Prognostic Value of the Immunological Subtypes of Adolescent and Adult T-Cell Lymphoblastic Lymphoma; an Ultra-High-Risk Pro-T/CD2(−) Subtype
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borowitz, M.J.; Chan, J.K.C. T lymphoblastic leukaemia/lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Eds.; IARC: Lyon, France, 2008; pp. 176–178. [Google Scholar]
- Borowitz, M.J.; Chan, J.K.C. T lymphoblastic leukaemia/lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Eds.; IARC: Lyon, France, 2017; pp. 209–212. [Google Scholar]
- Le Gouill, S.; Lepretre, S.; Briere, J.; Morel, P.; Bouabdallah, R.; Raffoux, E.; Sebban, C.; Lepage, E.; Brice, P. Adult lymphoblastic lymphoma: A retrospective analysis of 92 patients under 61 years included in the LNH87/93 trials. Leukemia 2003, 17, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, S.; Graux, C.; Touzart, A.; Macintyre, E.; Boissel, N. Adult T-type lymphoblastic lymphoma: Treatment advances and prognostic indicators. Exp. Hematol. 2017, 51, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, D.; Gokbuget, N.; Digel, W.; Faak, T.; Kneba, M.; Reutzel, R.; Romejko-Jarosinska, J.; Zwolinski, J.; Walewski, J. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002, 99, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; O’Brien, S.; Cortes, J.; Giles, F.J.; Faderl, S.; Verstovsek, S.; Ferrajoli, A.; Koller, C.; Beran, M.; Pierce, S.; et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004, 104, 1624–1630. [Google Scholar] [CrossRef]
- Cortelazzo, S.; Intermesoli, T.; Oldani, E.; Ciceri, F.; Rossi, G.; Pogliani, E.M.; Mattei, D.; Romani, C.; Cortelezzi, A.; Borlenghi, E.; et al. Results of a lymphoblastic leukemia-like chemotherapy program with risk-adapted mediastinal irradiation and stem cell transplantation for adult patients with lymphoblastic lymphoma. Ann. Hematol. 2012, 91, 73–82. [Google Scholar] [CrossRef][Green Version]
- Lepretre, S.; Touzart, A.; Vermeulin, T.; Picquenot, J.M.; Tanguy-Schmidt, A.; Salles, G.; Lamy, T.; Béné, M.-C.; Raffoux, E.; Huguet, F.; et al. Pediatric-Like Acute Lymphoblastic Leukemia Therapy in Adults With Lymphoblastic Lymphoma: The GRAALL-LYSA LL03 Study. J. Clin. Oncol. 2016, 34, 572–580. [Google Scholar] [CrossRef]
- Gökbuget, N.; Wolf, A.; Stelljes, M.; Hüttmann, A.; Buss, E.C.; Viardot, A.; Brandt, K.; de Wit, M.; Frickhofen, N.; Kebenko, M.; et al. Favorable Outcome in a Large Cohort of Prospectively Treated Adult Patients with T-Lymphoblastic Lymphoma (T-LBL) Despite Slowly Evolving Complete Remission Assessed By Conventional Radiography. Blood 2014, 124, 370. [Google Scholar] [CrossRef]
- El-Fattah, M.A. Prognostic Factors and Outcomes of Adult Lymphoblastic Lymphoma in the United States. Clin. Lymphoma Myeloma Leuk. 2017, 17, 498–505.e6. [Google Scholar] [CrossRef]
- Ellin, F.; Jerkeman, M.; Hagberg, H.; Relander, T. Treatment outcome in T-cell lymphoblastic lymphoma in adults—A population-based study from the Swedish Lymphoma Registry. Acta Oncol. 2014, 53, 927–934. [Google Scholar] [CrossRef]
- Marks, D.I.; Paietta, E.M.; Moorman, A.V.; Richards, S.M.; Buck, G.; DeWald, G.; Ferrando, A.; Fielding, A.K.; Goldstone, A.H.; Ketterling, R.P.; et al. T-cell acute lymphoblastic leukemia in adults: Clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood 2009, 114, 5136–5145. [Google Scholar] [CrossRef]
- Jain, N.; Lamb, A.V.; O’Brien, S.; Ravandi, F.; Konopleva, M.; Jabbour, E.; Zuo, Z.; Jorgensen, J.; Lin, P.; Pierce, S.; et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: A high-risk subtype. Blood 2016, 127, 1863–1869. [Google Scholar] [CrossRef]
- Hoelzer, D.; Thiel, E.; Arnold, R.; Beck, J.; Beelen, D.W.; Bornhäuser, M.; Bunjes, D.; Ditz, D.; Dührsen, U.; Finke, J.; et al. Successful Subtype Oriented Treatment Strategies in Adult T-All; Results of 744 Patients Treated in Three Consecutive GMALL Studies. Blood 2009, 114, 324. [Google Scholar] [CrossRef]
- Liu, Y.; Rao, J.; Li, J.; Wen, Q.; Wang, S.; Lou, S.; Yang, T.; Li, B.; Gao, L.; Zhanget, C.; et al. Tandem autologous hematopoietic stem cell transplantation for treatment of adult T-cell lymphoblastic lymphoma: A multiple center prospective study in China. Haematologica 2021, 106, 163–172. [Google Scholar] [CrossRef]
- Coustan-Smith, E.; Mullighan, C.G.; Onciu, M.; Behm, F.G.; Raimondi, S.C.; Pei, D.; Cheng, C.; Su, X.; Rubnitz, J.E.; Basso, G.; et al. Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009, 10, 147–156. [Google Scholar] [CrossRef]
- Bond, J.; Graux, C.; Lhermitte, L.; Lara, D.; Cluzeau, T.; Leguay, T.; Cieslak, A.; Trinquand, A.; Pastoret, C.; Belhocine, M.; et al. Early Response-Based Therapy Stratification Improves Survival in Adult Early Thymic Precursor Acute Lymphoblastic Leukemia: A Group for Research on Adult Acute Lymphoblastic Leukemia Study. J. Clin. Oncol. 2017, 35, 2683–2691. [Google Scholar] [CrossRef]
- Genesca, E.; Morgades, M.; Montesinos, P.; Barba, P.; Gil, C.; Guardia, R.; Moreno, M.-J.; Martínez-Carballeira, D.; García-Cadenas, I.; Vives, S.; et al. Unique clinico-biological, genetic and prognostic features of adult early T-cell precursor acute lymphoblastic leukemia. Haematologica 2020, 105, e294–e297. [Google Scholar] [CrossRef]
- Castaneda Puglianini, O.; Papadantonakis, N. Early precursor T-cell acute lymphoblastic leukemia: Current paradigms and evolving concepts. Ther. Adv. Hematol. 2020, 11, 2040620720929475. [Google Scholar] [CrossRef]
- Rymkiewicz, G.; Grygalewicz, B.; Chechlinska, M.; Blachnio, K.; Bystydzienski, Z.; Romejko-Jarosinska, J.; Woroniecka, R.; Zajdel, M.; Domanska-Czyz, K.; Martin-Garcia, D.; et al. A comprehensive flow-cytometry-based immunophenotypic characterization of Burkitt-like lymphoma with 11q aberration. Mod. Pathol. 2018, 31, 732–743. [Google Scholar] [CrossRef]
- Woroniecka, R.; Rymkiewicz, G.; Grygalewicz, B.; Blachnio, K.; Rygier, J.; Jarmuz-Szymczak, M.; Ratajczak, B.; Pieńkowska-Grela, B. Cytogenetic and flow cytometry evaluation of Richter syndrome reveals MYC, CDKN2A, IGH alterations with loss of CD52, CD62L and increase of CD71 antigen expression as the most frequent recurrent abnormalities. Am. J. Clin. Pathol. 2015, 143, 25–35. [Google Scholar] [CrossRef]
- Ostrowska, B.; Rymkiewicz, G.; Domanska-Czyz, K.; Romejko-Jarosińska, J.; Błachnio, K.; Popławska, L.; Michalski, W.; Walewski, J. Pro-T cell ALL/LBL: An ultra-high risk CD2-negative disease subtype in adults defined by flow cytometry. Haematologica 2017, 102, 30–31. [Google Scholar]
- Ostrowska, B.; Rymkiewicz, G.; Romejko-Jarosinska, J.; Blachnio, K.; Domanska-Czyz, K.; Osiadacz, W.; Osowiecki, M.; Paszkiewicz-Kozik, E.; Poplawska, L.; Rozewska, D.; et al. Fine Needle Aspiration Biopsy with Flow Cytometry is a Reliable Method for Defining Immunophenotype of Prognostic Value in T Lymphoblastic Lymphoma. Blood 2009, 114, 3928. [Google Scholar] [CrossRef]
- Gökbuget, N.; Arnold, R.; Böhme, A.; Fietkau, R.; Freund, M.; Ganser, A.; Kneba, M.; Lipp, T.; Ludwig, W.-D.; Maschmeyer, G.; et al. Treatment of Adult ALL According to Protocols of the German Multicenter Study Group for Adult ALL (GMALL). In Acute Leukemias; Springer: Berlin/Heidelberg, Germany, 2008; pp. 167–176. [Google Scholar]
- Czuczman, M.S.; Dodge, R.K.; Stewart, C.C.; Frankel, S.R.; Davey, F.R.; Powell, B.L.; Szatrowski, T.P.; Schiffer, C.A.; Larson, R.A.; Bloomfield, C.D. Value of immunophenotype in intensively treated adult acute lymphoblastic leukemia: Cancer and leukemia Group B study 8364. Blood 1999, 93, 3931–3939. [Google Scholar] [PubMed]
- Asnafi, V.; Radford-Weiss, I.; Dastugue, N.; Bayle, C.; Leboeuf, D.; Charrin, C.; Garand, R.; Lafage-Pochitaloff, M.; Delabesse, E.; Buzyn, A.; et al. CALM-AF10 is a common fusion transcript in T-ALL and is specific to the TCRgammadelta lineage. Blood 2003, 102, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Asnafi, V.; Buzyn, A.; Thomas, X.; Huguet, F.; Vey, N.; Boiron, J.M.; Reman, O.; Cayuela, J.-M.; Lheritier, V.; Vernant, J.-P.; et al. Impact of TCR status and genotype on outcome in adult T-cell acute lymphoblastic leukemia: A LALA-94 study. Blood 2005, 105, 3072–3078. [Google Scholar] [CrossRef]
- Baleydier, F.; Decouvelaere, A.V.; Bergeron, J.; Gaulard, P.; Canioni, D.; Bertrand, Y.; Lepretre, S.; Petit, B.; Dombret, H.; Beldjord, K.; et al. T cell receptor genotyping and HOXA/TLX1 expression define three T lymphoblastic lymphoma subsets which might affect clinical outcome. Clin. Cancer Res. 2008, 14, 692–700. [Google Scholar] [CrossRef]
- Moingeon, P.; Chang, H.C.; Wallner, B.P.; Stebbins, C.; Frey, A.Z.; Reinherz, E.L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature 1989, 339, 312–314. [Google Scholar] [CrossRef]
- Crawford, K.; Stark, A.; Kitchens, B.; Sternheim, K.; Pantazopoulos, V.; Triantafellow, E.; Wang, Z.; Vasir, B.; Larsen, C.E.; Gabuzdaet, D.; et al. CD2 engagement induces dendritic cell activation: Implications for immune surveillance and T-cell activation. Blood 2003, 102, 1745–1752. [Google Scholar] [CrossRef]

| Variables | n | OS | p | EFS | p | ||
|---|---|---|---|---|---|---|---|
| 5 Year (%) (95% CI) | 10 Year (%) (95% CI) | 5 Year (%) (95% CI) | 10 Year (%) (95% CI) | ||||
| 49 | 62 (48; 76) | 59 (44; 74) | - | 48 (33; 63) | 43 (28; 58) | - | |
| Age ≤ 35 Age > 35 | 35 14 | 76 (62, 91) 28 (5, 52) | 76 (62, 91) 14 (0, 37) | <0.001 | 73 (58, 89) 26 (2, 50) | 73 (58, 89) 13 (0, 34) | <0.001 |
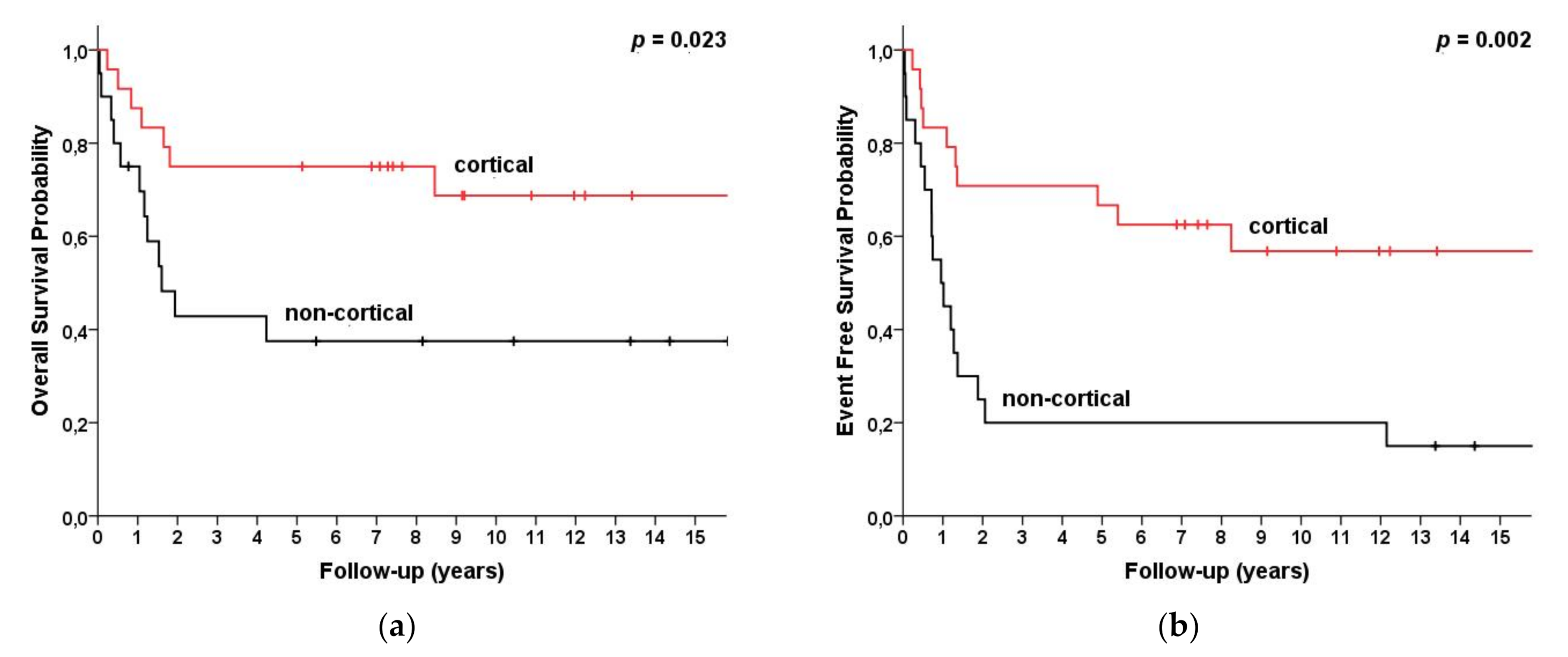
| Cortical Non-cortical | 28 19 | 75 (58, 92) 38 (16, 59) | 69 (49, 89) 38 (16, 59) | 0.023 | 75 (58, 92) 31 (8, 54) | 68 (48, 88) 31 (8, 54) | 0.002 |
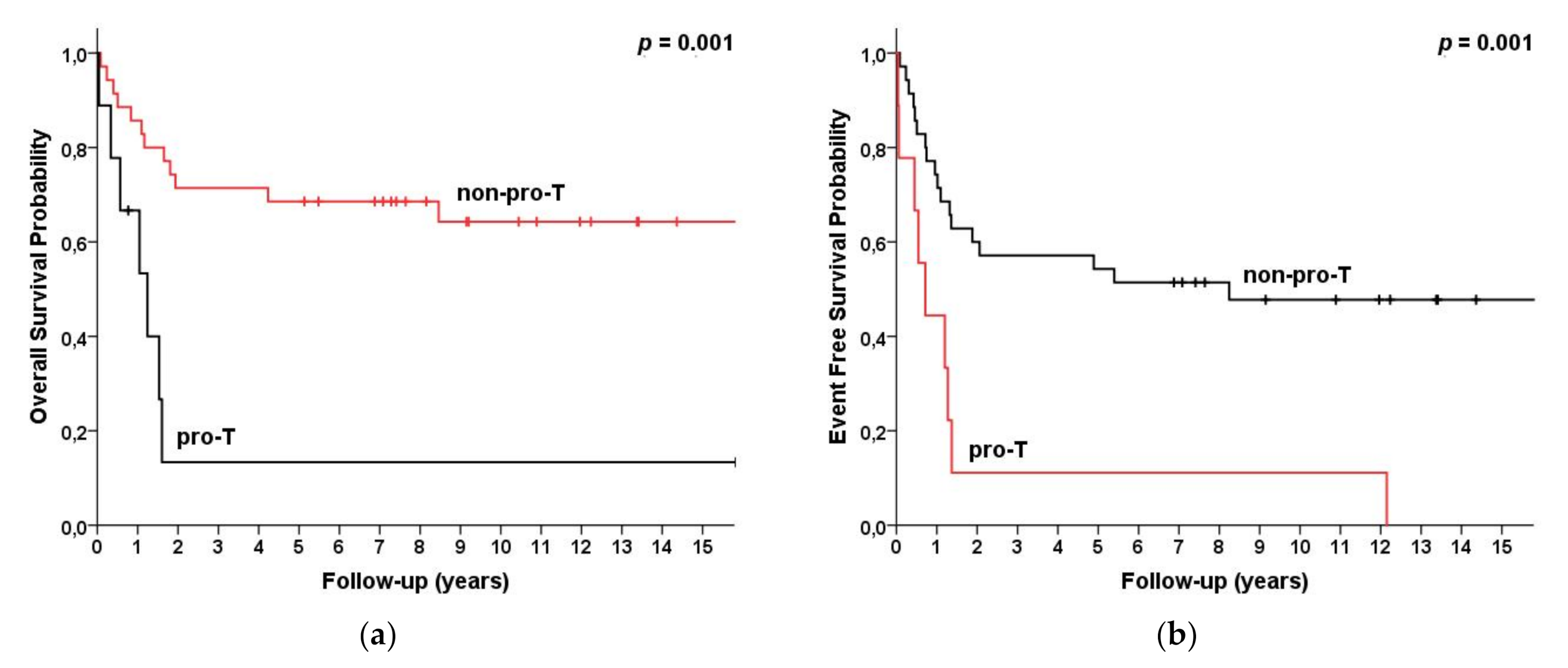
| Pro-T Non-proT | 9 35 | 13 (0, 38) 69 (53, 84) | 13 (0, 25) 64 (47, 81) | 0.001 | 13 (0, 38) 67 (51, 83) | 13 (0, 38) 63 (45, 80) | <0.001 |
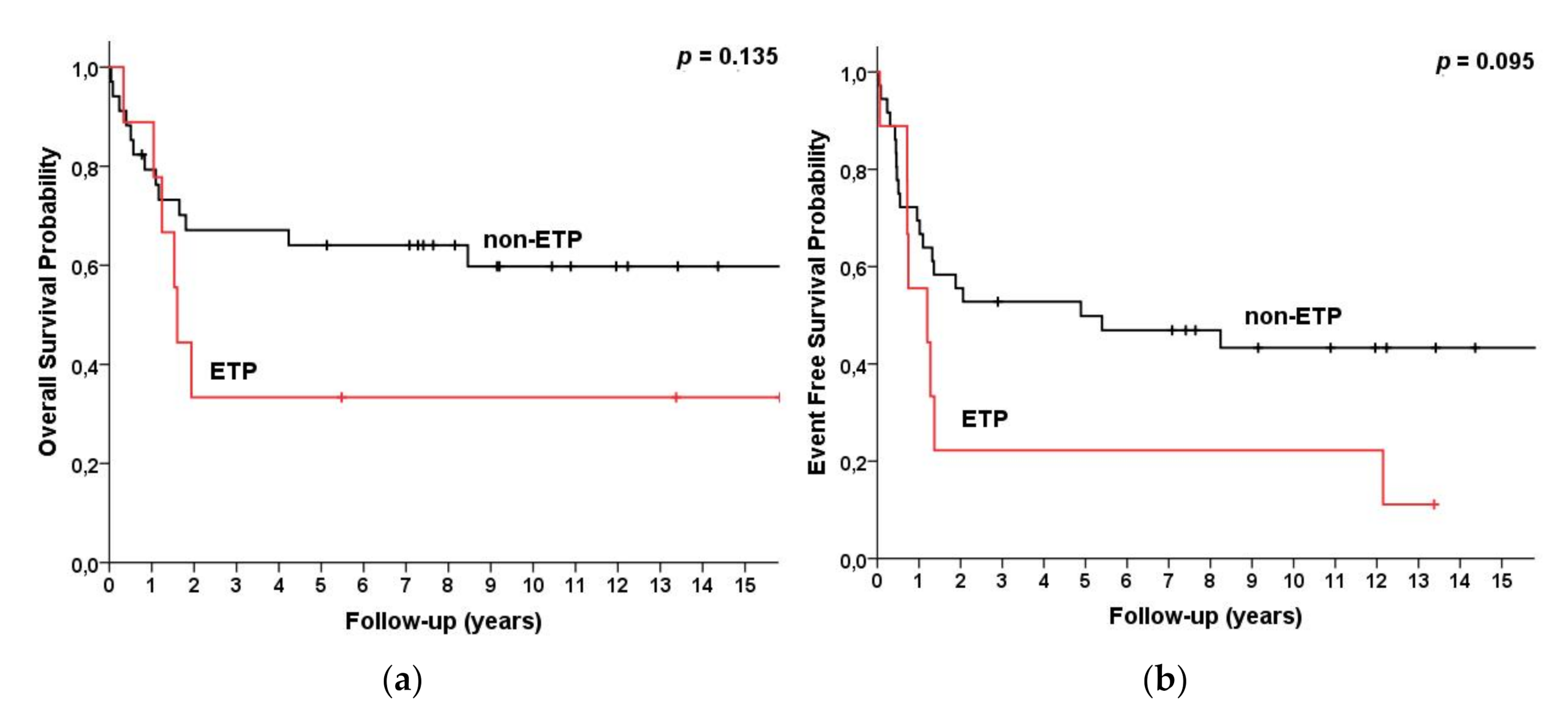
| ETP Non-ETP | 9 38 | 33 (3, 64) 66 (50, 82) | 33 (3, 64) 61 (45, 78) | 0.135 | 26 (0, 57) 63 (47, 80) | 26 (0, 57) 59 (41, 76) | 0.095 |
| CD1a(+) CD1a(−) | 28 19 | 78 (62, 94) 34 (12, 56) | 72 (54, 90) 34 (12, 56) | 0.002 | 76 (59, 93) 28 (6, 51) | 69 (49, 89) 28 (6, 51) | 0.001 |
| CD2(+) CD2(−) | 34 13 | 73 (58, 88) 26 (1, 51) | 68 (52, 85) 26 (1, 51) | 0.001 | 71 (55, 87) 19 (0, 43) | 66 (48, 84) 19 (0, 43) | <0.001 |
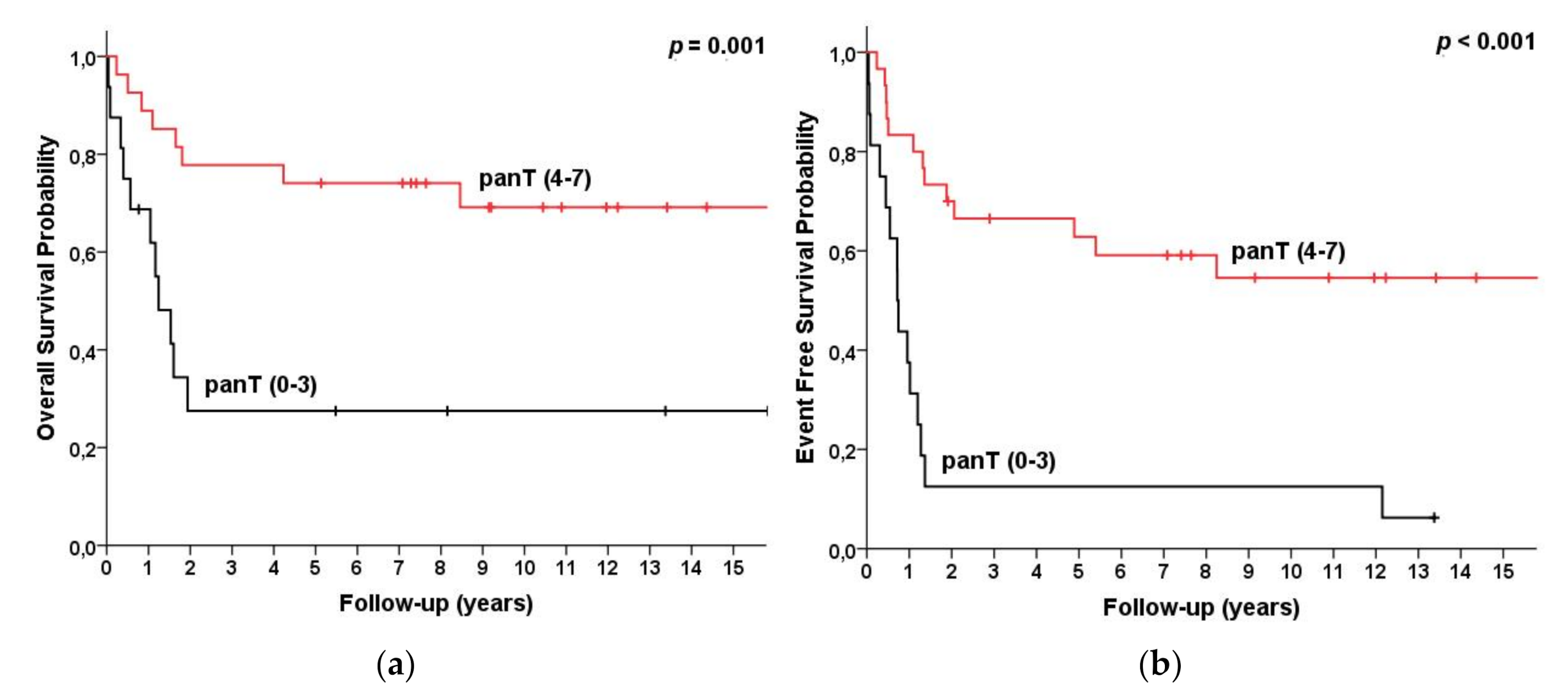
| 0–3 panT Ags expressed | 16 | 28 (5, 50) | 28 (5, 50) | 0.001 | 18 (0, 40) | 18 (0, 46) | <0.001 |
| 4–7 panT Ags expressed | 30 | 76 (61, 92) | 71(54, 89) | 0.001 | 74 (57, 72) | 68 (49, 87) | <0.001 |
| CPHM | Variables | HR | 95%CI | p |
|---|---|---|---|---|
| OS | Age > 35 CD2 negative | 5.39 5.10 | 2.10–13.8 1.93–13.49 | <0.001 0.001 |
| EFS | Age > 35 CD2 negative | 3.77 4.52 | 1.72–8.27 2.03–10.06 | 0.001 <0.001 |
| LRM | Variables | OR | 95%CI | p |
| CR | Age > 35 CD2 negative | 0.039 0.193 | 0.004–0.413 0.025–1.50 | 0.007 0.116 |
| n (%) | Total n = 47 (100%) | CD2(+) n = 34 (100%) | CD2(−) n = 13 (100%) |
|---|---|---|---|
| Gender, male | 36 (76.5%) | 24 (70.5%) | 12 (92%) |
| Age, median (range) <35 year | 28 (18, 58) 33 (70%) | 26 (18, 58) 25 (73.5%) | 30 (18, 57) 8 (61%) |
| Treatment | |||
| GMALL 05/93 GMALL 01/2004 | 18 (38%) 29 (62%) | 13 (38%) 21 (62%) | 5 (38.5%) 8 (61.5%) |
| Bone marrow involvement (<25%) | 11 (23%) | 7 (20.5%) | 4 (30.5%) |
| CNS involvement | 3 (6%) | 2 (6%) | 1 (7.5%) |
| median (range) | median (range) | median (range) | |
| WBC (G/L) HGB (g/dl) PLT (G/L) | 9.2 (2.5–21.2) 13.8 (9.1–17.7) 317 (151–788) | 8.6 (2.5–21.2) 13.4 (9.1–16.9) 296 (151–754) | 10 (3–18) 14 (9.3–17.7) 349 (198–788) |
| Immunophenotype | |||
| Pro-T Pre-T Cortical mature | 9 (19%) 7 (15%) 28 (59.5%) 3 (6.5) | 07 (20.5%) 26 (76.5%) 1 (3%) | 9 (69%) 0 2 (15.5%) 2 (15.5%) |
| ETP * | 9 (20%) | 3 (9%) | 6 (46%) |
| CR | 40 (85%) | 31 (91%) | 9 (69%) |
| progression | 17 (36%) | 7 (20.5%) | 10 (77%) |
| alive | 28 (59.5%) | 24 (70.5%) | 4 (30.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, B.; Rymkiewicz, G.; Chechlinska, M.; Blachnio, K.; Domanska-Czyz, K.; Bystydzienski, Z.; Romejko-Jarosinska, J.; Borysiuk, A.; Rybski, S.; Michalski, W.; et al. Prognostic Value of the Immunological Subtypes of Adolescent and Adult T-Cell Lymphoblastic Lymphoma; an Ultra-High-Risk Pro-T/CD2(−) Subtype. Cancers 2021, 13, 1911. https://doi.org/10.3390/cancers13081911
Ostrowska B, Rymkiewicz G, Chechlinska M, Blachnio K, Domanska-Czyz K, Bystydzienski Z, Romejko-Jarosinska J, Borysiuk A, Rybski S, Michalski W, et al. Prognostic Value of the Immunological Subtypes of Adolescent and Adult T-Cell Lymphoblastic Lymphoma; an Ultra-High-Risk Pro-T/CD2(−) Subtype. Cancers. 2021; 13(8):1911. https://doi.org/10.3390/cancers13081911
Chicago/Turabian StyleOstrowska, Beata, Grzegorz Rymkiewicz, Magdalena Chechlinska, Katarzyna Blachnio, Katarzyna Domanska-Czyz, Zbigniew Bystydzienski, Joanna Romejko-Jarosinska, Anita Borysiuk, Sebastian Rybski, Wojciech Michalski, and et al. 2021. "Prognostic Value of the Immunological Subtypes of Adolescent and Adult T-Cell Lymphoblastic Lymphoma; an Ultra-High-Risk Pro-T/CD2(−) Subtype" Cancers 13, no. 8: 1911. https://doi.org/10.3390/cancers13081911
APA StyleOstrowska, B., Rymkiewicz, G., Chechlinska, M., Blachnio, K., Domanska-Czyz, K., Bystydzienski, Z., Romejko-Jarosinska, J., Borysiuk, A., Rybski, S., Michalski, W., & Walewski, J. (2021). Prognostic Value of the Immunological Subtypes of Adolescent and Adult T-Cell Lymphoblastic Lymphoma; an Ultra-High-Risk Pro-T/CD2(−) Subtype. Cancers, 13(8), 1911. https://doi.org/10.3390/cancers13081911






