Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Scheme
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Geriatric Assessment
3.3. Chemotherapy Toxicities, Unplanned Hospitalizations and Death
3.4. Predictive Variables Associated with Grade 3–5 Toxicity, Unplanned Hospitalizations and Death
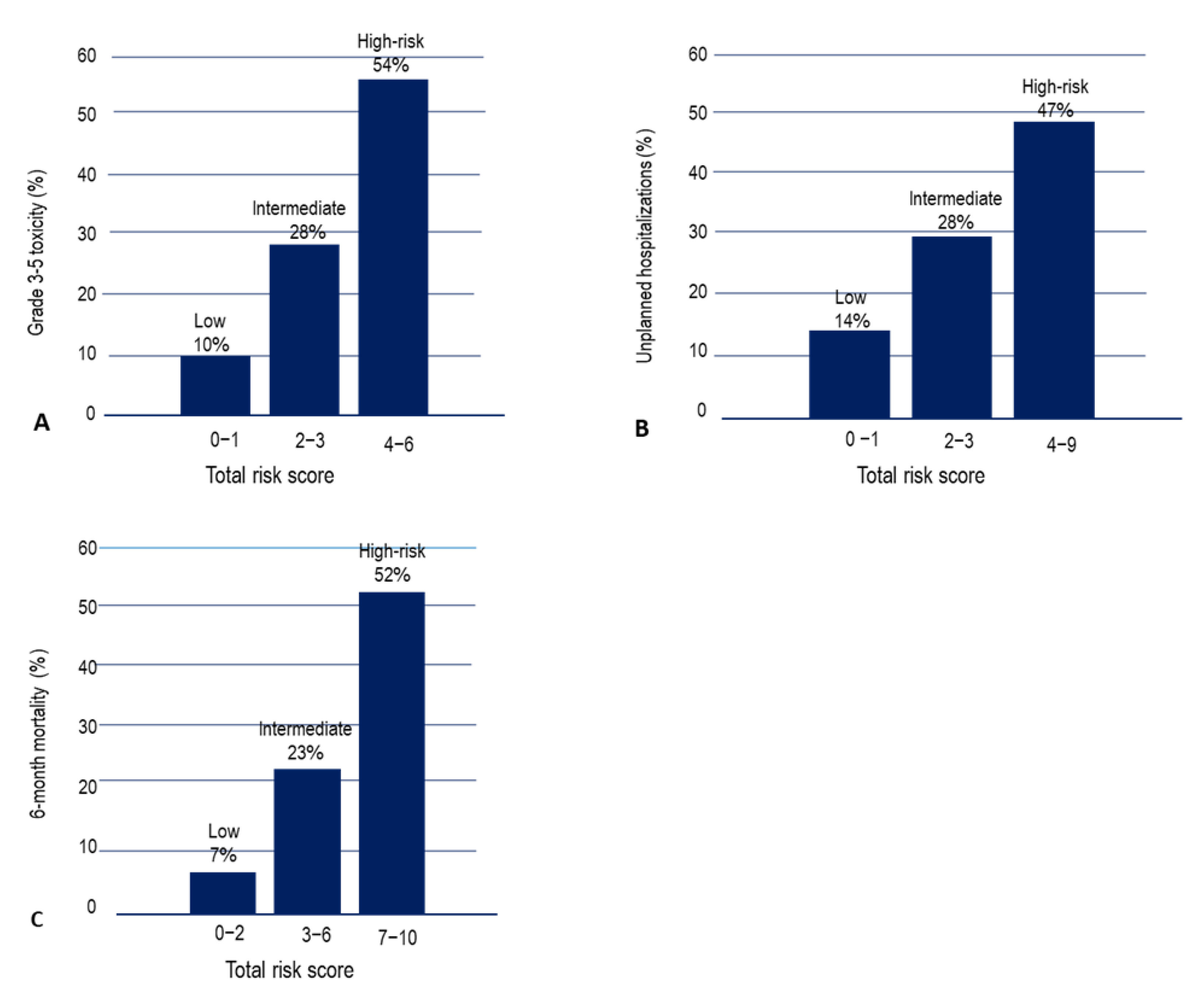
3.5. Predictive Model for Chemotherapy Grade 3–5 Toxicity, Unplanned Hospitalizations and Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Feliu, J.; Heredia-Soto, V.; Gironés, R.; Jiménez-Munarriz, B.; Saldaña, J.; Guillén-Ponce, C.; Molina-Garrido, M.J. Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients. Clin. Transl. Oncol. 2020, 22, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Scher, K.S.; Hurria, A. Under-representation of older adults in cancer registration trials: Known problem, little progress. J. Clin. Oncol. 2012, 30, 2036–2038. [Google Scholar] [CrossRef] [PubMed]
- Canouï-Poitrine, F.; Lièvre, A.; Dayde, F.; Lopez-Trabada-Ataz, D.; Baumgaertner, I.; Dubreuil, O.; Dubreuil, O.; Brunetti, F.; Coriat, R.; Maley, K.; et al. Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey. Oncologist 2019, 24, e1351–e1359. [Google Scholar] [CrossRef] [Green Version]
- Haller, D.G.; O’Connell, M.J.; Cartwright, T.H.; Twelves, C.J.; McKenna, E.F.; Sun, W.; Saif, M.W.; Lee, S.; Yothers, G.; Schmoll, H.J. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: A pooled analysis of individual patient data from four randomized, controlled trials. Ann. Oncol. 2015, 26, 715–724. [Google Scholar] [CrossRef]
- Langer, C.J.; Manola, J.; Bernardo, P.; Kugler, J.W.; Bonomi, P.; Cella, D.; Johnson, D.H. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: Implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J. Natl. Cancer Inst. 2002, 94, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Creditor, M.C. Hazards of hospitalization of the elderly. Ann. Intern. Med. 1993, 118, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.A.; Franke, T.; Inouye, S.K.; Landefeld, C.S.; Morgan, T.M.; Rudberg, M.A.; Sebens, H.; Winograd, C.H. Functional outcomes of acute medical illness and hospitalization in older persons. Arch. Intern. Med. 1996, 156, 645–652. [Google Scholar] [CrossRef]
- Feliu, J.; Heredia-Soto, V.; Gironés, R.; Jiménez-Munarriz, B.; Saldaña, J.; Guillén-Ponce, C.; Molina-Garrido, M.J. Can we avoid the toxicity of chemotherapy in elderly cancer patients? Crit. Rev. Oncol. Hematol. 2018, 131, 16–23. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Jerspensen, E.; Winther, S.B.; Minet, L.R.; Möller, S.; Pfeiffer, P. Frailty screening for predicting rapid functional decline, rapid progressive disease, and shorter overall survival in older patients with gastrointestinal cancer receiving palliative chemotherapy—A prospective, clinical study. J. Geriatr. Oncol. 2021, 12, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Retornaz, F.; Guillem, O.; Rousseau, F.; Morvan, F.; Rinaldi, Y.; Nahon, S.; Castagna, C.; Boulahssass, R.; Grino, M.; Gholam, D. Predicting Chemotherapy Toxicity and Death in Older Adults with Colon Cancer: Results of MOST Study. Oncologist 2020, 25, e85–e93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio, T.; Jouve, J.L.; Teillet, L.; Subtil, F.; Le Brun-Ly, V.; Cretin, J.; Locher, C.; Bouché, O.; Breysacher, G.; Charneau, J.; et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001–02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J. Clin. Oncol. 2013, 31, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Decoster, L.; Kenis, C.; Naessens, B.; Houbier, G.; De Man, M.; Lambrecht, G.; Monsaert, E.; Moons, V.; Vergauwe, P.; Prenen, H. Integrating geriatric assessment in the first line chemotherapy treatment in older patients with metastatic colorectal cancer: Results of a prospective observational cohort study (AVAPLUS). J. Geriatr. Oncol. 2018, 9, 93–101. [Google Scholar] [CrossRef]
- Aparicio, T.; Bouché, O.; Francois, E.; Retornaz, F.; Barbier, E.; Taieb, J.; Kirscher, S.; Etienne, P.L.; Faroux, R.; Khemissa Akouz, F.; et al. Geriatric analysis from PRODIGE 20 randomized phase II trial evaluating bevacizumab + chemotherapy versus chemotherapy alone in older patients with untreated metastatic colorectal cancer. Eur. J. Cancer 2018, 97, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.B.; Liposits, G.; Skuladottir, H.; Hofsli, E.; Shah, C.H.; Poulsen, L.Ø.; Ryg, J.; Osterlund, P.; Berglund, Å.; Qvortrup, C. Reduced-dose combination chemotherapy (S-1 plus oxaliplatin) versus full-dose monotherapy (S-1) in older vulnerable patients with metastatic colorectal cancer (NORDIC9): A randomised, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 376–388. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Richard, M.L.; Lubiner, E.T.; Pablo, R.; Schreiber, F.J., 3rd; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Roila, F.; Lupattelli, M.; Sassi, M.; Basurto, C.; Bracarda, S.; Picciafuoco, M.; Del Favero, A. Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann. Oncol. 1991, 2, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, E.A. A short portable mental status questionnaire for the assessment of organic brain deficits in elderly patients. J. Am. Geriatr. Soc. 1975, 22, 433. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL—A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Zigmod, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scan. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.S.; Skinner, K.; Lee, A.; Kazis, L. Social support, social selection and self-assessed health status: Results from the veterans health study in the United States. Soc. Sci. Med. 1999, 48, 1721–1734. [Google Scholar] [CrossRef]
- Costa-Requena, G.; Salamero, M.; Gil, F. Validity of the questionnaire MOS-SSS of social support in neoplastic patients. Med. Clin. 2007, 128, 687–691. [Google Scholar]
- Luciani, A.; Ascione, G.; Bertuzzi, C.; Marussi, D.; Codecà, C.; Di Maria, G.; Foa, P. Detecting Disabilities in Older Patients with Cancer: Comparison between Comprehensive Geriatric Assessment and Vulnerable Elders Survey-13. J. Clin. Oncol. 2010, 28, 2046–2050. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Extermann, M.; Chen, H.; Cantor, A.B.; Corcoran, M.B.; Meyer, J.; Grendys, E.; Cavanaugh, D.; Antonek, S.; Camarata, A.; Haley, W.E.; et al. Predictors of tolerance to chemotherapy in older cancer patients: A prospective pilot study. Eur. J. Cancer 2002, 38, 1466–1473. [Google Scholar] [CrossRef]
- Feliu, J.; Espinosa, E.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Antonio-Rebollo, M.; Losada, B.; Pinto, A.; Gironés, R.; et al. Undertreatment and overtreatment in older patients treated with chemotherapy. Geriatr. Oncol. 2021, 12, 381–387. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 4 April 2013).
- Landrum, L.; Weinrich, S. Readmission Data for Outcomes Measurement: Identifying and Strengthening the Empirical Base. Q. Manag. Health Care 2006, 15, 83–95. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. (Eds.) Applied Logistic Regression; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Concato, J.; Feinstein, A.R.; Holford, T.R. The risk of determining risk with multivariable models. Ann. Intern. Med. 1993, 118, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J. (Eds.) The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kim, J.W.; Lee, Y.G.; Hwang, I.G.; Song, H.S.; Koh, S.J.; Ko, Y.H.; Shin, S.H.; Woo, I.S.; Hong, S.; Kim, T.Y.; et al. Predicting cumulative incidence of adverse events in older patients with cancer undergoing first-line palliative chemotherapy: Korean Cancer Study Group (KCSG) multicentre prospective study. Br. J. Cancer 2018, 118, 1169–1175. [Google Scholar] [CrossRef]
- Gajra, A.; Jatoi, A. Non–small-cell lung cancer in elderly patients: A discussion of treatment options. J. Clin. Oncol. 2014, 32, 2562–2569. [Google Scholar] [CrossRef]
- Peterson, L.L.; Hurria, A.; Feng, T.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Glezerman, I.; et al. Association between renal function and chemotherapy-related toxicity in older adults with cancer. J. Geriatr. Oncol. 2017, 8, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Feliu, J.; Jiménez-Munárriz, B.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Antonio-Rebollo, M.; Losada, B.; Espinosa, E.; Gironés, R.; Custodio, A.B.; et al. Predicting chemotherapy toxicity in older patients with cancer: A multicenter prospective study. Oncologist 2020, 25, e1516–e1524. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.A.; Li, L.; Uno, H.; Hassett, M.J.; Landon, B.E.; Schrag, D. Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff. 2014, 33, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.A.; Kansagra, A.J.; Rao, S.R.; Weitzman, J.I.; Linden, E.A.; Jacobson, J.O. A clinical prediction model to assess risk for chemotherapy related hospitalization in patients initiating palliative chemotherapy. JAMA Oncol. 2015, 1, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Hassett, M.J.; Rao, S.R.; Brozovic, S.; Stahl, J.E.; Schwartz, J.H.; Maloney, B.; Jacobson, J.O. Chemotherapy-related hospitalization among community cancer center patients. Oncologist 2011, 16, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Du, X.L.; Osborne, C.; Goodwin, J.S. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J. Clin. Oncol. 2002, 20, 4636–4642. [Google Scholar] [CrossRef]
- Lodewijckx, E.; Kenis, C.; Flamaing, J.; Debruyne, P.; De Groof, I.; Focan, C.; Cornélis, F.; Verschaeve, V.; Bachmann, C.; Bron, D.; et al. Unplanned hospitalizations in older patients with cancer: Occurrence and predictive factors. J. Geriatr. Oncol. 2021, 12, 368–374. [Google Scholar] [CrossRef]
- Reed, M.; Patrick, C.; Quevillon, T.; Walde, N.; Voutsadakis, I.A. Prediction of hospital admissions and grade 3-4 toxicities in cancer patients 70 years old and older receiving chemotherapy. Eur. J. Cancer Care 2019, 28, e13144. [Google Scholar] [CrossRef]
- Feliu, J.; Espinosa, E.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Losada, B.; Pinto, A.; Custodio, A.B.; Muñoz, M.D.M.; et al. Prediction of Unplanned Hospitalizations in Older Patients Treated with Chemotherapy. Cancers 2021, 13, 1437. [Google Scholar] [CrossRef]
- Lichtman, S.M. Chemotherapy in the elderly. Sem. Oncol. 2004, 31, 160–174. [Google Scholar] [CrossRef]
- Den, E.; Morley, J.E.; Cruz-Jentoft, A.H.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef]
- Walter, L.C.; Brand, R.J.; Counsell, S.R.; Palmer, R.M.; Landefeld, C.S.; Fortinsky, R.H.; Covinsky, K.E. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 2001, 285, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lindquist, K.; Segal, M.R.; Covinsky, K.E. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006, 295, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Carey, E.C.; Covinsky, K.E.; Lui, L.Y.; Eng, C.; Sands, L.P.; Walter, L.C. Prediction of mortality in community-living frail elderly people with long-term care needs. J. Am. Geriatr. Soc. 2008, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Brunello, A.; Fontana, A.; Zafferri, V.; Panza, F.; Fiduccia, P.; Basso, U.; Copetti, M.; Lonard, S.; Roma, A.; Falci, C.; et al. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdel-Marchasson, I.; Diallo, A.; Ballera, C.; Blanc-Bisson, C.; Durrieu, J.; Germain, C.; Mathoulin-Pélissier, S.; Soubeyran, P.; Rainfray, M.; Foncket, M.; et al. One-year mortality older patients with cancer: Development and external validation of an MNA-based prognostic score. PLoS ONE 2016, 1, e0148523. [Google Scholar] [CrossRef]
- Soubeyran, P.; Fonck, M.; Blanc-Bisson, C.; Blanc, J.F.; Ceccaldi, J.; Mertens, C.; Rainfray, M. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J. Clin. Oncol. 2012, 30, 1829–1834. [Google Scholar] [CrossRef]
- Feliu, J.; Pinto, A.; Basterretxea, L.; López-San Vicente, B.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Antonio-Rebollo, M.; Losada, B.; Espinosa, E.; et al. Development and Validation of an Early Mortality Risk Score for Older Patients Treated with Chemotherapy for Cancer. J. Clin. Med. 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Boulahssass, R.; Gonfrier, S.; Ferrero, J.M.; Sanchez, M.; Mari, V.; Moranne, O.; Guerin, O. Predicting early death in older adults with cancer. Eur. J. Cancer 2018, 100, 65–74. [Google Scholar] [CrossRef]
- Angeli, E.; Chouahnia, K.; Canoui-Poitrine, F.; Duchemann, B.; Aparicio, T.; Paillaud, E.; Pamoukdjian, F. Development, Validation and Clinical Impact of a Prediction Model for 6-month Mortality in Older Cancer Patients: The GRADE. Aging 2020, 12, 4230–4246. [Google Scholar] [CrossRef]
- Kanesvaran, R.; Li, H.; Koo, K.N.; Poon, D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. Clin. Oncol. 2011, 29, 3620–3627. [Google Scholar] [CrossRef]
- Buck, I.; Morceau, F.; Grigorakaki, C.; Dicato, M.; Diederich, M. Linking anemia to inflammation and cancer: The crucial role of TNFalpha. Biochem. Pharmacol. 2009, 77, 1572–1579. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Total (n = 215) |
|---|---|
| Age, median (SD) | 78 (4.9) |
| Sex | |
| Male | 125 (58%) |
| Female | 90 (32%) |
| Metastatic status | |
| M0 | 110 (51%) |
| M1 | 105 (49%) |
| Chemotherapy | |
| Standard therapy | 100 (47%) |
| Reduced therapy or monotherapy | 115 (53%) |
| Capecitabine | 77 (36%) |
| Capecitabine-Oxaliplatin | 33 (15%) |
| Oxaliplatin-5FU-anti-EGFR | 32 (15%) |
| Oxalipatin-5FU-Bevacizumab | 40 (18%) |
| Oxaliplatin-Irinotecan-5FU | 15 (7%) |
| Irinotecan-anti-EGFR | 6 (3%) |
| Capecitabine-Bevacizumab | 12 (6%) |
| MAX2 index | |
| 0 | 73 (34%) |
| 1 | 127 (59%) |
| 2 | 15 (7%) |
| ECOG PS | |
| 0 | 58 (27%) |
| 1 | 144 (67%) |
| 2 | 13 (6%) |
| IADL | |
| 8 | 97 (45%) |
| ≤7 | 118 (55%) |
| ADL | |
| 6 | 181 (84%) |
| ≤5 | 34 (16%) |
| Number of falls in the past 6 months | |
| None | 183 (85%) |
| ≥1 | 32 (15%) |
| SPPB | |
| >7 | 185 (86%) |
| ≤6 | 30 (14%) |
| Charlson comorbidity score | |
| 0 | 80 (37%) |
| 1 | 64 (30%) |
| ≥2 | 71 (33%) |
| Pfeiffer test | |
| 0–2 errors | 185 (86%) |
| ≥3 errors | 30 (14%) |
| Unintentional weight loss % | |
| ≤10% | 181 (88%) |
| >10% | 34 (12%) |
| VES 13 | |
| 0–2 | 103 (48%) |
| ≥3 | 119 (52%) |
| Toxicity | |
| G3-5 | 73 (34%) |
| G0-2 | 142 (66%) |
| Early death < 6 months | |
| Yes | 47 (22%) |
| No | 168 (78%) |
| Unplanned hospitalizations | |
| Yes | 60 (28%) |
| No | 155 (67%) |
| Variable | Toxicity G3–4 | Early Death | Unplanned Hospitalizations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | |
| ECOG PS | 0.95 | 0.91 | 0.031 | ||||||
| 2 | 9 | 4 | 10 | 3 | 6 | 7 | |||
| 0–1 | 133 | 69 | 158 | 44 | 149 | 53 | |||
| IADL | 0.04 | 9672 | 0.20 | 0.0001 | |||||
| ≤7 | 85 | 33 | 22 | 98 | 20 | ||||
| 8 | 57 | 40 | 25 | 57 | 40 | ||||
| ADL | 0.85 | 0.038 | 0.004 | ||||||
| ≤5 | 22 | 12 | 22 | 12 | 16 | 18 | |||
| 6 | 120 | 61 | 146 | 35 | 139 | 42 | |||
| Charlson comorbidity score | 0.56 | 0.008 | 0.015 | ||||||
| ≥2 | 45 | 26 | 48 | 23 | 58 | 12 | |||
| 0–1 | 97 | 47 | 120 | 26 | 96 | 482 | |||
| Unintentional weight loss % | 0.012 | 0.000 | 0.0001 | ||||||
| >5% | 40 | 33 | 40 | 33 | 37 | 36 | |||
| ≤5% | 102 | 40 | 128 | 14 | 118 | 24 | |||
| Creatinine Clearance mL/min | 0.022 | 0.26 | 0.0001 | ||||||
| <50 | 42 | 33 | 60 | 21 | 36 | 46 | |||
| ≥50 | 100 | 40 | 108 | 26 | 119 | 14 | |||
| Albumin g/dL | 0.17 | 0.000 | 0.51 | ||||||
| ≤35 | 24 | 18 | 23 | 19 | 32 | 10 | |||
| >35 | 118 | 55 | 145 | 28 | 123 | 50 | |||
| Hemoglobin (g/dL) | 0.61 | 0.02 | 0.48 | ||||||
| <11 | 27 | 16 | 28 | 15 | 32 | 15 | |||
| ≥11 | 1157 | 57 | 140 | 32 | 123 | 45 | |||
| Metastatic status | 0.49 | 0.000 | 0.000 | ||||||
| M1 | 67 | 38 | 69 | 36 | 64 | 41 | |||
| M0 | 75 | 35 | 99 | 11 | 91 | 19 | |||
| Chemotherapy | 0.37 | 0.96 | 0.37 | ||||||
| Standard therapy | 63 | 37 | 78 | 22 | 75 | 25 | |||
| Reduced/monotherapy | 79 | 36 | 90 | 25 | 80 | 35 | |||
| MAX2 index | 0.03 | 0.73 | 0.84 | ||||||
| ≥0.45 | 87 | 55 | 110 | 32 | 103 | 39 | |||
| 0–0.44 | 55 | 18 | 58 | 15 | 52 | 21 | |||
| Variable | β | SE | p † | HR (95% CI) | Score |
|---|---|---|---|---|---|
| MAX2 index > 0.45 | 0.796 | 0.315 | 0.009 | 2.176 (1.143–4.213) | 2 |
| Weight loss > 5% | 0.709 | 0.315 | 0.03 | 2.014 (1.084–3.969) | 2 |
| IADL ≤ 7 | 0.455 | 0.284 | 0.04 | 1.293 (1.013–2.318) | 1 |
| Creatinine Clearance < 50 mL/min | 0.628 | 0.299 | 0.03 | 1.891 (1.061–3.384) | 1 |
| Variable | β | SE | p † | HR (95% CI) | Score |
|---|---|---|---|---|---|
| Stage IV | 0.997 | 0.312 | 0.001 | 2.732 (1.438–4.957) | 2 |
| Weight loss > 5% | 0.824 | 0.392 | 0.029 | 2.241 (1.067–4.921) | 2 |
| Albumin ≤ 3.5 g/dL | 0.739 | 0.399 | 0.045 | 2.012 (1.006–4.45) | 2 |
| Creatinine Clearance < 50 mL/min | 0.878 | 0.3719 | 0.013 | 2.219 (1.149–4.352) | 2 |
| Charlson score ≥ 2 | 0.215 | 0.181 | 0.045 | 1.239 (1.001–1.573) | 1 |
| Variable | β | SE | p † | HR (95% CI) | Score |
|---|---|---|---|---|---|
| Stage IV | 1.497 | 0.364 | 0.000 | 5.026 (10.109–2.486) | 5 |
| Weight loss > 5% | 0.9438 | 0.369 | 0.009 | 2.542 (1.243–5.106) | 2 |
| Hemoglobin ≤ 11 g/dL | 0.823 | 0.354 | 0.019 | 2.213 (1.1352–4.518) | 2 |
| ADL ≤ 5 | 0.437 | 0.311 | 0.045 | 1.104 (1.004–2.369) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliu, J.; Espinosa, E.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Antonio-Rebollo, M.; Losada, B.; Pinto, A.; Custodio, A.B.; et al. Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers 2022, 14, 127. https://doi.org/10.3390/cancers14010127
Feliu J, Espinosa E, Basterretxea L, Paredero I, Llabrés E, Jiménez-Munárriz B, Antonio-Rebollo M, Losada B, Pinto A, Custodio AB, et al. Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers. 2022; 14(1):127. https://doi.org/10.3390/cancers14010127
Chicago/Turabian StyleFeliu, Jaime, Enrique Espinosa, Laura Basterretxea, Irene Paredero, Elisenda Llabrés, Beatriz Jiménez-Munárriz, Maite Antonio-Rebollo, Beatriz Losada, Alvaro Pinto, Ana Belén Custodio, and et al. 2022. "Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy" Cancers 14, no. 1: 127. https://doi.org/10.3390/cancers14010127
APA StyleFeliu, J., Espinosa, E., Basterretxea, L., Paredero, I., Llabrés, E., Jiménez-Munárriz, B., Antonio-Rebollo, M., Losada, B., Pinto, A., Custodio, A. B., del Mar Muñoz, M., Gómez-Mediavilla, J., Torregrosa, M.-D., Soler, G., Cruz, P., Higuera, O., & Molina-Garrido, M.-J. (2022). Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers, 14(1), 127. https://doi.org/10.3390/cancers14010127






