Clinical Applications of Genomic Alterations in ATLL: Predictive Markers and Therapeutic Targets
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview of Genomic Alterations of ATLL
3. Mutations of Genes Involved in TCR/NF-κB Signaling
4. Immune Escape Mechanisms in ATLL
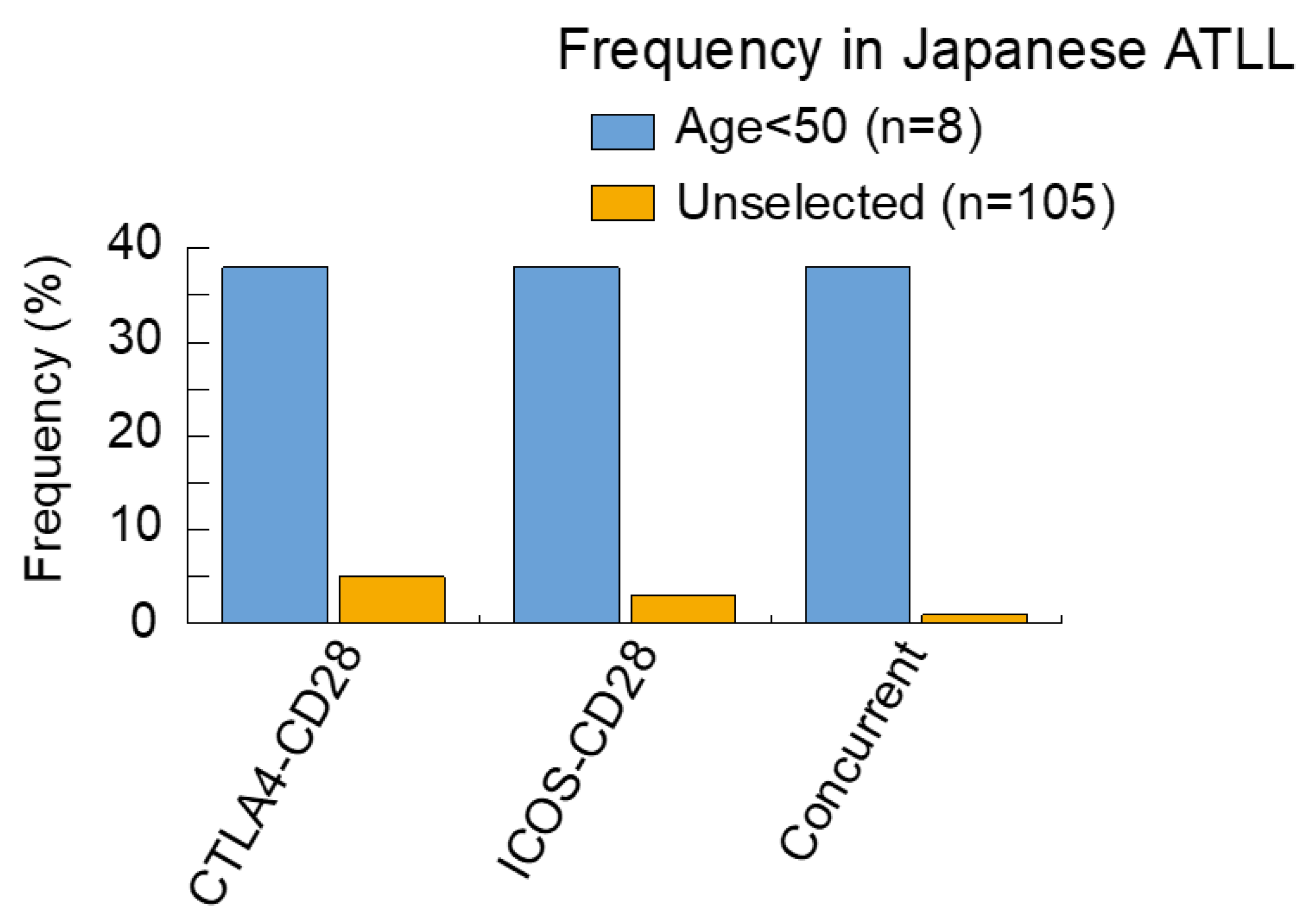
5. Heterogeneity of Genomic Alterations in ATLL: Geographic Region and Age at Diagnosis
6. Characteristic Genomic Alterations in ATLL Compared with Other PTCLs
7. Genomic Alterations in ATLL as Prognostic Markers
8. Precision Targets from ATLL Genomic Studies
8.1. Mogamulizumab
8.2. Immune Checkpoint Inhibitors
8.3. Lenalidomide
8.4. Other Potential Targeted Therapies
9. Targetable Super-Enhancers in ATLL
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef]
- Vose, J.; Armitage, J.; Weisenburger, D.; International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef]
- Edlich, R.F.; Arnette, J.A.; Williams, F.M. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J. Emerg. Med. 2000, 18, 109–119. [Google Scholar] [CrossRef]
- Ichimaru, M.; Ikeda, S.; Kinoshita, K.; Hino, S.; Tsuji, Y. Mother-to-child transmission of HTLV-1. Cancer Detect. Prev. 1991, 15, 177–181. [Google Scholar] [PubMed]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Tamura, K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014, 15, e517–e526. [Google Scholar] [CrossRef]
- Chihara, D.; Ito, H.; Matsuda, T.; Katanoda, K.; Shibata, A.; Taniguchi, S.; Utsunomiya, A.; Sobue, T.; Matsuo, K. Association between decreasing trend in the mortality of adult T-cell leukemia/lymphoma and allogeneic hematopoietic stem cell transplants in Japan: Analysis of Japanese vital statistics and Japan Society for Hematopoietic Cell Transplantation (JSHCT). Blood Cancer J. 2013, 3, e159. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Jo, T.; Takemoto, S.; Suzushima, H.; Uozumi, K.; Yamamoto, K.; Uike, N.; Saburi, Y.; Nosaka, K.; Utsunomiya, A.; et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br. J. Haematol. 2015, 169, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A. Progress in Allogeneic Hematopoietic Cell Transplantation in Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2019, 10, 2235. [Google Scholar] [CrossRef]
- Tokudome, S.; Tokunaga, O.; Shimamoto, Y.; Miyamoto, Y.; Sumida, I.; Kikuchi, M.; Takeshita, M.; Ikeda, T.; Fujiwara, K.; Yoshihara, M.; et al. Incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type I carriers in Saga, Japan. Cancer Res. 1989, 49, 226–228. [Google Scholar]
- Tajima, K. 4th nation-wide study of adult T-cell leukemia/lymphoma (ATL) in Japan: Estimates of risk of ATL and its geographical and clinical features. Int. J. Cancer 1990, 45, 237–243. [Google Scholar] [CrossRef]
- Arisawa, K.; Soda, M.; Endo, S.; Kurokawa, K.; Katamine, S.; Shimokawa, I.; Koba, T.; Takahashi, T.; Saito, H.; Doi, H.; et al. Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int. J. Cancer 2000, 85, 319–324. [Google Scholar] [CrossRef]
- Iwanaga, M. Epidemiology of HTLV-1 Infection and ATL in Japan: An Update. Front. Microbiol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Kondo, T.; Kono, H.; Miyamoto, N.; Yoshida, R.; Toki, H.; Matsumoto, I.; Hara, M.; Inoue, H.; Inatsuki, A.; Funatsu, T.; et al. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int. J. Cancer 1989, 43, 1061–1064. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.-R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: A nationwide prospective study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef]
- Sonoda, S.; Li, H.C.; Tajima, K. Ethnoepidemiology of HTLV-1 related diseases: Ethnic determinants of HTLV-1 susceptibility and its worldwide dispersal. Cancer Sci. 2011, 102, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, S.; Ichikawa, T.; Maneesaay, P.; Saito, Y.; Nagai, K.; Tamura, T.; Manachai, N.; Yamakawa, N.; Hamasaki, M.; Kitabayashi, I.; et al. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat. Commun. 2014, 5, 3393. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Karube, K.; Utsunomiya, A.; Tsukasaki, K.; Imaizumi, Y.; Taira, N.; Uike, N.; Umino, A.; Arita, K.; Suguro, M.; et al. Molecular Characterization of Chronic-type Adult T-cell Leukemia/Lymphoma. Cancer Res. 2014, 74, 6129–6138. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.-I.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.A.; Chung, E.Y.; Giricz, O.; Pradhan, K.; Kataoka, K.; Gordon-Mitchell, S.; Bhagat, T.D.; Mai, Y.; Wei, Y.; Ishida, E.; et al. North American ATLL has a distinct mutational and transcriptional profile and responds to epigenetic therapies. Blood 2018, 132, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Shigemori, K.; Donaldson, N.; Trevisani, C.; Cordero, N.A.; Stevenson, K.E.; Bu, X.; Arakawa, F.; Takeuchi, M.; Ohshima, K.; et al. Genomic landscape of young ATLL patients identifies frequent targetable CD28 fusions. Blood 2020, 135, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Kataoka, K. Genetic alterations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017, 108, 1719–1725. [Google Scholar] [CrossRef]
- Shaffer, A.L.; Emre, N.C.T.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nat. Cell Biol. 2008, 454, 226–231. [Google Scholar] [CrossRef]
- Yang, Y.; Shaffer, A.L., III; Emre, N.T.; Ceribelli, M.; Zhang, M.; Wright, G.; Xiao, W.; Powell, J.; Platig, J.; Kohlhammer, H.; et al. Exploiting Synthetic Lethality for the Therapy of ABC Diffuse Large B Cell Lymphoma. Cancer Cell 2012, 21, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Yoshida, N.; Christie, A.L.; Ghandi, M.; Dharia, N.V.; Dempster, J.; Murakami, M.; Shigemori, K.; Morrow, S.N.; Van Scoyk, A.; et al. Targetable vulnerabilities in T- and NK-cell lymphomas identified through preclinical models. Nat. Commun. 2018, 9, 2024. [Google Scholar] [CrossRef]
- Watatani, Y.; Sato, Y.; Miyoshi, H.; Sakamoto, K.; Nishida, K.; Gion, Y.; Nagata, Y.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 2019, 33, 2867–2883. [Google Scholar] [CrossRef]
- McKinney, M.; Moffitt, A.B.; Gaulard, P.; Travert, M.; De Leval, L.; Nicolae, A.; Raffeld, M.; Jaffe, E.S.; Pittaluga, S.; Xi, L.; et al. The Genetic Basis of Hepatosplenic T-cell Lymphoma. Cancer Discov. 2017, 7, 369–379. [Google Scholar] [CrossRef]
- Matsuoka, M.; Jeang, K.-T. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: Viral infectivity, Tax, HBZ and therapy. Oncogene 2010, 30, 1379–1389. [Google Scholar] [CrossRef]
- Kawano, N.; Shimoda, K.; Ishikawa, F.; Taketomi, A.; Yoshizumi, T.; Shimoda, S.; Yoshida, S.; Uozumi, K.; Suzuki, S.; Maehara, Y.; et al. Adult T-cell Leukemia Development from a Human T-cell Leukemia Virus Type I Carrier After a Living-Donor Liver Transplantation. Transplantation 2006, 82, 840–843. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Shirabe, K.; Ikegami, T.; Kayashima, H.; Yamashita, N.; Morita, K.; Masuda, T.; Hashimoto, N.; Taketomi, A.; Soejima, Y.; et al. Impact of Human T Cell Leukemia Virus Type 1 in Living Donor Liver Transplantation. Am. J. Transplant. 2012, 12, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 2016, 534, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Challa-Malladi, M.; Lieu, Y.K.; Califano, O.; Holmes, A.B.; Bhagat, G.; Murty, V.V.; Dominguez-Sola, D.; Pasqualucci, L.; Dalla-Favera, R. Combined Genetic Inactivation of β2-Microglobulin and CD58 Reveals Frequent Escape from Immune Recognition in Diffuse Large B Cell Lymphoma. Cancer Cell 2011, 20, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Ishida, T.; Inagaki, A.; Ishii, T.; Kusumoto, S.; Komatsu, H.; Iida, S.; Utsunomiya, A.; Ueda, R. Regulatory T-cell function of adult T-cell leukemia/lymphoma cells. Int. J. Cancer 2007, 120, 2052–2057. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Suarez, F.; Fields, P.; Hermine, O. How I treat adult T-cell leukemia/lymphoma. Blood 2011, 118, 1736–1745. [Google Scholar] [CrossRef]
- Karube, K.; Ohshima, K.; Tsuchiya, T.; Yamaguchi, T.; Kawano, R.; Suzumiya, J.; Utsunomiya, A.; Harada, M.; Kikuchi, M. Expression of FoxP3, a key molecule in CD4+ CD25+ regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br. J. Haematol. 2004, 126, 81–84. [Google Scholar] [CrossRef]
- Roncador, G.; Garcia, J.F.; Garcia, J.F.; Maestre, L.; Lucas, E.; Menarguez, J.; Ohshima, K.; Nakamura, S.; Banham, A.H.; A Piris, M. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia 2005, 19, 2247–2253. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shaffer, A.L., III; Ceribelli, M.; Zhang, M.; Wright, G.; Huang, D.W.; Xiao, W.; Powell, J.; Petrus, M.N.; Yang, Y.; et al. Targeting the HTLV-I-Regulated BATF3/IRF4 Transcriptional Network in Adult T Cell Leukemia/Lymphoma. Cancer Cell 2018, 34, 286–297. [Google Scholar] [CrossRef]
- Coelho, M.A.; de Carne Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099. [Google Scholar] [CrossRef]
- Tagaya, Y.; Matsuoka, M.; Gallo, R. 40 years of the human T-cell leukemia virus: Past, present, and future. F1000Research 2019, 8, 228. [Google Scholar] [CrossRef]
- Yoshida, N.; Chihara, D. Incidence of Adult T-Cell Leukemia/Lymphoma in Nonendemic Areas. Curr. Treat. Options Oncol. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-Cell Leukemia: A Review of Epidemiological Evidence. Front. Microbiol. 2012, 3, 322. [Google Scholar] [CrossRef] [PubMed]
- Chiu, E.; Samra, B.; Tam, E.; Baseri, B.; Lin, B.; Luhrs, C.; Gonsky, J.; Sawas, A.; Taiwo, E.; Sidhu, G. Clinical Characteristics and Outcomes of Caribbean Patients with Adult T-Cell Lymphoma/Leukemia at Two Affiliated New York City Hospitals. JCO Glob. Oncol. 2020, 6, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580. [Google Scholar] [CrossRef]
- Choudhuri, J.; Geiser Roberts, L.; Zhang, Y.; Wang, Y.; Fang, Y. A Rare CD4−CD8+ Adult T-Cell Leukemia/Lymphoma with Unique Molecular Mutations: A Case Report with Literature Review. Case Rep. Hematol. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Hashemi Zonouz, T.; Abdulbaki, R.; Bandyopadhyay, B.; Nava, V. Novel Mutations in a Lethal Case of Lymphomatous Adult T Cell Lymphoma with Cryptic Myocardial Involvement. Curr. Oncol. 2021, 28, 79. [Google Scholar] [CrossRef] [PubMed]
- Rusch, M.; Nakitandwe, J.; Shurtleff, S.; Newman, S.; Zhang, Z.; Edmonson, M.N.; Parker, M.; Jiao, Y.; Ma, X.; Liu, Y.; et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat. Commun. 2018, 9, 3962. [Google Scholar] [CrossRef]
- Iacobucci, I.; Wen, J.; Meggendorfer, M.; Choi, J.K.; Shi, L.; Pounds, S.B.; Carmichael, C.L.; Masih, K.E.; Morris, S.M.; Lindsley, R.C.; et al. Genomic subtyping and therapeutic targeting of acute erythroleukemia. Nat. Genet. 2019, 51, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci. 2007, 98, 772–778. [Google Scholar] [CrossRef]
- Nakagawa, M.; Nakagawa-Oshiro, A.; Karnan, S.; Tagawa, H.; Utsunomiya, A.; Nakamura, S.; Takeuchi, I.; Ohshima, K.; Seto, M. Array Comparative Genomic Hybridization Analysis of PTCL-U Reveals a Distinct Subgroup with Genetic Alterations Similar to Lymphoma-Type Adult T-Cell Leukemia/Lymphoma. Clin. Cancer Res. 2009, 15, 30–38. [Google Scholar] [CrossRef]
- Umino, A.; Nakagawa, M.; Utsunomiya, A.; Tsukasaki, K.; Taira, N.; Katayama, N.; Seto, M. Clonal evolution of adult T-cell leukemia/lymphoma takes place in the lymph nodes. Blood 2011, 117, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Umino, A.; Liu, F.; Arita, K.; Karube, K.; Tsuzuki, S.; Ohshima, K.; Seto, M. Identification of multiple subclones in peripheral T-cell lymphoma, not otherwise specified with genomic aberrations. Cancer Med. 2012, 1, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Su, T.T.; Guo, B.; Kawakami, Y.; Sommer, K.; Chae, K.; Humphries, L.A.; Kato, R.M.; Kang, S.; Patrone, L.; Wall, R.; et al. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002, 3, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, K.; Gomez, F.; White, B.S.; Matlock, M.; Miller, C.A.; Trani, L.; Fronick, C.C.; Fulton, R.S.; Kreisel, F.; Cashen, A.F.; et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood 2017, 129, 473–483. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Ho, I.-C.; Tai, T.-S.; Pai, S.-Y. GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009, 9, 125–135. [Google Scholar] [CrossRef]
- Belver, L.; Yang, A.Y.; Albero, R.; Herranz, D.; Brundu, F.G.; Quinn, S.A.; Pérez-Durán, P.; Álvarez, S.; Gianni, F.; Rashkovan, M.; et al. GATA3-Controlled Nucleosome Eviction Drives MYC Enhancer Activity in T-cell Development and Leukemia. Cancer Discov. 2019, 9, 1774–1791. [Google Scholar] [CrossRef]
- Katsuya, H.; Yamanaka, T.; Ishitsuka, K.; Utsunomiya, A.; Sasaki, H.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; et al. Prognostic Index for Acute- and Lymphoma-Type Adult T-Cell Leukemia/Lymphoma. J. Clin. Oncol. 2012, 30, 1635–1640. [Google Scholar] [CrossRef]
- Fukushima, T.; Nomura, S.; Shimoyama, M.; Shibata, T.; Imaizumi, Y.; Moriuchi, Y.; Tomoyose, T.; Uozumi, K.; Kobayashi, Y.; Fukushima, N.; et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A). Br. J. Haematol. 2014, 166, 739–748. [Google Scholar] [CrossRef]
- Katsuya, H.; Shimokawa, M.; Ishitsuka, K.; Kawai, K.; Amano, M.; Utsunomiya, A.; Hino, R.; Hanada, S.; Jo, T.; Tsukasaki, K.; et al. Prognostic index for chronic- and smoldering-type adult T-cell leukemia-lymphoma. Blood 2017, 130, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Miyoshi, H.; Asano, N.; Yoshida, N.; Yamada, K.; Yanagida, E.; Moritsubo, M.; Nakata, M.; Umeno, T.; Suzuki, T.; et al. Human leukocyte antigen class II expression is a good prognostic factor in adult T-cell leukemia/lymphoma. Haematologica 2019, 104, 1626–1632. [Google Scholar] [CrossRef]
- Steidl, C.; Shah, S.P.; Woolcock, B.W.; Rui, L.; Kawahara, M.; Farinha, P.; Johnson, N.A.; Zhao, Y.; Telenius, A.; Ben Neriah, S.; et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nat. Cell Biol. 2011, 471, 377–381. [Google Scholar] [CrossRef]
- Christopher, M.J.; Petti, A.A.; Rettig, M.P.; Miller, C.A.; Chendamarai, E.; Duncavage, E.J.; Klco, J.M.; Helton, N.M.; O’Laughlin, M.; Fronick, C.C.; et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N. Engl. J. Med. 2018, 379, 2330–2341. [Google Scholar] [CrossRef]
- Ennishi, D.; Takata, K.; Béguelin, W.; Duns, G.; Mottok, A.; Farinha, P.; Bashashati, A.; Saberi, S.; Boyle, M.; Meissner, B.; et al. Molecular and Genetic Characterization of MHC Deficiency Identifies EZH2 as Therapeutic Target for Enhancing Immune Recognition. Cancer Discov. 2019, 9, 546–563. [Google Scholar] [CrossRef]
- Sasaki, D.; Imaizumi, Y.; Hasegawa, H.; Osaka, A.; Tsukasaki, K.; Choi, Y.L.; Mano, H.; Marquez, V.E.; Hayashi, T.; Yanagihara, K.; et al. Overexpression of enhancer of zeste homolog 2 with trimethylation of lysine 27 on histone H3 in adult T-cell leukemia/lymphoma as a target for epigenetic therapy. Haematologica 2011, 96, 712–719. [Google Scholar] [CrossRef]
- Yamagishi, M.; Nakano, K.; Miyake, A.; Yamochi, T.; Kagami, Y.; Tsutsumi, A.; Matsuda, Y.; Sato-Otsubo, A.; Muto, S.; Utsunomiya, A.; et al. Polycomb-Mediated Loss of miR-31 Activates NIK-Dependent NF-κB Pathway in Adult T Cell Leukemia and Other Cancers. Cancer Cell 2012, 21, 121–135. [Google Scholar] [CrossRef]
- Yamagishi, M.; Hori, M.; Fujikawa, D.; Ohsugi, T.; Honma, D.; Adachi, N.; Katano, H.; Hishima, T.; Kobayashi, S.; Nakano, K.; et al. Targeting Excessive EZH1 and EZH2 Activities for Abnormal Histone Methylation and Transcription Network in Malignant Lymphomas. Cell Rep. 2019, 29, 2321–2337. [Google Scholar] [CrossRef]
- Kataoka, K.; Iwanaga, M.; Yasunaga, J.-I.; Nagata, Y.; Kitanaka, A.; Kameda, T.; Yoshimitsu, M.; Shiraishi, Y.; Sato-Otsubo, A.; Sanada, M.; et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood 2018, 131, 215–225. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kiyasu, J.; Kato, T.; Yoshida, N.; Shimono, J.; Yokoyama, S.; Taniguchi, H.; Sasaki, Y.; Kurita, D.; Kawamoto, K.; et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 2016, 128, 1374–1381. [Google Scholar] [CrossRef]
- Asano, N.; Miyoshi, H.; Kato, T.; Shimono, J.; Yoshida, N.; Kurita, D.; Sasaki, Y.; Kawamoto, K.; Ohshima, K.; Seto, M. Expression pattern of immunosurveillance-related antigen in adult T cell leukaemia/lymphoma. Histopathology 2018, 72, 945–954. [Google Scholar] [CrossRef]
- Yoshida, N.; Weinstock, D.M. Clinicogenetic risk modeling in ATL. Blood 2018, 131, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Dillon, R.; Witkover, A.; Melamed, A.; Demontis, M.-A.; Gillet, N.A.; Mun, L.J.; Bangham, C.R.M.; Cook, L.B.; Fields, P.A.; et al. Evolution of retrovirus-infected premalignant T-cell clones prior to adult T-cell leukemia/lymphoma diagnosis. Blood 2020, 135, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Utsunomiya, A.; Iida, S.; Inagaki, H.; Takatsuka, Y.; Kusumoto, S.; Takeuchi, G.; Shimizu, S.; Ito, M.; Komatsu, H.; et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003, 9, 3625–3634. [Google Scholar] [PubMed]
- Nakagawa, M.; Schmitz, R.; Xiao, W.; Goldman, C.K.; Xu, W.; Yang, Y.; Yu, X.; Waldmann, T.A.; Staudt, L.M. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J. Exp. Med. 2014, 211, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Miyoshi, H.; Kato, T.; Sakata-Yanagimoto, M.; Niino, D.; Taniguchi, H.; Moriuchi, Y.; Miyahara, M.; Kurita, D.; Sasaki, Y.; et al. CCR4frameshift mutation identifies a distinct group of adult T cell leukaemia/lymphoma with poor prognosis. J. Pathol. 2016, 238, 621–626. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishida, T.; Masaki, A.; Murase, T.; Yonekura, K.; Tashiro, Y.; Tokunaga, M.; Utsunomiya, A.; Ito, A.; Kusumoto, S.; et al. CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood 2018, 132, 758–761. [Google Scholar] [CrossRef]
- Kawano, N.; Yoshida, N.; Kawano, S.; Arakawa, F.; Miyoshi, H.; Yamada, K.; Nakashima, K.; Yoshida, S.; Kuriyama, T.; Tochigi, T.; et al. Clinical Features, Pathological Features, and Treatment Outcomes of 22 Patients with Aggressive Adult T-cell Leukemia-lymphoma Treated with a Humanized CCR4 Antibody (Mogamulizumab) at a Single Institution during a 6-year Period (2012–2018). Intern. Med. 2019, 58, 2159–2166. [Google Scholar] [CrossRef]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef]
- Rauch, D.A.; Conlon, K.C.; Janakiram, M.; Brammer, J.E.; Harding, J.C.; Ye, B.H.; Zang, X.; Ren, X.; Olson, S.; Cheng, X.; et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019, 134, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Patterson, S.J.; Levings, M.K. The Role of the PI3K Signaling Pathway in CD4+ T Cell Differentiation and Function. Front. Immunol. 2012, 3, 245. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.J.; Tan, T.K.; Amanda, S.; Ngoc, P.C.T.; Leong, W.Z.; Tan, S.H.; Asamitsu, K.; Hibi, Y.; Ueda, R.; Okamoto, T.; et al. Feed-forward regulatory loop driven by IRF4 and NF-κB in adult T-cell leukemia/lymphoma. Blood 2020, 135, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Braggio, E.; Shi, C.-X.; Bruins, L.A.; Schmidt, J.E.; Van Wier, S.; Chang, X.-B.; Bjorklund, C.C.; Fonseca, R.; Bergsagel, P.L.; et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011, 118, 4771–4779. [Google Scholar] [CrossRef]
- Ishida, T.; Fujiwara, H.; Nosaka, K.; Taira, N.; Abe, Y.; Imaizumi, Y.; Moriuchi, Y.; Jo, T.; Ishizawa, K.; Tobinai, K.; et al. Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J. Clin. Oncol. 2016, 34, 4086–4093. [Google Scholar] [CrossRef]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef]
- Krönke, J.; Fink, E.C.; Hollenbach, P.W.; Macbeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef]
- Carvajal, L.A.; Ben Neriah, D.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.-R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-Enhancers in the Control of Cell Identity and Disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective Inhibition of Tumor Oncogenes by Disruption of Super-Enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master Transcription Factors and Mediator Establish Super-Enhancers at Key Cell Identity Genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Chipumuro, E.; Marco, E.; Christensen, C.L.; Kwiatkowski, N.; Zhang, T.; Hatheway, C.M.; Abraham, B.J.; Sharma, B.; Yeung, C.; Altabef, A.; et al. CDK7 Inhibition Suppresses Super-Enhancer-Linked Oncogenic Transcription in MYCN-Driven Cancer. Cell 2014, 159, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, N.P.; Zhang, T.; Rahl, P.B.; Abraham, B.J.; Reddy, J.; Ficarro, S.B.; Dastur, A.; Amzallag, A.; Ramaswamy, S.; Tesar, B.; et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 2014, 511, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.J.; Ngoc, P.C.T.; Leong, W.Z.; Yam, A.W.Y.; Zhang, T.; Asamitsu, K.; Iida, S.; Okamoto, T.; Ueda, R.; Gray, N.S.; et al. Enhancer profiling identifies critical cancer genes and characterizes cell identity in adult T-cell leukemia. Blood 2017, 130, 2326–2338. [Google Scholar] [CrossRef]

| Gene | ATLL Entire Japan [20] (n = 370) | ATLL North America [21] (n = 30) | ATLL Young Japanese [22] (n = 8) | PTCL-NOS [27] (n = 133) | AITL [27] (n = 26) | ALCL [27] (n = 23) | ENKTL [27] (n = 25) | HSTL [28] (n = 68) |
|---|---|---|---|---|---|---|---|---|
| PLCG1 | 36 | 0 | 0 | 8 | 8 | 0 | 0 | 6 |
| PRKCB | 33 | NE | 13 | 0 | 0 | 0 | 0 | NA |
| CCR4 | 29 | NE | 38 | 4 | 0 | 0 | 0 | 2 |
| CARD11 | 24 | 7 | 38 | 3 | 0 | 0 | 0 | 2 |
| STAT3 | 21 | 0 | 0 | 5 | 8 | 13 | 12 | 10 |
| TP53 | 18 | 23 | 13 | 17 | 0 | 9 | 12 | 10 |
| VAV1 | 18 | NE | 0 | 6 | 8 | 0 | 0 | NA |
| TBL1XR1 | 17 | 13 | 0 | 4 | 0 | 0 | 0 | NA |
| NOTCH1 | 15 | 20 | 25 | 5 | 0 | 0 | 0 | NA |
| GATA3 | 15 | 7 | 0 | 0 | 0 | 0 | 0 | NA |
| IRF4 | 14 | NE | 13 | 1 | 0 | 0 | 0 | 16 |
| FAS | 11 | 0 | 0 | 4 | 0 | 4 | 0 | NA |
| CCR7 | 11 | NE | 25 | 2 | 0 | 0 | 0 | NA |
| POT1 | 10 | 7 | 0 | 2 | 4 | 4 | 0 | NA |
| IRF2BP2 | 8 | NE | NE | 2 | 0 | 0 | 0 | NA |
| TET2 | 8 | 7 | 13 | 43 | 88 | 4 | 0 | 6 |
| RHOA | 8 | 3 | 13 | 26 | 81 | 0 | 0 | 2 |
| HLA-B | 6 | NE | 0 | 5 | 4 | 4 | 0 | NA |
| HNRNPA2B1 | 6 | NE | 0 | 0 | 0 | 4 | 0 | NA |
| EP300 | 6 | 20 | 0 | 2 | 0 | 0 | 8 | NA |
| CD58 | 5 | NE | 0 | 4 | 0 | 9 | 0 | 0 |
| GPR183 | 5 | NE | NE | 1 | 0 | 0 | 0 | NA |
| CSNK1A1 | 5 | NE | NE | 0 | 0 | 0 | 0 | NA |
| CSNK2B | 5 | NE | NE | 1 | 0 | 0 | 0 | NA |
| CBLB | 4 | NE | 0 | 0 | 0 | 0 | 0 | NA |
| FYN | 4 | NE | 13 | 2 | 0 | 0 | 0 | 3 |
| B2M | 4 | 0 | 0 | 5 | 8 | 0 | 0 | NA |
| SETD2 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 22 |
| Alteration | Targets | Clinical Outcome | Reference |
|---|---|---|---|
| CNA of cell cycle-related genes | Chronic type | Early acute transformation | [19] |
| Del of CD58 | Chronic type | Early acute transformation | [19] |
| PRKCB mutation | Aggressive type | Poor prognosis | [70] |
| Amp of PD-L1 | Aggressive type | Poor prognosis | [70] |
| IRF4 mutation | Indolent type | Poor prognosis | [70] |
| Amp; PD-L1 | Indolent type | Poor prognosis | [70] |
| Del; CDKN2A | Indolent type | Poor prognosis | [70] |
| Putative Targets | Potential Drugs | Active, or Recruiting Trials Including the Potential Drugs |
|---|---|---|
| CCR4 | Mogamulizumab | NCT04185220 |
| PD1/PD-L1 | Pembrolizumab | |
| IRF4 | Lenalidomide | NCT04301076 |
| proteolysis targeting chimera | ||
| EZH1/2 | Valemetostat Tosylate | NCT04102150 |
| Histone deacetylases | Belinostat | NCT02737046 |
| Romidepsin | NCT04639843 | |
| Phosphatidylinositol 3-kinase | Duvelisib | NCT04639843 |
| CD30 | Brentuximab | NCT03113500 |
| Anti-CD30 CAR-T | NCT04008394 | |
| DNA methylation | 5-azacitidine | NCT04639843 |
| OR-2100 | ||
| PRKCB | MS-533 | |
| proteolysis targeting chimera | ||
| MALT1 | JNJ-67856633 | |
| MDM2/MDM4 | ALRN-6924 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, N.; Miyoshi, H.; Ohshima, K. Clinical Applications of Genomic Alterations in ATLL: Predictive Markers and Therapeutic Targets. Cancers 2021, 13, 1801. https://doi.org/10.3390/cancers13081801
Yoshida N, Miyoshi H, Ohshima K. Clinical Applications of Genomic Alterations in ATLL: Predictive Markers and Therapeutic Targets. Cancers. 2021; 13(8):1801. https://doi.org/10.3390/cancers13081801
Chicago/Turabian StyleYoshida, Noriaki, Hiroaki Miyoshi, and Koichi Ohshima. 2021. "Clinical Applications of Genomic Alterations in ATLL: Predictive Markers and Therapeutic Targets" Cancers 13, no. 8: 1801. https://doi.org/10.3390/cancers13081801
APA StyleYoshida, N., Miyoshi, H., & Ohshima, K. (2021). Clinical Applications of Genomic Alterations in ATLL: Predictive Markers and Therapeutic Targets. Cancers, 13(8), 1801. https://doi.org/10.3390/cancers13081801






