Does an Endometrial Cancer Diagnosis among Asymptomatic Patients Improve Prognosis?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
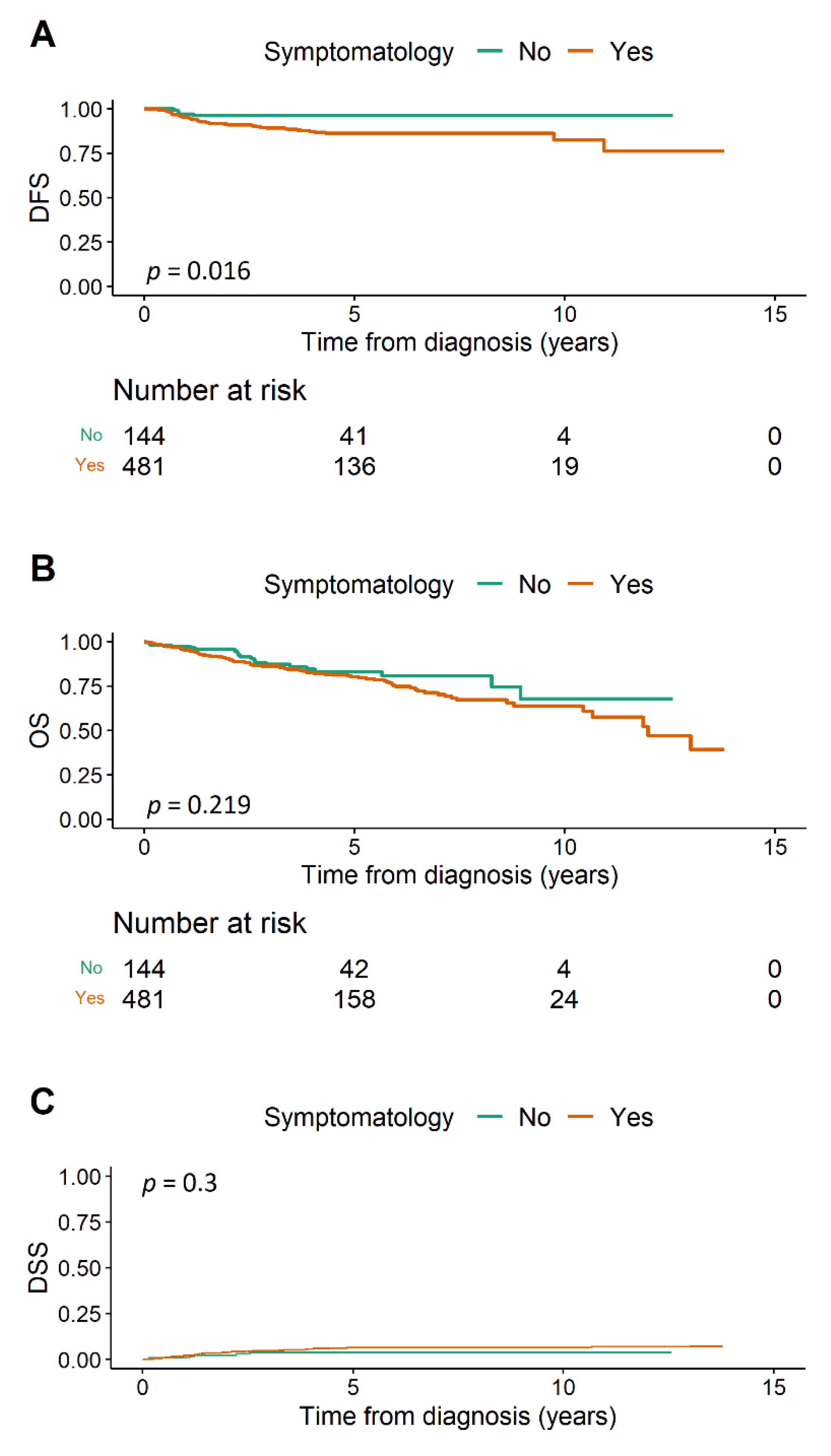
3.1. Disease-Free Survival
3.2. Overall Survival
3.3. Disease-Specific Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFS | disease-free survival |
| DSS | disease-specific survival |
| EC | endometrial cancer |
| FIGO | The International Federation of Gynecology and Obstetrics |
| CHRT | chemoradiotherapy |
| CHT | chemotherapy |
| LVSI | lymphovascular space invasion |
| NEC | non-endometrioid carcinoma |
| OS | overall survival |
| RT | radiotherapy |
| HR | hazard ratio |
| CI | confidence interval |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant, M.; Lopez, M.D.C.; Agresti, R.; Pérez, M.J.S.; Holleczek, B.; Bielska-Lasota, M.; Dimitrova, N.; Innos, K.; Katalinic, A.; Langseth, H.; et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.; Nyan, H.Y.; Pecorelli, S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S105–S143. [Google Scholar]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Martín, A.G.; Ledermann, J.; Marth, C.; Nout, R.A.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrede, S.; van Weelden, W.; Visser, N.; Bulten, J.; van der Putten, L.; van de Vijver, K.; Santacana, M.; Colas, E.; Gil-Moreno, A.; Moiola, C.; et al. Immunohistochemical biomarkers are prognostic relevant in addition to the ESMO-ESGO-ESTRO risk classification in endometrial cancer. Gynecol. Oncol. 2021, 161, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; Fulton, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Uglietti, A.; Buggio, L.; Farella, M.; Chiaffarino, F.; Dridi, D.; Vercellini, P.; Parazzini, F. The risk of malignancy in uterine polyps: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Bonilla, L.; Marucco, J.; Padilla, A.I.; Chacón, E.; Manzour, N.; Salas, A. Risk of endometrial cancer and endometrial hyperplasia with atypia in asymptomatic postmenopausal women with endometrial thickness ≥11 mm: A systematic review and meta-analysis. J. Clin. Ultrasound 2018, 46, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Onkogynekologie.com. Available online: http://www.onkogynekologie.com/ (accessed on 19 October 2021).
- NCI Dictionary of Cancer Terms—National Cancer Institute. Published 2 February 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/ (accessed on 26 June 2021).
- Jokubkiene, L.; Sladkevicius, P.; Valentin, L. Transvaginal ultrasound examination of the endometrium in postmenopausal women without vaginal bleeding. Ultrasound Obstet. Gynecol. 2016, 48, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Weiss, E.; Feldstein, V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet. Gynecol. 2004, 24, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Vinklerová, P.; Felsinger, M.; Frydová, S.; Ovesná, P.; Hausnerová, J.; Weinberger, V. Is the finding of endometrial hyperplasia or corporal polyp an mandatory indication for biopsy? Ceska Gynekol. 2020, 85, 84–93. [Google Scholar] [PubMed]
- Ferrazzi, E.; Zupi, E.; Leone, F.P.; Savelli, L.; Omodei, U.; Moscarini, M.; Barbieri, M.; Cammareri, G.; Capobianco, G.; Cicinelli, E.; et al. How often are endometrial polyps malignant in asymptomatic postmenopausal women? A multicenter study. Am. J. Obstet. Gynecol. 2009, 200, 235.e1–235.e6. [Google Scholar] [CrossRef] [PubMed]
- Scrimin, F.; Wiesenfeld, U.; Galati, E.F.; Monasta, L.; Ricci, G. Hysteroscopic chasing for endometrial cancer in a low-risk population: Risks of overinvestigation. Arch. Gynecol. Obstet. 2016, 293, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Vinklerová, P.; Bednaříková, M.; Minář, L.; Felsinger, M.; Hausnerová, J.; Ovesná, P.; Weinberger, V. Tumor Characteristic Variations between Symptomatic and Asymptomatic Endometrial Cancer. Healthcare 2021, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Gemer, O.; Segev, Y.; Helpman, L.; Hag-Yahia, N.; Eitan, R.; Raban, O.; Vaknin, Z.; Leytes, S.; Ben Arie, A.; Amit, A.; et al. Is there a survival advantage in diagnosing endometrial cancer in asymptomatic postmenopausal patients? An Israeli Gynecology Oncology Group study. Am. J. Obstet. Gynecol. 2018, 219, 181.e1–181.e6. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, V.; Schmid, M.; Polterauer, S.; Hefler-Frischmuth, K.; Leipold, H.; Concin, N.; Reinthaller, A.; Hefler, L. The presence of postmenopausal bleeding as prognostic parameter in patients with endometrial cancer: A retrospective multi-center study. BMC Cancer 2009, 9, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barak, F.; Kalichman, L.; Gdalevich, M.; Milgrom, R.; Laitman, Y.; Piura, B.; Lavie, O.; Gemer, O. The influence of early diagnosis of endometrioid endometrial cancer on disease stage and survival. Arch. Gynecol. Obstet. 2013, 288, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Namazov, A.; Helpman, L.; Eitan, R.; Vaknin, Z.; Lavie, O.; Ben-Arie, A.; Amit, A.; Levy, T.; Volodarsky, M.; Atlas, I.; et al. The diagnosis of endometrial cancer in women with asymptomatic endometrial polyp does not increase survival rates: An Israel gynecologic oncology group study. Maturitas 2021, 148, 18–23. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Asymptomatic (n = 144) | Symptomatic (n = 481) | p-Value | |
|---|---|---|---|---|
| Age (years) | <50 | 13 (9.0%) | 46 (9.6%) | 0.32 |
| 51–60 | 27 (18.8%) | 95 (19.8%) | ||
| 61–70 | 68 (47.2%) | 191 (39.7%) | ||
| 71–80 | 32 (22.2%) | 117 (24.3%) | ||
| >80 | 4 (2.8%) | 32 (6.7%) | ||
| Age (years) | Mean (SD) | 64.5 (9.3%) | 65.2 (10.6%) | 0.476 |
| Lymphadenectomy | No | 124 (86.1%) | 335 (69.6%) | <0.001 |
| Yes | 20 (13.9%) | 146 (30.4%) | ||
| Adjuvant therapy | None | 111 (77.6%) | 264 (56.4%) | <0.001 |
| RT | 25 (17.5%) | 166 (35.5%) | ||
| CHT | 4 (2.8%) | 19 (4.1%) | ||
| CHRT | 3 (2.1%) | 19 (4.1%) | ||
| LVSI | No | 138 (95.8%) | 398 (83.3%) | <0.001 |
| Yes | 6 (4.2%) | 80 (16.7%) | ||
| Histology + grade | Endometrioid G1 | 60 (41.7%) | 86 (17.9%) | <0.001 |
| Endometrioid G2 | 70 (48.6%) | 270 (56.1%) | ||
| Endometrioid G3 | 5 (3.5%) | 70 (14.6%) | ||
| Non-endometrioid | 9 (6.2%) | 55 (11.4%) | ||
| FIGO stage | Ia | 117 (81.2%) | 277 (57.6%) | <0.001 |
| Ib | 13 (9.0%) | 89 (18.5%) | ||
| II | 10 (6.9%) | 60 (12.5%) | ||
| IIIa | 1 (0.7%) | 12 (2.5%) | ||
| IIIb | 2 (1.4%) | 8 (1.7%) | ||
| IIIc | 1 (0.7%) | 26 (5.4%) | ||
| IVa | 0 (0.0%) | 0 (0.0%) | ||
| IVb | 0 (0.0%) | 9 (1.9%) |
| Clinical Characteristics | Crude HR (95% CI, p-Value) | Adjusted HR (95% CI, p-Value) | |
|---|---|---|---|
| Symptomatology | No | 1 | 1 |
| Yes | 3.1 (1.24–7.77, p = 0.016) | 2.03 (0.79–5.24, p = 0.144) | |
| Age (years) | <50 | 1 | |
| 51–60 | 2.18 (0.47–10.08, p = 0.320) | ||
| 61–70 | 2.49 (0.58–10.58, p = 0.217) | ||
| 71–80 | 3.11 (0.71–13.68, p = 0.134) | ||
| >80 | 9.91 (2.14–45.92, p = 0.003) | ||
| Age (years) | Mean (SD) | 1.05 (1.02–1.08, p = 0.002) | 1.04 (1.01–1.07, p = 0.013) |
| Lymphadenectomy | No | 1 | |
| Yes | 1.75 (1.02–3, p = 0.042) | ||
| Adjuvant therapy | None | 1 | 1 |
| RT | 1.47 (0.81–2.69, p = 0.209) | 0.82 (0.42–1.61, p = 0.569) | |
| CHT | 9.61 (4.43–20.86, p < 0.001) | 1.68 (0.50–5.63, p = 0.404) | |
| CHRT | 2.18 (0.65–7.25, p = 0.205) | 0.36 (0.08–1.63, p = 0.186) | |
| LVSI | No | 1 | 1 |
| Yes | 3.75 (2.09–6.73, p < 0.001) | 1.34 (0.58–3.06, p = 0.494) | |
| Histology + grade | Endometrioid G1 | 1 | |
| Endometrioid G2 | 3.52 (1.06–11.63, p = 0.039) | 1 (ref. G1 + G2) | |
| Endometrioid G3 | 7.15 (1.99–25.64, p = 0.003) | 1.70 (0.76–3.79, p = 0.194) | |
| Non-endometrioid | 15.61 (4.55–53.61, p < 0.001) | 3.20 (1.59–6.43, p = 0.001) | |
| FIGO stage | Ia | 1 | |
| Ib | 2.69 (1.33–5.47, p = 0.006) | ||
| II | 3 (1.36–6.63, p = 0.007) | ||
| IIIa | 6.51 (2.21–19.22, p = 0.001) | ||
| IIIb | 4.11 (0.55–30.82, p = 0.169) | ||
| IIIc | 7.60 (3.03–19.07, p < 0.001) | ||
| IVa | NA | ||
| IVb | 21.67 (7.32–64.17, p < 0.001) | ||
| FIGO stage | I–II | 1 | 1 |
| III–IV | 5.37 (2.96–9.73, p < 0.001) | 3.55 (1.40–8.96, p = 0.007) |
| Clinical Characteristics | Crude HR (95% CI, p-Value) | Adjusted HR (95% CI, p-Value) | |
|---|---|---|---|
| Symptomatology | No | 1 | 1 |
| Yes | 1.35 (0.84–2.19, p = 0.219) | 0.72 (0.43–1.21, p = 0.216) | |
| Age (years) | <50 | 1 | |
| 51–60 | 1.4 (0.46–4.27, p = 0.551) | ||
| 61–70 | 1.9 (0.68–5.33, p = 0.222) | ||
| 71–80 | 4.63 (1.66–12.89, p = 0.003) | ||
| >80 | 7.44 (2.48–22.31, p < 0.001) | ||
| Age (years) | Mean (SD) | 1.07 (1.05–1.09, p < 0.001) | 1.07 (1.05–1.10, p < 0.001) |
| Lymphadenectomy | No | 1 | |
| Yes | 1.42 (0.98–2.07, p = 0.066) | ||
| Adjuvant therapy | None | 1 | 1 |
| RT | 0.98 (0.64–1.51, p = 0.938) | 0.64 (0.40–1.03, p = 0.067) | |
| CHT | 5.91 (3.19–10.96, p < 0.001) | 1.16 (0.50–2.73, p = 0.727) | |
| CHRT | 1.65 (0.66–4.14, p = 0.284) | 0.28 (0.09–0.85, p = 0.024) | |
| LVSI | No | 1 | 1 |
| Yes | 4.55 (3.06–6.75, p < 0.001) | 2.05 (1.13–3.72, p = 0.018) | |
| Histology + grade | Endometrioid G1 | 1 | |
| Endometrioid G2 | 1.17 (0.66–2.07, p = 0.584) | 1 (ref. G1 + G2) | |
| Endometrioid G3 | 2.63 (1.4–4.95, p = 0.003) | 2.05 (1.17–3.61, p = 0.013 | |
| Non-endometrioid | 5.43 (2.97–9.95, p < 0.001) | 2.89 (1.77–4.72, p < 0.001) | |
| FIGO stage | Ia | 1 | |
| Ib | 2.38 (1.45–3.91, p = 0.001) | ||
| II | 2.54 (1.44–4.49, p = 0.001) | ||
| IIIa | 3.78 (1.60–8.94, p = 0.003 | ||
| IIIb | 16.01 (7.06–36.3, p < 0.001) | ||
| IIIc | 8.7 (4.71–16.09, p < 0.001) | ||
| IVa | NA | ||
| IVb | 14.89 (6.58–33.72, p < 0.001) | ||
| FIGO stage | I–II | 1 | 1 |
| III–IV | 5.69 (3.80–8.52, p < 0.001) | 3.63 (1.93–6.85, p < 0.001) |
| Clinical Characteristics | Crude HR (95% CI, p-Value) | |
|---|---|---|
| Symptomatology | No | 1 |
| Yes | 1.66 (0.64–4.28, p = 0.300) | |
| Age (years) | <50 | 1 |
| 51–60 | 1.14 (0.22–5.89, p = 0.870) | |
| 61–70 | 1.48 (0.34–6.37, p = 0.600) | |
| 71–80 | 1.7 (0.37–7.74, p = 0.500) | |
| >80 | 3.94 (0.77–20.05, p = 0.099) | |
| Age (years) | Mean (SD) | 1.03 (0.99–1.07, p = 0.170) |
| Lymphadenectomy | No | 1 |
| Yes | 2.20 (1.14–4.26, p = 0.019) | |
| Adjuvant therapy | None | 1 |
| RT | 1.12 (0.49–2.53, p = 0.790) | |
| CHT | 11.93 (5.17–27.53, p < 0.001) | |
| CHRT | 3.75 (1.1–12.74, p = 0.034) | |
| LVSI | No | 1 |
| Yes | 8.08 (4.21–15.48, p < 0.001) | |
| Histology + grade | Endometrioid G1 + 2 | 1 |
| Endometrioid G3 | 5.19 (2.22–12.13, p < 0.001) | |
| Non-endometrioid | 8.74 (4.06–18.78, p < 0.001) | |
| FIGO stage | I–II | 1 |
| III–IV | 10.33 (5.36–19.90, p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinklerová, P.; Ovesná, P.; Bednaříková, M.; Minář, L.; Felsinger, M.; Hausnerová, J.; Weinberger, V. Does an Endometrial Cancer Diagnosis among Asymptomatic Patients Improve Prognosis? Cancers 2022, 14, 115. https://doi.org/10.3390/cancers14010115
Vinklerová P, Ovesná P, Bednaříková M, Minář L, Felsinger M, Hausnerová J, Weinberger V. Does an Endometrial Cancer Diagnosis among Asymptomatic Patients Improve Prognosis? Cancers. 2022; 14(1):115. https://doi.org/10.3390/cancers14010115
Chicago/Turabian StyleVinklerová, Petra, Petra Ovesná, Markéta Bednaříková, Luboš Minář, Michal Felsinger, Jitka Hausnerová, and Vít Weinberger. 2022. "Does an Endometrial Cancer Diagnosis among Asymptomatic Patients Improve Prognosis?" Cancers 14, no. 1: 115. https://doi.org/10.3390/cancers14010115
APA StyleVinklerová, P., Ovesná, P., Bednaříková, M., Minář, L., Felsinger, M., Hausnerová, J., & Weinberger, V. (2022). Does an Endometrial Cancer Diagnosis among Asymptomatic Patients Improve Prognosis? Cancers, 14(1), 115. https://doi.org/10.3390/cancers14010115






