MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Literature Research
3. MR-Guided Radiotherapy: Present Evidence
4. MR-Guided Radiotherapy: Future Directions
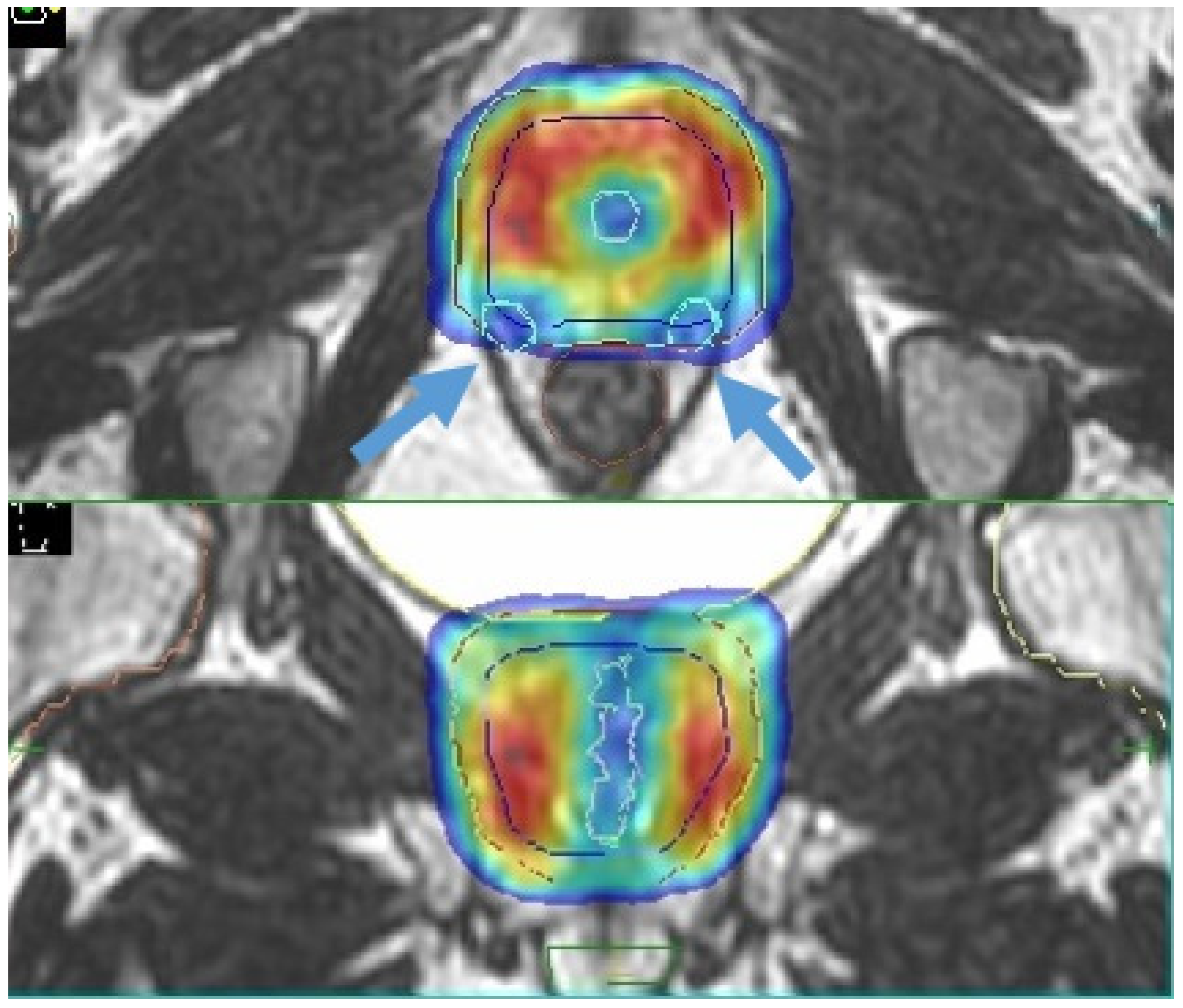
4.1. Boost of the Dominant Intraprostatic Lesion
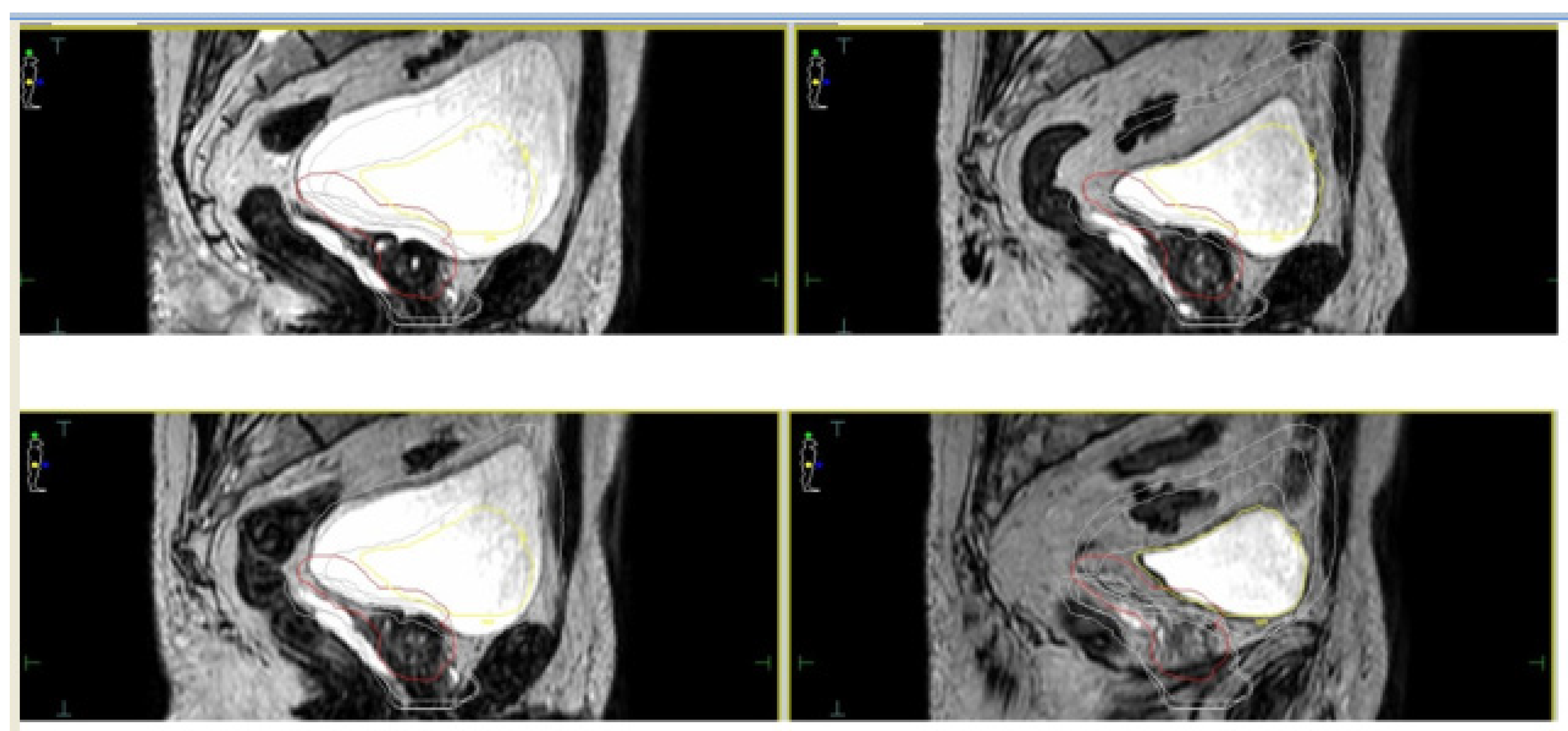
4.2. Margin Reduction/Single Shot Treatments
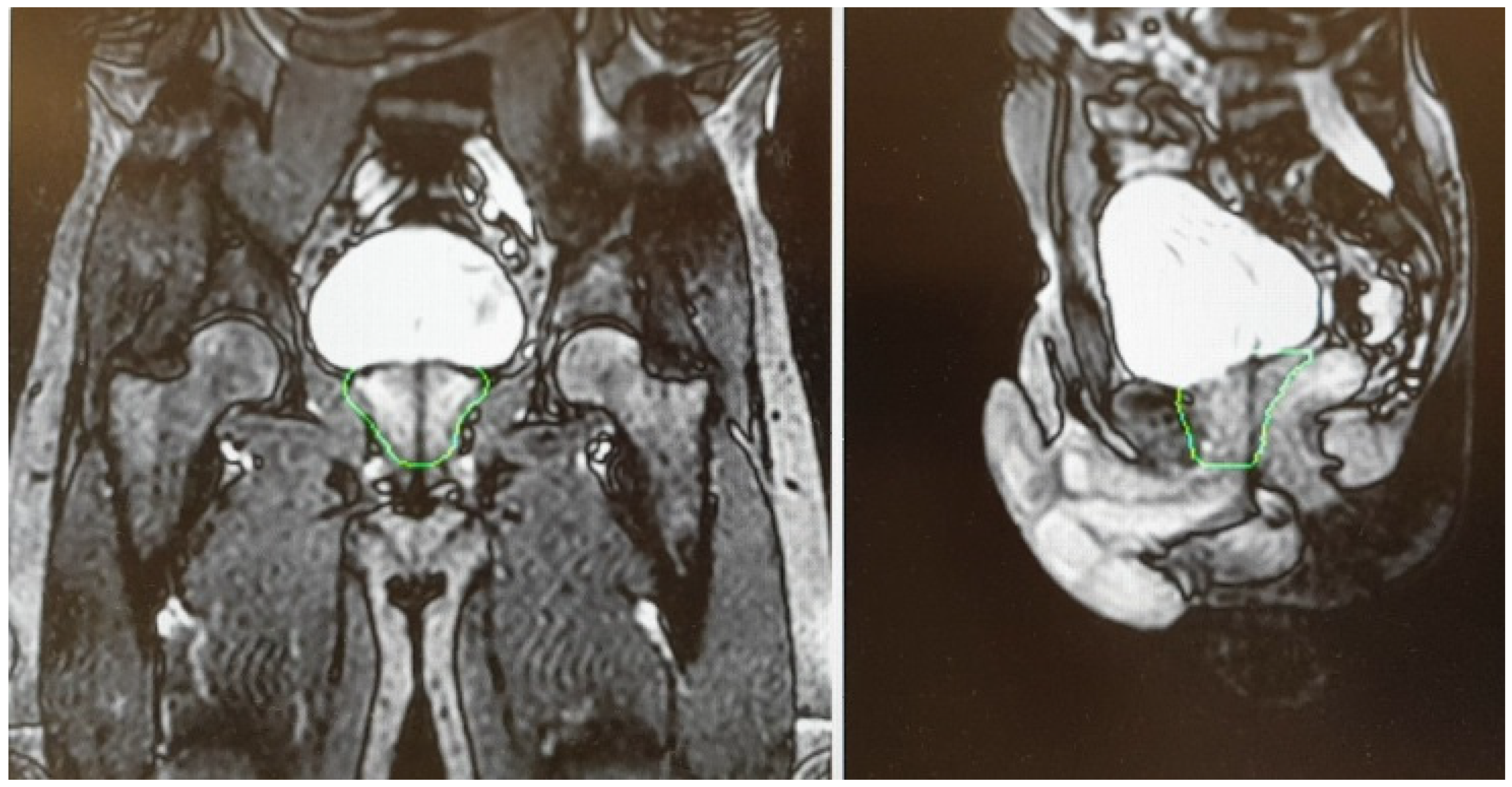
4.3. Sexual Function Preservation
4.4. Re-Irradiation
5. Patient Selection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Comission. Estimates of Cancer Incidence and Mortality in 2020 for All Countries; European Comission: Bruxelles, Belgium, 2020. [Google Scholar]
- Bryant, R.J.; Oxley, J.; Young, G.J.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Goepel, J.R.; Varma, M.; et al. The ProtecT trial: Analysis of the patient cohort, baseline risk stratification and disease progression. BJU Int. 2020, 125, 506–514. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Pra, A.D.; Abramowitz, M.C.; Stoyanova, R.; Pollack, A. Contemporary role of postoperative radiotherapy for prostate cancer. Transl. Androl. Urol. 2018, 7, 399–413. [Google Scholar] [CrossRef]
- Wilkins, A.; Naismith, O.; Brand, D.; Fernandez, K.; Hall, E.; Dearnaley, D.; Gulliford, S. Derivation of Dose/Volume Constraints for the Anorectum from Clinician- and Patient-Reported Outcomes in the CHHiP Trial of Radiation Therapy Fractionation. Int. J. Radiat. Oncol. 2020, 106, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Bari, B.; Fiorentino, A.; Arcangeli, S.; Franco, P.; D’Angelillo, R.M.; Alongi, F. From radiobiology to technology: What is changing in radiotherapy for prostate cancer. Expert Rev. Anticancer. Ther. 2014, 14, 553–564. [Google Scholar] [CrossRef]
- Morgan, S.C.; Hoffman, K.; Loblaw, D.A.; Buyyounouski, M.K.; Patton, C.; Barocas, D.; Bentzen, S.; Chang, M.; Efstathiou, J.; Greany, P.; et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: An ASTRO, ASCO, and AUA Evidence-Based Guideline. J. Clin. Oncol. 2018, 36, 3411–3430. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Cuccia, F.; Mortellaro, G.; Trapani, G.; Valenti, V.; Ognibene, L.; De Gregorio, G.; Quartuccio, E.; Luca, N.; Tripoli, A.; Serretta, V.; et al. Acute and late toxicity and preliminary outcomes report of moderately hypofractionated helical tomotherapy for localized prostate cancer: A mono-institutional analysis. Radiol. Med. 2019, 125, 220–227. [Google Scholar] [CrossRef]
- Catton, C.N.; Lukka, H.; Gu, C.S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.P.; Ahmed, S.; Cheung, P.; et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef]
- Incrocci, L.; Wortel, R.C.; Alemayehu, W.G.; Aluwini, S.; Schimmel, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Heemsbergen, W.; Heijmen, B.; et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1061–1069. [Google Scholar] [CrossRef]
- Arcangeli, S.; Scorsetti, M.; Alongi, F. Will SBRT replace conventional radiotherapy in patients with low-intermediate risk prostate cancer? A review. Crit. Rev. Oncol. 2012, 84, 101–108. [Google Scholar] [CrossRef]
- De Bari, B.; Arcangeli, S.; Ciardo, D.; Mazzola, R.; Alongi, F.; Russi, E.G.; Santoni, R.; Magrini, S.M.; Jereczek-Fossa, B.A. Extreme hypofractionation for early prostate cancer: Biology meets technology. Cancer Treat. Rev. 2016, 50, 48–60. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Brand, D.H.; Tree, A.C.; Ostler, P.; Van Der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef]
- Kishan, A.U.; King, C.R. Stereotactic Body Radiotherapy for Low- and Intermediate-Risk Prostate Cancer. Semin. Radiat. Oncol. 2017, 27, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Tree, A.C. Prostate cancer: Advantages and disadvantages of MR-guided RT. Clin. Transl. Radiat. Oncol. 2019, 18, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Salembier, C.; Villeirs, G.; De Bari, B.; Hoskin, P.; Pieters, B.R.; Van Vulpen, M.; Khoo, V.; Henry, A.; Bossi, A.; De Meerleer, G.; et al. ESTRO ACROP consensus guideline on CT-and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother. Oncol. 2018, 127, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Hörner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Jmour, O.; Benna, M.; Champagnol, P.; Mrad, B.M.; Hamrouni, A.; Obeid, L.; Lahmamssi, C.; Bousarsar, A.; Vial, N.; Rehailia-Blanchard, A.; et al. CBCT evaluation of inter- and intra-fraction motions during prostate stereotactic body radiotherapy: A technical note. Radiat. Oncol. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Levin-Epstein, R.; Qiao-Guan, G.; Juarez, J.E.; Shen, Z.; Steinberg, M.L.; Ruan, D.; Valle, L.; Nickols, N.G.; Kupelian, P.A.; King, C.R.; et al. Clinical Assessment of Prostate Displacement and Planning Target Volume Margins for Stereotactic Body Radiotherapy of Prostate Cancer. Front. Oncol. 2020, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Alongi, F.; Andratschke, N.; Azria, D.; Bohoudi, O.; Boldrini, L.; Bruynzeel, A.; Hörner-Rieber, J.; Jürgenliemk-Schulz, I.; Lagerwaard, F.; et al. ESTRO-ACROP recom-mendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol. 2021, 21, 06162–06164. [Google Scholar] [CrossRef]
- Hehakaya, C.; Van Der Voort Van Zyp, J.R.; Lagendijk, J.J.W.; Grobbee, D.E.; Verkooijen, H.M.; Moors, E.H.M. Problems and Promises of Introducing the Magnetic Resonance Imaging Linear Accelerator Into Routine Care: The Case of Prostate Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Botman, R.; Tetar, S.; Palacios, M.; Slotman, B.; Lagerwaard, F.; Bruynzeel, A. The clinical introduction of MR-guided radiation therapy from a RTT perspective. Clin. Transl. Radiat. Oncol. 2019, 18, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.E.D.; Pra, A.D.; Hoffe, E.S.; Mellon, E.A. Toxicity reduction required for MRI-guided radiotherapy to be cost-effective in the treatment of localized prostate cancer. Br. J. Radiol. 2020, 93, 20200028. [Google Scholar] [CrossRef]
- Alongi, F.; Arcangeli, S.; Cuccia, F.; D’Angelillo, R.M.; Di Muzio, N.G.; Filippi, A.R.; Jereczek-Fossa, B.A.; Livi, L.; Pergolizzi, S.; Scorsetti, M.; et al. In reply to Fiorino et al.: The central role of the radiation oncologist in the multidisciplinary & multiprofessional model of modern radiation therapy. Radiother. Oncol. 2021, 155, e20–e21. [Google Scholar] [CrossRef]
- Delgadillo, R.; Ford, J.C.; Abramowitz, M.C.; Pra, A.D.; Pollack, A.; Stoyanova, R. The role of radiomics in prostate cancer radiotherapy. Strahlenther. Onkol. 2020, 196, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Aznar, M.; Dubec, M.; Parker, G.; O’Connor, J. Delivering Functional Imaging on the MRI-Linac: Current Challenges and Potential Solutions. Clin. Oncol. 2018, 30, 702–710. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; Van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.; Eppinga, W.S.; Tijssen, R.H.; Kerkmeijer, L.G.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance-Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.; Spoelstra, F.O.; Piet, A.H.; Meijnen, P.; van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. 2019, 105, 1086–1094. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bruynzeel, A.M.; Oei, S.S.; Senan, S.; Fraikin, T.; Slotman, B.J.; van Moorselaar, R.J.A.; Lagerwaard, F.J. Magnetic Resonance-guided Stereotactic Radiotherapy for Localized Prostate Cancer: Final Results on Patient-reported Outcomes of a Prospective Phase 2 Study. Eur. Urol. Oncol. 2020, 11, 30061–30064. [Google Scholar] [CrossRef]
- Cuccia, F.; Mazzola, R.; Nicosia, L.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Vitale, C.; Mantoan, B.; De Simone, A.; et al. Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat. Oncol. 2020, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, R.; Rigo, M.; Naccarato, S.; Gurrera, D.; Figlia, V.; Mazzola, R.; Ricchetti, F.; Nicosia, L.; Giaj-Levra, N.; Cuccia, F.; et al. Adaptive SBRT by 1.5 T MR-linac for prostate cancer: On the accuracy of dose delivery in view of the prolonged session time. Phys. Med. 2020, 80, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S.; et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Sahin, B.; Mustafayev, T.Z.; Gungor, G.; Aydin, G.; Yapici, B.; Atalar, B.; Ozyar, E. First 500 Fractions Delivered with a Magnetic Resonance-guided Radiotherapy System: Initial Experience. Cureus 2019, 11, e6457. [Google Scholar] [CrossRef] [PubMed]
- Ugurluer, G.; Atalar, B.; Mustafayev, T.Z.; Gungor, G.; Aydin, G.; Sengoz, M.; Abacioglu, U.; Tuna, M.B.; Kural, A.R.; Ozyar, E. Magnetic resonance image-guided adaptive stereotactic body radiotherapy for prostate cancer: Preliminary results of outcome and toxicity. Br. J. Radiol. 2021, 94, 20200696. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Sicignano, G.; Rigo, M.; Figlia, V.; Cuccia, F.; De Simone, A.; Giaj-Levra, N.; Mazzola, R.; Naccarato, S.; Ricchetti, F.; et al. Daily dosimetric variation between image-guided volumetric modulated arc radiotherapy and MR-guided daily adaptive radiotherapy for prostate cancer stereotactic body radiotherapy. Acta Oncol. 2021, 60, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Klüter, S. Technical design and concept of a 0.35 T MR-Linac. Clin. Transl. Radiat. Oncol. 2019, 18, 98–101. [Google Scholar] [CrossRef]
- Keizer, D.D.M.; Kerkmeijer, L.; Willigenburg, T.; Van Lier, A.; Hartogh, M.D.; Van Der Voort Van Zyp, J.R.N.; Breugel, E.D.G.V.; Raaymakers, B.; Lagendijk, J.; De Boer, J. Prostate intrafraction motion during the preparation and delivery of MR-guided radiotherapy sessions on a 1.5T MR-Linac. Radiother. Oncol. 2020, 151, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, R.; Naccarato, S.; Stavrev, P.; Stavreva, N.; Fersino, S.; Levra, N.G.; Mazzola, R.; Mancosu, P.; Scorsetti, M.; Alongi, F. Volumetric-modulated arc stereotactic body radiotherapy for prostate cancer: Dosimetric impact of an increased near-maximum target dose and of a rectal spacer. Br. J. Radiol. 2015, 88, 20140736. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Vitale, C.; Sicignano, G.; De Simone, A.; et al. Rectal spacer hydrogel in 1.5T MR-guided and daily adapted SBRT for prostate cancer: Dosimetric analysis and preliminary patient-reported outcomes. Br. J. Radiol. 2021, 94, 20200848. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Alongi, F. Reply to Ghaffari et al. In regard to Cuccia et al.: Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat. Oncol. 2020, 15, 1–2. [Google Scholar] [CrossRef]
- Ardekani, M.A.; Ghaffari, H.; Navaser, M.; Moghaddam, S.H.Z.; Refahi, S. Effectiveness of rectal displacement devices in managing prostate motion: A systematic review. Strahlenther. Onkol. 2021, 197, 97–115. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Jameson, M.G.; Rivest-Henault, D.; Keats, S.; Rai, R.; Arumugam, S.; Wilton, L.; Ngo, D.; Liney, G.; Moses, D.; et al. Reduced motion and improved rectal dosimetry through endorectal immobilization for prostate stereotactic body radiotherapy. Br. J. Radiol. 2019, 92, 20190056. [Google Scholar] [CrossRef]
- Huang, C.C.; Deng, F.M.; Kong, M.X.; Ren, Q.; Melamed, J.; Zhou, M. Reevaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Arch. Int. J. Pathol. 2014, 464, 589–594. [Google Scholar] [CrossRef]
- Feutren, T.; Herrera, F.G. Prostate irradiation with focal dose escalation to the intraprostatic dominant nodule: A systematic review. Prostate Int. 2018, 6, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Arrayeh, E.; Westphalen, A.C.; Kurhanewicz, J.; Roach, M., 3rd; Jung, A.J.; Carroll, P.R.; Fergus, V.C. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e787–e793. [Google Scholar] [CrossRef]
- Cellini, N.; Morganti, A.G.; Mattiucci, G.C.; Valentini, V.; Leone, M.; Luzi, S.; Manfredi, R.; Dinapoli, N.; Digesu’, C.; Smaniotto, D. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int. J. Radiat. Oncol. 2002, 53, 595–599. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Hindley, R.G.; Dickinson, L.; Freeman, A.; Kirkham, A.P.; Sahu, M.; Scott, R.; Allen, C.; Van der Meulen, J.; Emberton, M. Focal therapy for localised unifocal and multifocal prostate cancer: A prospective development study. Lancet Oncol. 2012, 13, 622–632. [Google Scholar] [CrossRef]
- Pucar, D.; Hricak, H.; Shukla-Dave, A.; Kuroiwa, K.; Drobnjak, M.; Eastham, J.; Scardino, P.T.; Zelefsky, M.J. Clinically Significant Prostate Cancer Local Recurrence After Radiation Therapy Occurs at the Site of Primary Tumor: Magnetic Resonance Imaging and Step-Section Pathology Evidence. Int. J. Radiat. Oncol. 2007, 69, 62–69. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose Escalated External Beam Boost for High-and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; Van Der Voort Van Zyp, J.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 786–796. [Google Scholar] [CrossRef]
- Hötker, A.M.; Mazaheri, Y.; Zheng, J.; Moskowitz, C.S.; Berkowitz, J.; Lantos, J.E.; Pei, X.; Zelefsky, M.J.; Hricak, H.; Akin, O. Prostate Cancer: Assessing the effects of androgen-deprivation therapy using quantitative diffusion-weighted and dynamic contrast-enhanced MRI. Eur. Radiol. 2015, 25, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, M.A.; Van Houdt, P.J.; Ghobadi, G.; Pos, F.J.; Walraven, I.; De Boer, H.C.J.; Van Den Berg, C.A.T.; Smeenk, R.J.; Kerkmeijer, L.G.W.; Van Der Heide, U.A. Quantitative MRI Changes During Weekly Ultra-Hypofractionated Prostate Cancer Radiotherapy With Integrated Boost. Front. Oncol. 2019, 9, 1264. [Google Scholar] [CrossRef] [PubMed]
- Sogono, P.; Bressel, M.; David, S.; Shaw, M.; Chander, S.; Chu, J.; Plumridge, N.; Byrne, K.; Hardcastle, N.; Kron, T.; et al. Safety, Efficacy, and Patterns of Failure After Single-Fraction Stereotactic Body Radiation Therapy (SBRT) for Oligometastases. Int. J. Radiat. Oncol. Biol. Phys. 2020, 109, 756–763. [Google Scholar] [CrossRef]
- Zilli, T.; Scorsetti, M.; Zwahlen, D.; Franzese, C.; Förster, R.; Giaj-Levra, N.; Koutsouvelis, N.; Bertaut, A.; Zimmermann, M.; D’Agostino, G.R.; et al. ONE SHOT—Single shot radiotherapy for localized prostate cancer: Study protocol of a single arm, multicenter phase I/II trial. Radiat. Oncol. 2018, 13, 166. [Google Scholar] [CrossRef]
- Zilli, T.; Franzese, C.; Bottero, M.; Giaj-Levra, N.; Förster, R.; Zwahlen, D.; Koutsouvelis, N.; Bertaut, A.; Blanc, J.; D’Agostino, G.R.; et al. Single fraction urethra-sparing prostate cancer SBRT: Phase I results of the ONE SHOT trial. Radiother. Oncol. 2019, 139, 83–86. [Google Scholar] [CrossRef]
- Dunlop, A.; Mitchell, A.; Tree, A.; Barnes, H.; Bower, L.; Chick, J.; Goodwin, E.; Herbert, T.; Lawes, R.; McNair, H.; et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin. Transl. Radiat. Oncol. 2020, 23, 35–42. [Google Scholar] [CrossRef]
- Mannerberg, A.; Persson, E.; Jonsson, J.; Gustafsson, C.J.; Gunnlaugsson, A.; Olsson, L.E.; Ceberg, S. Dosimetric effects of adaptive prostate cancer radiotherapy in an MR-linac workflow. Radiat. Oncol. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Mottet, N.; Van Den Bergh, R.C.N.; Briers, E.; Broeck, T.V.D.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Ramirez-Fort, M.K.; Rogers, M.J.; Santiago, R.; Mahase, S.S.; Mendez, M.; Zheng, Y.; Kong, X.; Kashanian, J.A.; Niaz, M.J.; McClelland, S.; et al. Prostatic irradiation-induced sexual dysfunction: A review and multidisciplinary guide to management in the radical radiotherapy era (Part I defining the organ at risk for sexual toxicities). Rep. Pr. Oncol. Radiother. 2020, 25, 367–375. [Google Scholar] [CrossRef]
- Ramirez-Fort, M.K.; Suarez, P.; Carrion, M.; Weiner, D.; Postl, C.; Arribas, R.; Sayyah, M.; Forta, D.V.; Niaz, M.J.; Feily, A.; et al. Prostatic irradiation-induced sexual dysfunction: A review and multidisciplinary guide to management in the radical radiotherapy era (Part III on Psychosexual Therapy and the Masculine Self-Esteem). Rep. Pract. Oncol. Radiother. 2020, 25, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, A.R.; et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef]
- Pinkawa, M. Current role of spacers for prostate cancer radiotherapy. World J. Clin. Oncol. 2015, 6, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Lee, J.Y.; Dess, R.T.; Narayana, V.; Evans, C.; Liss, A.; Winfield, R.; Schipper, M.J.; Lawrence, T.S.; McLaughlin, P.W. Vessel-sparing Radiotherapy for Localized Prostate Cancer to Preserve Erectile Function: A Single-arm Phase 2 Trial. Eur. Urol. 2017, 72, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Ramirez-Fort, M.K.; Kashanian, J.A.; Broster, S.A.; Matta, J.; Mahase, S.S.; Fort, D.V.; Niaz, M.J.; McClelland, S.; Bander, N.H.; et al. Prostatic irradiation-induced sexual dysfunction: A review and multidisciplinary guide to management in the radical radiotherapy era (Part II on Urological Management). Rep. Pract. Oncol. Radiother. 2020, 25, 619–624. [Google Scholar] [CrossRef]
- Cuccia, F.; Mazzola, R.; Nicosia, L.; Giaj-Levra, N.; Figlia, V.; Ricchetti, F.; Rigo, M.; Vitale, C.; Corradini, S.; Alongi, F. Prostate reirradiation: Current concerns and future perspectives. Expert Rev. Anticancer Ther. 2020, 20, 947–956. [Google Scholar] [CrossRef]
- Leroy, T.; Lacornerie, T.; Bogart, E.; Nickers, P.; Lartigau, E.; Pasquier, D. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: Preliminary results of the Oscar Lambret Center. Radiat. Oncol. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Loi, M.; Di Cataldo, V.; Simontacchi, G.; Detti, B.; Bonomo, P.; Masi, L.; Desideri, I.; Greto, D.; Francolini, G.; Carfora, V.; et al. Robotic Stereotactic Retreatment for Biochemical Control in Previously Irradiated Patients Affected by Recurrent Prostate Cancer. Clin. Oncol. 2018, 30, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Miszczyk, L.; Stąpór-Fudzińska, M.; Miszczyk, M.; Maciejewski, B.; Tukiendorf, A. Salvage CyberKnife-Based Re-irradiation of Patients with Recurrent Prostate Cancer: The Single-Center Experience. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.; Basson, L.; Puech, P.; Lacornerie, T.; Villers, A.; Wallet, J.; Lartigau, E.; Pasquier, D. Stereotactic Re-irradiation for Local Recurrence in the Prostatic Bed After Prostatectomy: Preliminary Results. Front. Oncol. 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, D.; Martinage, G.; Janoray, G.; Rojas, D.P.; Zerini, D.; Goupy, F.; De Crevoisier, R.; Bogart, E.; Calais, G.; Toledano, A.; et al. Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: A Retrospective Multicenter Study of the GETUG. Int. J. Radiat. Oncol. 2019, 105, 727–734. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.R.; Di Brina, L.; Mancosu, P.; Franzese, C.; Iftode, C.; Franceschini, D.; Clerici, E.; Tozzi, A.; Navarria, P.; Scorsetti, M. Reirradiation of Locally Recurrent Prostate Cancer With Volumetric Modulated Arc Therapy. Int. J. Radiat. Oncol. 2019, 104, 614–621. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Rojas, D.P.; Zerini, D.; Fodor, C.; Viola, A.; Fanetti, G.; Volpe, S.; Luraschi, R.; Bazani, A.; Rondi, E.; et al. Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br. J. Radiol. 2019, 92, 20180494. [Google Scholar] [CrossRef]
- Fuller, D.; Wurzer, J.; Shirazi, R.; Bridge, S.; Law, J.; Crabtree, T.; Mardirossian, G. Retreatment for Local Recurrence of Prostatic Carcinoma After Prior Therapeutic Irradiation: Efficacy and Toxicity of HDR-Like SBRT. Int. J. Radiat. Oncol. 2020, 106, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Nicosia, L.; Mazzola, R.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Vitale, C.; Corradini, S.; Ruggieri, R.; et al. Linac-based SBRT as a feasible salvage option for local recurrences in previously irradiated prostate cancer. Strahlenther. Onkol. 2020, 196, 628–636. [Google Scholar] [CrossRef]
- Zerini, D.; Jereczek-Fossa, B.A.; Ciabattoni, A.; Mirri, A.; Bertoni, F.; Fersino, S.; D’Agostino, G.; Lohr, F.; Mortellaro, G.; Triggiani, L.; et al. PROLAPSE: Survey about local prostate cancer relapse salvage treatment with external beam re-irradiation: Results of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). J. Cancer Res. Clin. Oncol. 2020, 146, 2311–2317. [Google Scholar] [CrossRef]
- Lieng, H.; Hayden, A.J.; Christie, D.R.; Davis, B.J.; Eade, T.N.; Emmett, L.; Holt, T.; Hruby, G.; Pryor, D.; Shakespeare, T.P.; et al. Radiotherapy for recurrent prostate cancer: 2018 Recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother. Oncol. 2018, 129, 377–386. [Google Scholar] [CrossRef]
- Mazzola, R.; Cuccia, F.; Figlia, V.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Rigo, M.; Pasinetti, N.; Salgarello, M.; Alongi, F. New metabolic tracers for detectable PSA levels in the post-prostatectomy setting: Is the era of melting glaciers upcoming? Transl. Androl. Urol. 2019, 8, S538–S541. [Google Scholar] [CrossRef]
- Zattoni, F.; Kawashima, A.; Morlacco, A.; Davis, B.J.; Nehra, A.K.; Mynderse, L.A.; Froemming, A.T.; Karnes, R.J. Detection of recurrent prostate cancer after primary radiation therapy: An evaluation of the role of multiparametric 3T magnetic resonance imaging with endorectal coil. Pract. Radiat. Oncol. 2017, 7, 42–49. [Google Scholar] [CrossRef]
- Sala, E.; Eberhardt, S.C.; Akin, O.; Moskowitz, C.S.; Onyebuchi, C.N.; Kuroiwa, K.; Ishill, N.; Zelefsky, M.J.; Eastham, J.A.; Hricak, H. Endorectal MR Imaging before Salvage Prostatectomy: Tumor Localization and Staging. Radiology 2006, 238, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Fiorentino, A.; De Bari, B. SBRT and extreme hypofractionation: A new era in prostate cancer treatments? Rep. Pract. Oncol. Radiother. 2015, 20, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, R.C.; Kane, B.L.; Medbery, C.A.; Underhill, K.J.; Gray, J.R.; Peddada, A.V.; Fuller, D.B. Patient and Dosimetric Predictors of Genitourinary and Bowel Quality of Life After Prostate SBRT: Secondary Analysis of a Multi-institutional Trial. Int. J. Radiat. Oncol. 2018, 102, 1430–1437. [Google Scholar] [CrossRef]
- Pepin, A.; Aghdam, N.; Shah, S.; Kataria, S.; Tsou, H.J.; Datta, S.; Danner, M.; Ayoob, M.; Yung, T.; Lei, S.; et al. Urinary Morbidity in Men Treated With Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer Following Transurethral Resection of the Prostate (TURP). Front. Oncol. 2020, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, N.; Pepin, A.; Buchberger, D.; Hirshberg, J.; Lei, S.; Ayoob, M.; Danner, M.; Yung, T.; Kumar, D.; Collins, B.T.; et al. Stereotactic Body Radiation Therapy (SBRT) for Prostate Cancer in Men With a High Baseline International Prostate Symptom Score (IPSS ≥ 15). Front. Oncol. 2020, 10, 1060. [Google Scholar] [CrossRef]
- Chaddad, A.; Kucharczyk, M.J.; Niazi, T. Multimodal Radiomic Features for the Predicting Gleason Score of Prostate Cancer. Cancers 2018, 10, 249. [Google Scholar] [CrossRef]
- Park, J.E.; Park, S.Y.; Kim, H.J.; Kim, H.S. Reproducibility and Generalizability in Radiomics Modeling: Possible Strategies in Radiologic and Statistical Perspectives. Korean J. Radiol. 2019, 20, 1124–1137. [Google Scholar] [CrossRef]
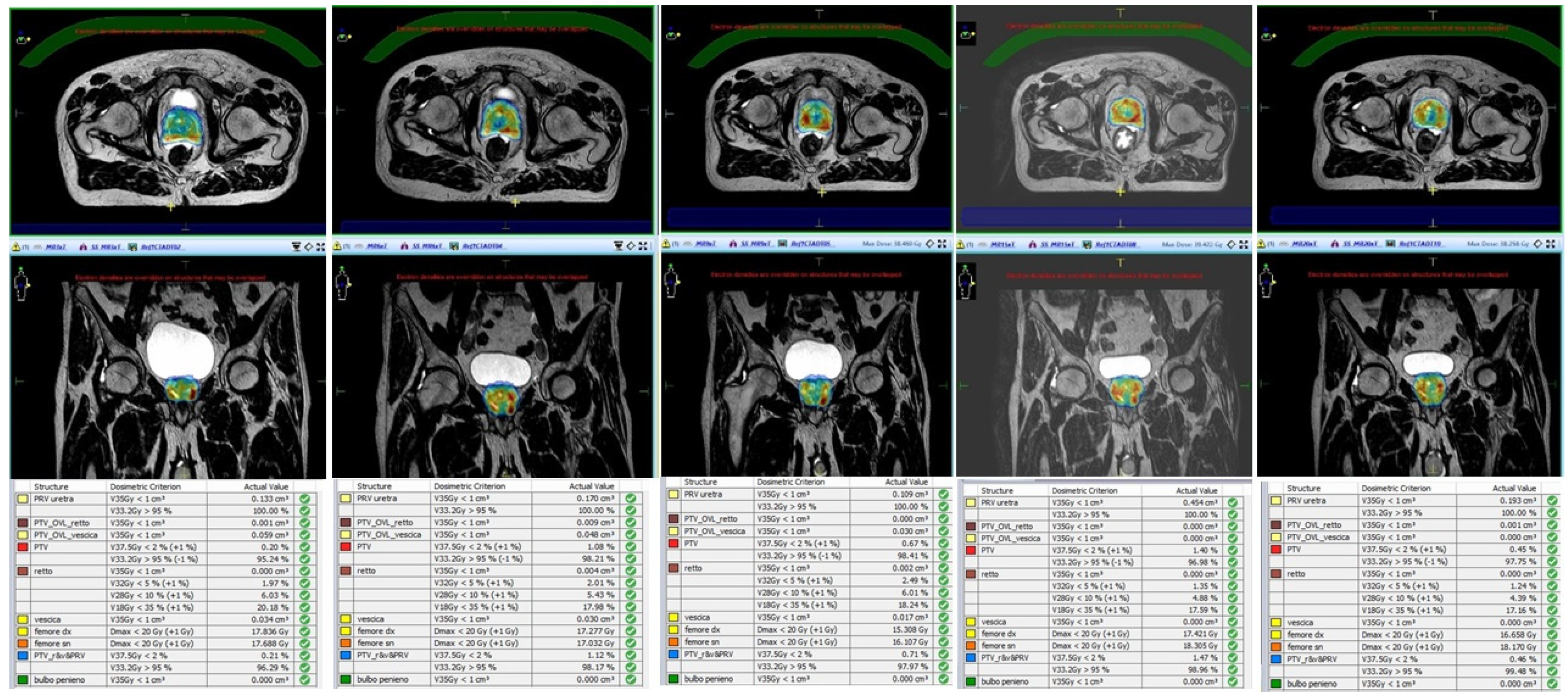
| Author | N° of Patients | MR-Linac Device | SBRT Schedule | Main Endpoint of the Study | Results |
|---|---|---|---|---|---|
| Alongi et al. [36] | 20 | Elekta Unity | 35 Gy/5 fractions | Dosimetric analysis and preliminary PROMs report | Hydrogel improves rectal sparing with minimal impact on QoL |
| Bruynzeel et al. [32] | 101 | Viewray MRIdian | 36.25 Gy/5 fractions | Early toxicity analysis | G ≥ 2 GU = 23.8% (including 5.9% of G3 according to RTOG criteria); ≥2 GI = 5.0% |
| Cuccia et al. [34] | 20 | Elekta Unity | 35 Gy/5 fractions | Assessment of the impact of rectal spacer on prostate motion | Significant impact on rotational antero-posterior shifts with consequently reduced prostate motion |
| Tetar et al. [33] | 101 | Viewray MRIdian | 36.25 Gy/5 fractions | PROMs analysis | After one year, only 2.2% of cases reported a relevant impact on daily activities due to GI toxicity |
| Nicosia et al. [39] | 10 | Elekta Unity | 35 Gy/5 fractions | Dosimetric comparison between MR-guided SBRT and conventional Linacs SBRT | MR-guided SBRT resulted in lower constraint violation rates |
| Sahin et al. [37] | 24 | Viewray MRIdian | 36.25 Gy/5 fractions | Preliminary report of feasibility | Substantial feasibility of MR-adaptive SBRT with acceptable time schedules |
| Ugurluer et al. [38] | 50 | Viewray MRIdian | 36.25 Gy/5 fractions | Early toxicity analysis | Acute G2 GU = 28%; Late G2 GU = 6%; Late GI GU = 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuccia, F.; Corradini, S.; Mazzola, R.; Spiazzi, L.; Rigo, M.; Bonù, M.L.; Ruggieri, R.; Buglione di Monale e Bastia, M.; Magrini, S.M.; Alongi, F. MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer. Cancers 2021, 13, 1791. https://doi.org/10.3390/cancers13081791
Cuccia F, Corradini S, Mazzola R, Spiazzi L, Rigo M, Bonù ML, Ruggieri R, Buglione di Monale e Bastia M, Magrini SM, Alongi F. MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer. Cancers. 2021; 13(8):1791. https://doi.org/10.3390/cancers13081791
Chicago/Turabian StyleCuccia, Francesco, Stefanie Corradini, Rosario Mazzola, Luigi Spiazzi, Michele Rigo, Marco Lorenzo Bonù, Ruggero Ruggieri, Michela Buglione di Monale e Bastia, Stefano Maria Magrini, and Filippo Alongi. 2021. "MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer" Cancers 13, no. 8: 1791. https://doi.org/10.3390/cancers13081791
APA StyleCuccia, F., Corradini, S., Mazzola, R., Spiazzi, L., Rigo, M., Bonù, M. L., Ruggieri, R., Buglione di Monale e Bastia, M., Magrini, S. M., & Alongi, F. (2021). MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer. Cancers, 13(8), 1791. https://doi.org/10.3390/cancers13081791








