al. Slug (SNAI2) down-regulation by RNA interference facilitates apoptosis and inhibits invasive growth in neuroblastoma preclinical models. Clin. Cancer Res. 2008, 14, 4622–4630. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Tsai, M.F.; Su, K.Y.; Wu, S.G.; Huang, C.P.; Yu, S.L.; Yu, Y.-L.; Lan, C.C.; Yang, C.-H.; Lin, S.-B.; et al. Slug confers resistance to the epidermal growth factor receptor tyrosine kinase inhibitor. Am. J. Respir. Crit. Care Med. 2011, 183, 1071–1079. [Google Scholar] [CrossRef]
- Yang, H.W.; Menon, L.G.; Black, P.M.; Carroll, R.S.; Johnson, M.D. SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer 2010, 10, 301. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Appaiah, H.; Ballas, C.; Pick-Franke, P.; Goulet, R., Jr.; Badve, S.; Srour, E.F.; Nakshatri, H. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype. BMC Cancer 2010, 10, 411. [Google Scholar] [CrossRef]
- Shih, J.-Y.; Tsai, M.-F.; Chang, T.-H.; Chang, Y.-L.; Yuan, A.; Yu, C.-J.; Lin, S.-B.; Liou, G.-Y.; Lee, M.-L.; Chen, J.J.W.; et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin. Cancer Res. 2005, 11, 8070–8078. [Google Scholar] [CrossRef]
- Shioiri, M.; Shida, T.; Koda, K.; Oda, K.; Seike, K.; Nishimura, M.; Takano, S.; Miyazaki, M. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br. J. Cancer 2006, 94, 1816–1822. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Heller, G.; Halabi, S.; Monk, J.P., 3rd; Beltran, H.; Gleave, M.; Evans, C.P.; Clinton, S.K.; Szmulewitz, R.Z.; Coleman, J.; et al. Cancer and Leukemia Group B 90203 (Alliance): Radical Prostatectomy with or Without Neoadjuvant Chemohormonal Therapy in Localized, High-Risk Prostate Cancer. J. Clin. Oncol. 2020, 38, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Taplin, M.E.; Montgomery, B.; Logothetis, C.J.; Bubley, G.J.; Richie, J.P.; Dalkin, B.L.; Sanda, M.G.; Davis, J.W.; Loda, M.; True, L.D.; et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 2014, 32, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).