Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer
Abstract
Simple Summary
Abstract
1. Introduction
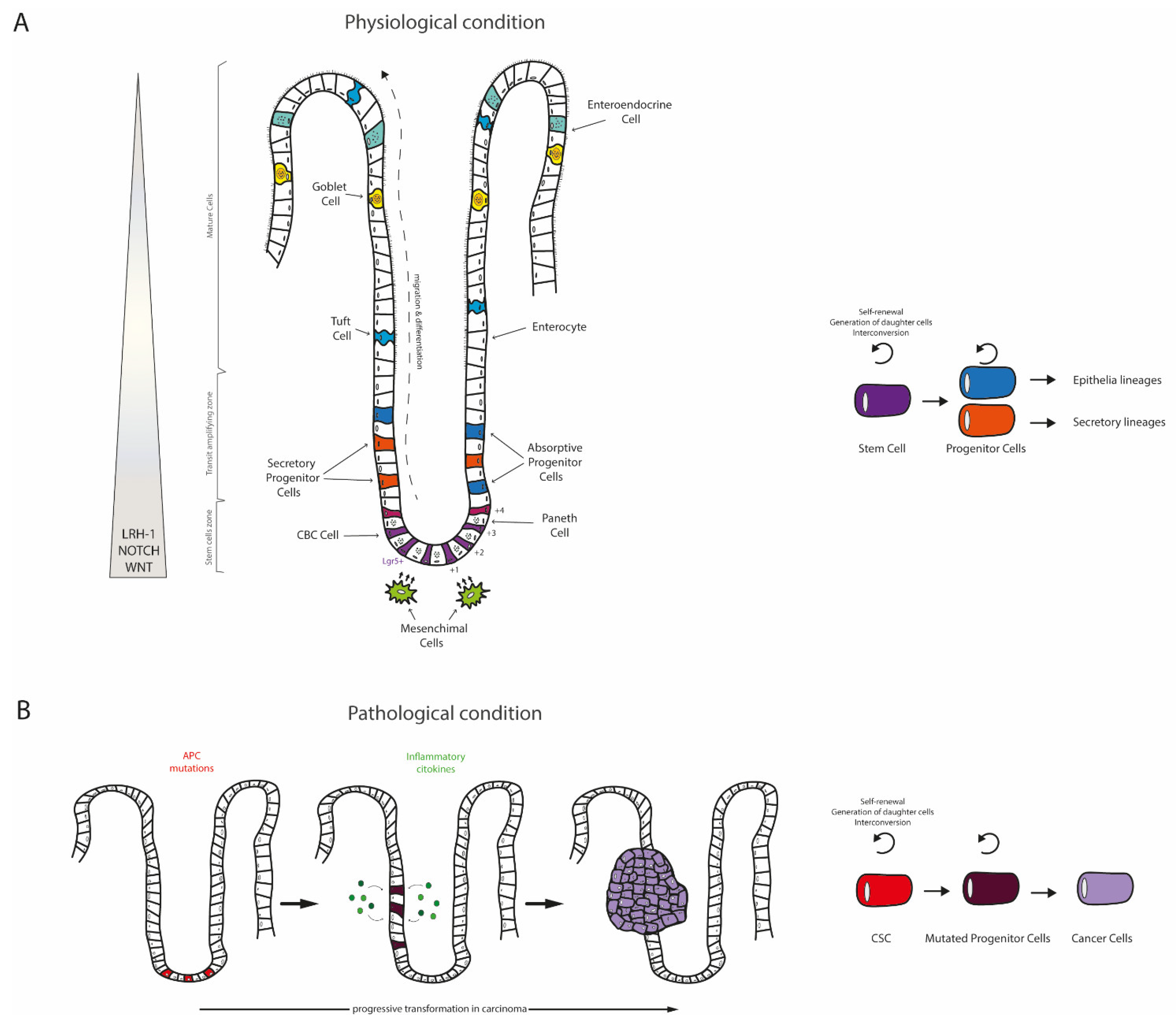
2. The Intestine: A Well-Organized Architecture behind Multiple Functions
3. Disruptions of Intestinal Homeostasis: The Colorectal Cancer (CRC)
4. LRH-1: An Ex-Orphan Nuclear Receptor
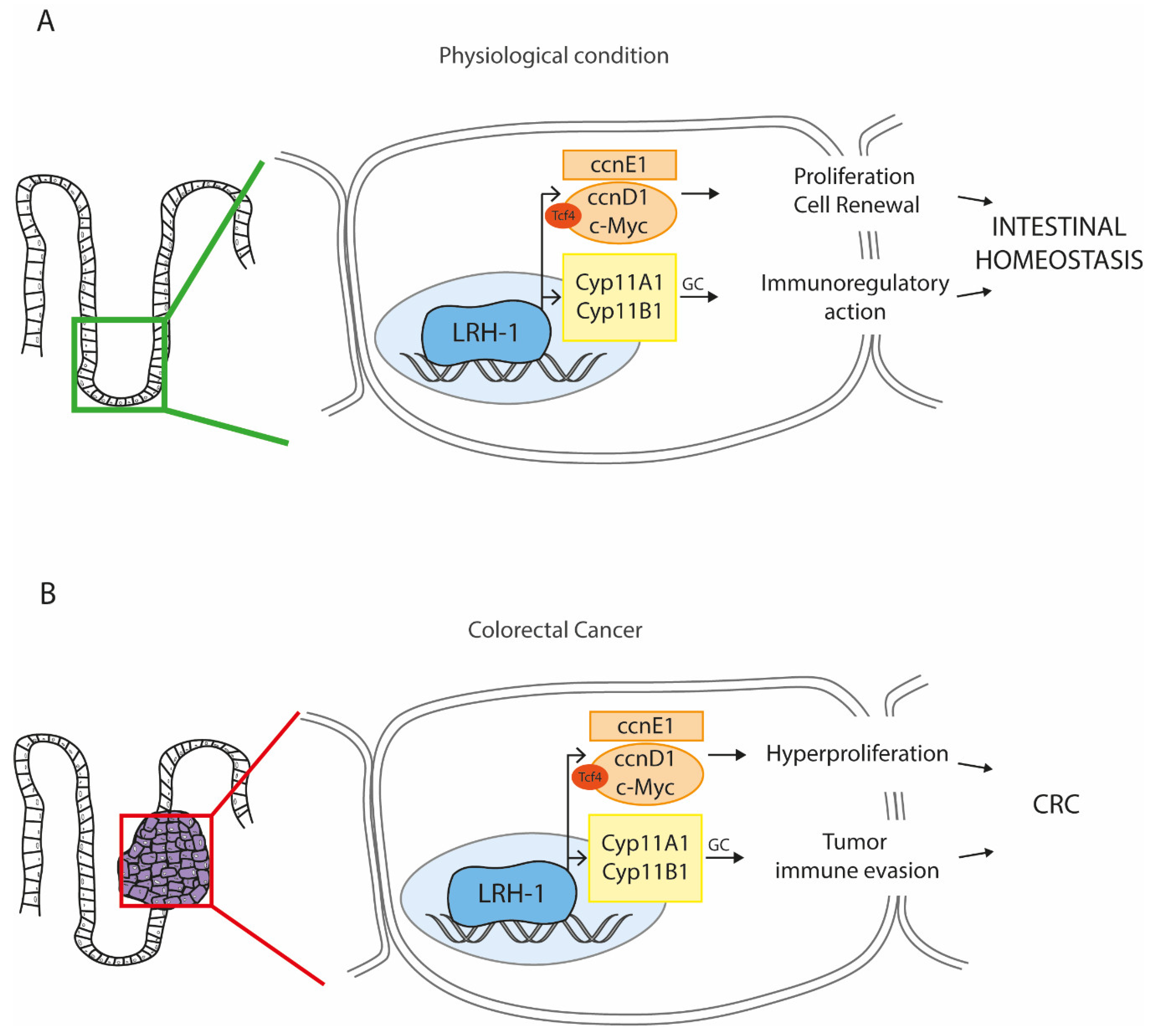
4.1. LRH-1 in Stemness: A Boost for Cell Proliferation
4.2. LRH-1 in Inflammation: A Shield against Intestinal Bowel Diseases
4.3. LRH-1 in Cancer: A Harmful Piece in the CRC Puzzle
4.4. LRH-1 in the Gut: Connecting the Dots to Look Forward
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- Dhiman, V.K.; Bolt, M.J.; White, K.P. Nuclear receptors in cancer—Uncovering new and evolving roles through genomic analysis. Nat. Rev. Genet. 2018, 19, 160–174. [Google Scholar] [CrossRef]
- McKenna, N.J.; Cooney, A.J.; DeMayo, F.J.; Downes, M.; Glass, C.K.; Lanz, R.B.; Lazar, M.A.; Mangelsdorf, D.J.; Moore, D.D.; Qin, J.; et al. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol. Endocrinol. 2009, 23, 740–746. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Fayard, E.; Auwerx, J.; Schoonjans, K. LRH-1: An orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004, 14, 250–260. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J.; Schoonjans, K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim. Biophys. Acta 2011, 1812, 947–955. [Google Scholar] [CrossRef]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Frohlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of Deposition, Lung Surface Area and Lung Fluid for Simulation of Inhaled Compounds. Front. Pharmacol. 2016, 7, 181. [Google Scholar] [CrossRef]
- Daulagala, A.C.; Bridges, M.C.; Kourtidis, A. E-cadherin Beyond Structure: A Signaling Hub in Colon Homeostasis and Disease. Int. J. Mol. Sci. 2019, 20, 2756. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974, 141, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 1999, 116, 7–14. [Google Scholar] [CrossRef]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab. Dispos. Biol. Fate Chem. 2014, 42, 2016–2022. [Google Scholar] [CrossRef]
- Winton, D.J.; Blount, M.A.; Ponder, B.A. A clonal marker induced by mutation in mouse intestinal epithelium. Nature 1988, 333, 463–466. [Google Scholar] [CrossRef]
- D’Errico, I.; Moschetta, A. Nuclear receptors, intestinal architecture and colon cancer: An intriguing link. Cell. Mol. Life Sci. CMLS 2008, 65, 1523–1543. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 167, 1137. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Nystrom, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Hocker, M.; Wiedenmann, B. Molecular mechanisms of enteroendocrine differentiation. Ann. N. Y. Acad. Sci. 1998, 859, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Marshman, E.; Booth, C.; Potten, C.S. The intestinal epithelial stem cell. Bioessays News Rev. Mol. Cell. Dev. Biol. 2002, 24, 91–98. [Google Scholar] [CrossRef]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Batlle, E.; Henderson, J.T.; Beghtel, H.; van den Born, M.M.; Sancho, E.; Huls, G.; Meeldijk, J.; Robertson, J.; van de Wetering, M.; Pawson, T.; et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002, 111, 251–263. [Google Scholar] [CrossRef]
- Waterman, M.L. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- De Lau, W.; Clevers, H. LEF1 turns over a new leaf. Nat. Genet. 2001, 28, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Aoki, R.; Shoshkes-Carmel, M.; Gao, N.; Shin, S.; May, C.L.; Golson, M.L.; Zahm, A.M.; Ray, M.; Wiser, C.L.; Wright, C.V.; et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 175–188. [Google Scholar] [CrossRef]
- Stzepourginski, I.; Nigro, G.; Jacob, J.M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl. Acad. Sci. USA 2017, 114, E506–E513. [Google Scholar] [CrossRef]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Greicius, G.; Kabiri, Z.; Sigmundsson, K.; Liang, C.; Bunte, R.; Singh, M.K.; Virshup, D.M. PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E3173–E3181. [Google Scholar] [CrossRef] [PubMed]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Toth, B.; Kondo, A.; Massasa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Jain, R.; LeBoeuf, M.R.; Wang, Q.; Lu, M.M.; Epstein, J.A. Interconversion between intestinal stem cell populations in distinct niches. Science 2011, 334, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; de Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar] [CrossRef]
- Yan, K.S.; Chia, L.A.; Li, X.; Ootani, A.; Su, J.; Lee, J.Y.; Su, N.; Luo, Y.; Heilshorn, S.C.; Amieva, M.R.; et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar] [CrossRef]
- Van Es, J.H.; Sato, T.; van de Wetering, M.; Lyubimova, A.; Yee Nee, A.N.; Gregorieff, A.; Sasaki, N.; Zeinstra, L.; van den Born, M.; Korving, J.; et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012, 14, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Buczacki, S.J.; Zecchini, H.I.; Nicholson, A.M.; Russell, R.; Vermeulen, L.; Kemp, R.; Winton, D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013, 495, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, P.W.; Basak, O.; Farin, H.F.; Wiebrands, K.; Kretzschmar, K.; Begthel, H.; van den Born, M.; Korving, J.; de Sauvage, F.; van Es, J.H.; et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 2016, 18, 203–213. [Google Scholar] [CrossRef]
- De Sousa, E.M.F.; Wang, X.; Jansen, M.; Fessler, E.; Trinh, A.; de Rooij, L.P.; de Jong, J.H.; de Boer, O.J.; van Leersum, R.; Bijlsma, M.F.; et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 2013, 19, 614–618. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357. [Google Scholar] [CrossRef]
- Fodde, R. The APC gene in colorectal cancer. Eur. J. Cancer 2002, 38, 867–871. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Shih, I.M.; Wang, T.L.; Traverso, G.; Romans, K.; Hamilton, S.R.; Ben-Sasson, S.; Kinzler, K.W.; Vogelstein, B. Top-down morphogenesis of colorectal tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Toyama, K.; Shioya, H.; Ito, M.; Hirota, M.; Hasegawa, S.; Matsumoto, H.; Takano, H.; Akiyama, T.; Toyoshima, K.; et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997, 278, 120–123. [Google Scholar] [CrossRef]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Van der Heijden, M.; Zimberlin, C.D.; Nicholson, A.M.; Colak, S.; Kemp, R.; Meijer, S.L.; Medema, J.P.; Greten, F.R.; Jansen, M.; Winton, D.J.; et al. Bcl-2 is a critical mediator of intestinal transformation. Nat. Commun. 2016, 7, 10916. [Google Scholar] [CrossRef]
- Yang, G.Y.; Taboada, S.; Liao, J. Inflammatory bowel disease: A model of chronic inflammation-induced cancer. Methods Mol. Biol. 2009, 511, 193–233. [Google Scholar] [CrossRef]
- Jess, T.; Frisch, M.; Simonsen, J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 43–48. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
- Falender, A.E.; Lanz, R.; Malenfant, D.; Belanger, L.; Richards, J.S. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 2003, 144, 3598–3610. [Google Scholar] [CrossRef]
- Lee, Y.K.; Moore, D.D. Liver receptor homolog-1, an emerging metabolic modulator. Front. Biosci. A J. Virtual Libr. 2008, 13, 5950–5958. [Google Scholar] [CrossRef]
- Becker-Andre, M.; Andre, E.; DeLamarter, J.F. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem. Biophys. Res. Commun. 1993, 194, 1371–1379. [Google Scholar] [CrossRef]
- Sablin, E.P.; Krylova, I.N.; Fletterick, R.J.; Ingraham, H.A. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol. Cell 2003, 11, 1575–1585. [Google Scholar] [CrossRef]
- Krylova, I.N.; Sablin, E.P.; Moore, J.; Xu, R.X.; Waitt, G.M.; MacKay, J.A.; Juzumiene, D.; Bynum, J.M.; Madauss, K.; Montana, V.; et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 2005, 120, 343–355. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Marimuthu, A.; Krupka, H.I.; Tabrizizad, M.; Shelloe, R.; Mehra, U.; Eng, K.; Nguyen, H.; Settachatgul, C.; et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc. Natl. Acad. Sci. USA 2005, 102, 7505–7510. [Google Scholar] [CrossRef]
- Ortlund, E.A.; Lee, Y.; Solomon, I.H.; Hager, J.M.; Safi, R.; Choi, Y.; Guan, Z.; Tripathy, A.; Raetz, C.R.; McDonnell, D.P.; et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat. Struct. Mol. Biol. 2005, 12, 357–363. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, Y.K.; Mamrosh, J.L.; Busby, S.A.; Griffin, P.R.; Pathak, M.C.; Ortlund, E.A.; Moore, D.D. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature 2011, 474, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Whitby, R.J.; Dixon, S.; Maloney, P.R.; Delerive, P.; Goodwin, B.J.; Parks, D.J.; Willson, T.M. Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J. Med. Chem. 2006, 49, 6652–6655. [Google Scholar] [CrossRef]
- Whitby, R.J.; Stec, J.; Blind, R.D.; Dixon, S.; Leesnitzer, L.M.; Orband-Miller, L.A.; Williams, S.P.; Willson, T.M.; Xu, R.; Zuercher, W.J.; et al. Small molecule agonists of the orphan nuclear receptors steroidogenic factor-1 (SF-1, NR5A1) and liver receptor homologue-1 (LRH-1, NR5A2). J. Med. Chem. 2011, 54, 2266–2281. [Google Scholar] [CrossRef] [PubMed]
- Mays, S.G.; Flynn, A.R.; Cornelison, J.L.; Okafor, C.D.; Wang, H.; Wang, G.; Huang, X.; Donaldson, H.N.; Millings, E.J.; Polavarapu, R.; et al. Development of the First Low Nanomolar Liver Receptor Homolog-1 Agonist through Structure-guided Design. J. Med. Chem. 2019, 62, 11022–11034. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M.; Ku, S.; Brown, C.; Okamoto, A.Y.; Shan, B. CPF: An orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6660–6665. [Google Scholar] [CrossRef]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Schoonjans, K.; Annicotte, J.S.; Huby, T.; Botrugno, O.A.; Fayard, E.; Ueda, Y.; Chapman, J.; Auwerx, J. Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 2002, 3, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Botrugno, O.A.; Fayard, E.; Annicotte, J.S.; Haby, C.; Brennan, T.; Wendling, O.; Tanaka, T.; Kodama, T.; Thomas, W.; Auwerx, J.; et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell 2004, 15, 499–509. [Google Scholar] [CrossRef]
- Pare, J.F.; Malenfant, D.; Courtemanche, C.; Jacob-Wagner, M.; Roy, S.; Allard, D.; Belanger, L. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J. Biol. Chem. 2004, 279, 21206–21216. [Google Scholar] [CrossRef]
- Marikawa, Y.; Alarcon, V.B. Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol. Reprod. Dev. 2009, 76, 1019–1032. [Google Scholar] [CrossRef]
- Sung, B.; Do, H.J.; Park, S.W.; Huh, S.H.; Oh, J.H.; Chung, H.J.; Kang, M.J.; Kim, J.H.; Kim, N.H.; Kim, J.H. Regulation of OCT4 gene expression by liver receptor homolog-1 in human embryonic carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 427, 315–320. [Google Scholar] [CrossRef]
- Gu, P.; Goodwin, B.; Chung, A.C.; Xu, X.; Wheeler, D.A.; Price, R.R.; Galardi, C.; Peng, L.; Latour, A.M.; Koller, B.H.; et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 2005, 25, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.C.; Feng, B.; Han, J.; Jiang, J.; Kraus, P.; Ng, J.H.; Orlov, Y.L.; Huss, M.; Yang, L.; Lufkin, T.; et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 2010, 6, 167–174. [Google Scholar] [CrossRef]
- Rausa, F.M.; Galarneau, L.; Belanger, L.; Costa, R.H. The nuclear receptor fetoprotein transcription factor is coexpressed with its target gene HNF-3beta in the developing murine liver, intestine and pancreas. Mech. Dev. 1999, 89, 185–188. [Google Scholar] [CrossRef]
- Zhai, G.; Song, J.; Shu, T.; Yan, J.; Jin, X.; He, J.; Yin, Z. LRH-1 senses signaling from phosphatidylcholine to regulate the expansion growth of digestive organs via synergy with Wnt/beta-catenin signaling in zebrafish. J. Genet. Genom. Yi Chuan Xue Bao 2017, 44, 307–317. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Wang, H.; Nattiv, R.; Suzawa, M.; Escusa, H.S.; Fletterick, R.J.; Klein, O.D.; Moore, D.D.; Ingraham, H.A. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat. Commun. 2018, 9, 4055. [Google Scholar] [CrossRef] [PubMed]
- Noti, M.; Sidler, D.; Brunner, T. Extra-adrenal glucocorticoid synthesis in the intestinal epithelium: More than a drop in the ocean? Semin. Immunopathol. 2009, 31, 237–248. [Google Scholar] [CrossRef]
- Cima, I.; Corazza, N.; Dick, B.; Fuhrer, A.; Herren, S.; Jakob, S.; Ayuni, E.; Mueller, C.; Brunner, T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004, 200, 1635–1646. [Google Scholar] [CrossRef]
- Mueller, M.; Cima, I.; Noti, M.; Fuhrer, A.; Jakob, S.; Dubuquoy, L.; Schoonjans, K.; Brunner, T. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 2006, 203, 2057–2062. [Google Scholar] [CrossRef]
- Coste, A.; Dubuquoy, L.; Barnouin, R.; Annicotte, J.S.; Magnier, B.; Notti, M.; Corazza, N.; Antal, M.C.; Metzger, D.; Desreumaux, P.; et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2007, 104, 13098–13103. [Google Scholar] [CrossRef]
- Carulli, A.J.; Keeley, T.M.; Demitrack, E.S.; Chung, J.; Maillard, I.; Samuelson, L.C. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev. Biol. 2015, 402, 98–108. [Google Scholar] [CrossRef]
- Lefevre, L.; Authier, H.; Stein, S.; Majorel, C.; Couderc, B.; Dardenne, C.; Eddine, M.A.; Meunier, E.; Bernad, J.; Valentin, A.; et al. LRH-1 mediates anti-inflammatory and antifungal phenotype of IL-13-activated macrophages through the PPARgamma ligand synthesis. Nat. Commun. 2015, 6, 6801. [Google Scholar] [CrossRef]
- Schwaderer, J.; Gaiser, A.K.; Phan, T.S.; Delgado, M.E.; Brunner, T. Liver receptor homolog-1 (NR5a2) regulates CD95/Fas ligand transcription and associated T-cell effector functions. Cell Death Dis. 2017, 8, e2745. [Google Scholar] [CrossRef]
- Seitz, C.; Huang, J.; Geiselhoringer, A.L.; Galbani-Bianchi, P.; Michalek, S.; Phan, T.S.; Reinhold, C.; Dietrich, L.; Schmidt, C.; Corazza, N.; et al. The orphan nuclear receptor LRH-1/NR5a2 critically regulates T cell functions. Sci. Adv. 2019, 5, eaav9732. [Google Scholar] [CrossRef]
- Schwaderer, J.; Phan, T.S.; Glockner, A.; Delp, J.; Leist, M.; Brunner, T.; Delgado, M.E. Pharmacological LRH-1/Nr5a2 inhibition limits pro-inflammatory cytokine production in macrophages and associated experimental hepatitis. Cell Death Dis. 2020, 11, 154. [Google Scholar] [CrossRef]
- Benod, C.; Vinogradova, M.V.; Jouravel, N.; Kim, G.E.; Fletterick, R.J.; Sablin, E.P. Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, 16927–16931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, Y.; Wang, H.; Yan, S.; Wang, X.; Cai, L. LRH1 as a promising prognostic biomarker and predictor of metastasis in patients with non-small cell lung cancer. Thorac. Cancer 2018, 9, 1725–1732. [Google Scholar] [CrossRef]
- Annicotte, J.S.; Chavey, C.; Servant, N.; Teyssier, J.; Bardin, A.; Licznar, A.; Badia, E.; Pujol, P.; Vignon, F.; Maudelonde, T.; et al. The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene 2005, 24, 8167–8175. [Google Scholar] [CrossRef]
- Miki, Y.; Clyne, C.D.; Suzuki, T.; Moriya, T.; Shibuya, R.; Nakamura, Y.; Ishida, T.; Yabuki, N.; Kitada, K.; Hayashi, S.; et al. Immunolocalization of liver receptor homologue-1 (LRH-1) in human breast carcinoma: Possible regulator of insitu steroidogenesis. Cancer Lett. 2006, 244, 24–33. [Google Scholar] [CrossRef]
- Wu, C.; Feng, J.; Li, L.; Wu, Y.; Xie, H.; Yin, Y.; Ye, J.; Li, Z. Liver receptor homologue 1, a novel prognostic marker in colon cancer patients. Oncol. Lett. 2018, 16, 2833–2838. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Mukkamala, S.; Sablin, E.P.; Webb, P.; Fletterick, R.J. Silencing LRH-1 in colon cancer cell lines impairs proliferation and alters gene expression programs. Proc. Natl. Acad. Sci. USA 2015, 112, 2467–2472. [Google Scholar] [CrossRef]
- Qu, R.; Hao, S.; Jin, X.; Shi, G.; Yu, Q.; Tong, X.; Guo, D. MicroRNA-374b reduces the proliferation and invasion of colon cancer cells by regulation of LRH-1/Wnt signaling. Gene 2018, 642, 354–361. [Google Scholar] [CrossRef]
- Yuan, Q.; Cao, G.; Li, J.; Zhang, Y.; Yang, W. MicroRNA-136 inhibits colon cancer cell proliferation and invasion through targeting liver receptor homolog-1/Wnt signaling. Gene 2017, 628, 48–55. [Google Scholar] [CrossRef]
- Kramer, H.B.; Lai, C.F.; Patel, H.; Periyasamy, M.; Lin, M.L.; Feller, S.M.; Fuller-Pace, F.V.; Meek, D.W.; Ali, S.; Buluwela, L. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016, 44, 582–594. [Google Scholar] [CrossRef]
- Lai, H.T.; Tseng, W.K.; Huang, S.W.; Chao, T.C.; Su, Y. MicroRNA-203 diminishes the stemness of human colon cancer cells by suppressing GATA6 expression. J. Cell. Physiol. 2020, 235, 2866–2880. [Google Scholar] [CrossRef]
- Safi, R.; Kovacic, A.; Gaillard, S.; Murata, Y.; Simpson, E.R.; McDonnell, D.P.; Clyne, C.D. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005, 65, 11762–11770. [Google Scholar] [CrossRef]
- Lai, H.T.; Chiang, C.T.; Tseng, W.K.; Chao, T.C.; Su, Y. GATA6 enhances the stemness of human colon cancer cells by creating a metabolic symbiosis through upregulating LRH-1 expression. Mol. Oncol. 2020, 14, 1327–1347. [Google Scholar] [CrossRef]
- Schoonjans, K.; Dubuquoy, L.; Mebis, J.; Fayard, E.; Wendling, O.; Haby, C.; Geboes, K.; Auwerx, J. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 2058–2062. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Piccinin, E.; Pinto, C.; Peres, C.; Bellafante, E.; Moschetta, A. Biliary Phospholipids Sustain Enterocyte Proliferation and Intestinal Tumor Progression via Nuclear Receptor Lrh1 in mice. Sci. Rep. 2016, 6, 39278. [Google Scholar] [CrossRef]
- Sidler, D.; Renzulli, P.; Schnoz, C.; Berger, B.; Schneider-Jakob, S.; Fluck, C.; Inderbitzin, D.; Corazza, N.; Candinas, D.; Brunner, T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncoimmunology 2012, 1, 529–530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sidler, D.; Renzulli, P.; Schnoz, C.; Berger, B.; Schneider-Jakob, S.; Fluck, C.; Inderbitzin, D.; Corazza, N.; Candinas, D.; Brunner, T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene 2011, 30, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, Y.H.; Chua, S.; Park, Y.J.; Moore, D.D. Phosphorylation of the hinge domain of the nuclear hormone receptor LRH-1 stimulates transactivation. J. Biol. Chem. 2006, 281, 7850–7855. [Google Scholar] [CrossRef] [PubMed]
- Boivin, M.A.; Ye, D.; Kennedy, J.C.; Al-Sadi, R.; Shepela, C.; Ma, T.Y. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G590–G598. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerlotin, R.; Arconzo, M.; Piccinin, E.; Moschetta, A. Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer. Cancers 2021, 13, 896. https://doi.org/10.3390/cancers13040896
Zerlotin R, Arconzo M, Piccinin E, Moschetta A. Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer. Cancers. 2021; 13(4):896. https://doi.org/10.3390/cancers13040896
Chicago/Turabian StyleZerlotin, Roberta, Maria Arconzo, Elena Piccinin, and Antonio Moschetta. 2021. "Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer" Cancers 13, no. 4: 896. https://doi.org/10.3390/cancers13040896
APA StyleZerlotin, R., Arconzo, M., Piccinin, E., & Moschetta, A. (2021). Another One Bites the Gut: Nuclear Receptor LRH-1 in Intestinal Regeneration and Cancer. Cancers, 13(4), 896. https://doi.org/10.3390/cancers13040896







