MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Pathways Involved in the Metastatic Process and the Role/Importance of Plasticity
2.1. Molecular Regulation of Tumor Microenvironment
2.1.1. Cadherins and Catenins
microRNA, Cadherins, and Catenin in BLCA
2.1.2. Integrins in BLCA
Integrins and miRNA in BLCA
2.1.3. CD44 in BLCA
CD44 and miRNA in BLCA
2.2. The Interplay between Extracellular Matrix (ECM) and miRNAs
Matrix Metalloproteinases (MMPs)
2.3. Mechanism of Metastatic Inhibition or Induction in BLCA Via miRNA
2.4. Mechanism of Angiogenesis Inhibition Via miRNA in BLCA
3. Exosomes and miRNAs in Metastasis and Angiogenesis of BLCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enokida, H.; Yoshino, H.; Matsushita, R.; Nakagawa, M. The role of microRNAs in bladder cancer. Investig. Clin. Urol. 2016, 57 (Suppl. 1), S60. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W. Molecular Subtypes of Bladder Cancer. Curr. Oncol. Rep. 2018, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N. Muscle invasive and metastatic bladder cancer. Ann. Oncol. 2006, 17, x23–x30. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wang, J.; Zhao, Y.; Li, S.; Zhou, B.; Su, Z.; Xu, C.; Xia, Y.; Qian, H.; et al. Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. IJMS 2020, 21, 5840. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xu, T.; Shen, Y.; Zhong, S.; Chen, S.; Ding, Q.; Shen, Z. Dysregulation of miRNAs in bladder cancer: Altered expression with aberrant biogenesis procedure. Oncotarget 2017, 8, 27547–27568. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Radojicic, J.; Lambrou, G.I.; Volanis, D.; Delakas, D.; Stathopoulos, E.N.; Spandidos, D.A. Expression of miRNAs Involved in Angiogenesis, Tumor Cell Proliferation, Tumor Suppressor Inhibition, Epithelial-Mesenchymal Transition and Activation of Metastasis in Bladder Cancer. J. Urol. 2012, 188, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, Q.-F.; Liu, X.-Q.; Guo, Z.-J.; Li, C.-Y.; Sun, G. MiR-122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. Am. J. Transl. Res. 2016, 8, 3056–3066. [Google Scholar] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Ravegnini, G.; Cargnin, S.; Sammarini, G.; Zanotti, F.; Bermejo, J.L.; Hrelia, P.; Terrazzino, S.; Angelini, S. Prognostic Role of miR-221 and miR-222 Expression in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 970. [Google Scholar] [CrossRef]
- Amuran, G.G.; Eyuboglu, I.P.; Tinay, I.; Akkiprik, M. New Insights in Bladder Cancer Diagnosis: Urinary miRNAs and Proteins. Med. Sci. 2018, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019, 110, 408–419. [Google Scholar] [CrossRef]
- Mihnea, D.; Baoqing, C.; Xiao, F.; George, A.C. Key questions about the checkpoint blockade-are microRNAs an answer? Cancer Biol. Med. 2018, 15, 103. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- van der Horst, G.; Bos, L.; van der Pluijm, G. Epithelial Plasticity, Cancer Stem Cells, and the Tumor-Supportive Stroma in Bladder Carcinoma. Mol. Cancer Res. 2012, 10, 995–1009. [Google Scholar] [CrossRef]
- Bryan, R.T.; Tselepis, C. Cadherin Switching and Bladder Cancer. J. Urol. 2010, 184, 423–431. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Thege, F.I.; Gruber, C.N.; Cardle, I.I.; Cong, S.H.; Lannin, T.B.; Kirby, B.J. anti-EGFR capture mitigates EMT- and chemoresistance-associated heterogeneity in a resistance-profiling CTC platform. Anal. Biochem. 2019, 577, 26–33. [Google Scholar] [CrossRef]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. [Google Scholar] [CrossRef]
- Godin, L.; Balsat, C.; Van Eycke, Y.-R.; Allard, J.; Royer, C.; Remmelink, M.; Pastushenko, I.; D’Haene, N.; Blanpain, C.; Salmon, I.; et al. A Novel Approach for Quantifying Cancer Cells Showing Hybrid Epithelial/Mesenchymal States in Large Series of Tissue Samples: Towards a New Prognostic Marker. Cancers 2020, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Bocci, F.; Tripathi, S.C.; Vilchez Mercedes, S.A.; George, J.T.; Casabar, J.P.; Wong, P.K.; Hanash, S.M.; Levine, H.; Onuchic, J.N.; Jolly, M.K. NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr. Biol. 2019, 11, 251–263. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef]
- Bryan, R.T. Cell adhesion and urothelial bladder cancer: The role of cadherin switching and related phenomena. Philos. Trans. R. Soc. B 2015, 370, 20140042. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Haitel, A.; Moschini, M.; Gust, K.; Foerster, B.; Özsoy, M.; D’Andrea, D.; Karakiewicz, P.I.; Rouprêt, M.; Briganti, A.; et al. Prognostic Role of N-cadherin Expression in Patients With Invasive Bladder Cancer. Clin. Genitourin. Cancer 2018, 16, e73–e78. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Choi, W.; Marquis, L.; Martin, F.; Williams, M.B.; Shah, J.; Svatek, R.; Das, A.; Adam, L.; Kamat, A.; et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009, 28, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.; Miao, S.; Li, C.; Chen, Z.; Li, F. Enhancing E-cadherin expression via promoter-targeted miR-373 suppresses bladder cancer cells growth and metastasis. Oncotarget 2017, 8, 93969–93983. [Google Scholar] [CrossRef]
- Liu, W.; Qi, L.; Lv, H.; Zu, X.; Chen, M.; Wang, J.; Liu, L.; Zeng, F.; Li, Y. MiRNA-141 and miRNA-200b are closely related to invasive ability and considered as decision-making biomarkers for the extent of PLND during cystectomy. BMC Cancer 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Chang, Z.; Wu, C.; Guo, H. microRNA-145 Modulates Migration and Invasion of Bladder Cancer Cells by Targeting N-Cadherin. Mol. Med. Rep. 2018, 17, 8450–8456. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-W.; Xiao, J.-Q.; Li, Z.-Y.; Zheng, Y.-C.; Zhang, N. Effects of microRNA-135a on the epithelial–mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 2018, 50, e429. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Zhong, M.; Choi, W.; Qi, W.; Nicoloso, M.; Arora, A.; Calin, G.; Wang, H.; Siefker-Radtke, A.; McConkey, D.; et al. miR-200 Expression Regulates Epithelial-to-Mesenchymal Transition in Bladder Cancer Cells and Reverses Resistance to Epidermal Growth Factor Receptor Therapy. Clin. Cancer Res. 2009, 15, 5060–5072. [Google Scholar] [CrossRef]
- Zhang, Q.; Miao, S.; Han, X.; Li, C.; Zhang, M.; Cui, K.; Xiong, T.; Chen, Z.; Wang, C.; Xu, H. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Qin, L.; Zhu, Z.; Wang, X.; Liu, Y.; Fan, Y.; Zhong, S.; Wang, X.; Zhang, X.; Xia, L.; et al. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin α5. Oncotarget 2016, 7, 27445–27457. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, H.; Gao, Z.; Li, J.; Zhuang, J.; Dong, Y.; Shen, B.; Li, M.; Zhou, H.; Guo, H.; et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018, 293, 6693–6706. [Google Scholar] [CrossRef]
- Derycke, L.D.M.; Bracke, M.E. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004, 48, 463–476. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. Illustrating the interplay between the extracellular matrix and microRNAs. Int. J. Exp. Path. 2014, 95, 158–180. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Yoshino, H.; Yonemori, M.; Miyamoto, K.; Sugita, S.; Matsushita, R.; Itesako, T.; Tatarano, S.; Nakagawa, M.; Enokida, H. Regulation of ITGA3 by the dual-stranded microRNA-199 family as a potential prognostic marker in bladder cancer. Br. J. Cancer 2017, 116, 1077–1087. [Google Scholar] [CrossRef]
- Vallo, S.; Rutz, J.; Kautsch, M.; Winkelmann, R.; Michaelis, M.; Wezel, F.; Bartsch, G.; Haferkamp, A.; Rothweiler, F.; Blaheta, R.; et al. Blocking integrin β1 decreases adhesion in chemoresistant urothelial cancer cell lines. Oncol. Lett. 2017, 14, 5513–5518. Available online: http://www.spandidos-publications.com/10.3892/ol.2017.6883 (accessed on 3 November 2020). [CrossRef] [PubMed]
- Liu, S.; Chen, L.; Zhao, H.; Li, Q.; Hu, R.; Wang, H. Integrin β8 facilitates tumor growth and drug resistance through a Y-box binding protein 1-dependent signaling pathway in bladder cancer. Cancer Sci. 2020, 111, 2423–2430. [Google Scholar] [CrossRef]
- Gontero, P.; Banisadr, S.; Frea, B.; Brausi, M. Metastasis Markers in Bladder Cancer: A Review of the Literature and Clinical Considerations. Eur. Urol. 2004, 46, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-R.; Liu, B.; Zhou, L.; Huang, Y.-X. MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer. CBM 2019, 24, 159–172. [Google Scholar] [CrossRef]
- Mamuya, F.A.; Duncan, M.K. aV integrins and TGF-β-induced EMT: A circle of regulation. J. Cell. Mol. Med. 2012, 16, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Lin, W.-Y.; Chang, Y.-H.; Chen, W.-C.; Chen, M.-F. Impact of CD44 expression on radiation response for bladder cancer. J. Cancer 2017, 8, 1137–1144. [Google Scholar] [CrossRef]
- Yu, G.; Yao, W.; Xiao, W.; Li, H.; Xu, H.; Lang, B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J. Exp. Clin. Cancer Res. 2014, 33, 779. [Google Scholar] [CrossRef]
- Wu, C.-T.; Lin, W.-Y.; Chen, W.-C.; Chen, M.-F. Predictive Value of CD44 in Muscle-Invasive Bladder Cancer and Its Relationship with IL-6 Signaling. Ann. Surg. Oncol. 2018, 25, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Zeng, F.; Qi, L.; Zu, X.B.; Wang, J.; Liu, L.F.; Li, Y. Transforming growth factor-β1 induces epithelial-mesenchymal transition and increased expression of matrix metalloproteinase-16 via miR-200b downregulation in bladder cancer cells. Mol. Med. Rep. 2014, 10, 1549–1554. [Google Scholar] [CrossRef]
- Yonemori, M.; Seki, N.; Yoshino, H.; Matsushita, R.; Miyamoto, K.; Nakagawa, M.; Enokida, H. Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016, 107, 1233–1242. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Xu, B.; Wang, P.; Fan, W.; Cai, Y.; Gu, X.; Meng, F. miR-124 exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015, 282, 4376–4388. [Google Scholar] [CrossRef]
- Yan, T.; Ye, X.-X. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5139–5178. [Google Scholar]
- Zhang, L.; Wang, C.-Z.; Ma, M.; Shao, G.-F. MiR-15 suppressed the progression of bladder cancer by targeting BMI1 oncogene via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8813–8822. [Google Scholar] [PubMed]
- Zhang, S.; Zhang, C.; Liu, W.; Zheng, W.; Zhang, Y.; Wang, S.; Huang, D.; Liu, X.; Bai, Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015, 47, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Deng, G.; Chang, I.; Greene, K.; Tanaka, Y.; Dahiya, R.; Yamamura, S. MicroRNA-23b Functions as a Tumor Suppressor by Regulating Zeb1 in Bladder Cancer. PLoS ONE 2013, 8, e67686. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Xiao, M.; Yang, J.; Zhang, N. miR-203 Suppresses Bladder Cancer Cell Growth and Targets Twist1. Oncol. Res. 2018, 26, 1155–1165. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Wang, X.; Meng, S.; Shen, J.; Wang, S.; Xu, X.; Xie, B.; Liu, B.; Xie, L. MiR-22 suppresses epithelial–mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018, 9, 209. [Google Scholar] [CrossRef]
- Wei, X.C.; Lv, Z.H. MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition via TGFβ1/Smad2 signaling pathway in human bladder cancer. OTT 2019, 12, 5937–5945. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Q.; Wang, S.; Zhang, J. miR-485-5p inhibits bladder cancer metastasis by targeting HMGA2. Int. J. Mol. Med. 2015, 36, 1136–1142. [Google Scholar] [CrossRef]
- Jia, A.Y.; Castillo-Martin, M.; Bonal, D.M.; Sánchez-Carbayo, M.; Silva, J.M.; Cordon-Cardo, C. MicroRNA-126 inhibits invasion in bladder cancer via regulation of ADAM9. Br. J. Cancer 2014, 110, 2945–2954. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Hu, L.; Liu, F.; Zhang, Q.; Zhang, D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016, 16, 64. [Google Scholar] [CrossRef]
- Ueno, K.; Hirata, H.; Majid, S.; Yamamura, S.; Shahryari, V.; Tabatabai, Z.L.; Hinoda, Y.; Dahiya, R. Tumor Suppressor MicroRNA-493 Decreases Cell Motility and Migration Ability in Human Bladder Cancer Cells by Downregulating RhoC and FZD4. Mol. Cancer Ther. 2012, 11, 244–253. [Google Scholar] [CrossRef]
- Itesako, T.; Seki, N.; Yoshino, H.; Chiyomaru, T.; Yamasaki, T.; Hidaka, H.; Yonezawa, T.; Nohata, N.; Kinoshita, T.; Nakagawa, M.; et al. The MicroRNA Expression Signature of Bladder Cancer by Deep Sequencing: The Functional Significance of the miR-195/497 Cluster. PLoS ONE 2014, 9, e84311. [Google Scholar] [CrossRef]
- He, C.; Zhang, Q.; Gu, R.; Lou, Y.; Liu, W. miR-96 regulates migration and invasion of bladder cancer through epithelial-mesenchymal transition in response to transforming growth factor-β1. J. Cell. Biochem. 2018, 119, 7807–7817. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Zhao, X. miR-221 facilitates the TGFbeta1-induced epithelial-mesenchymal transition in human bladder cancer cells by targeting STMN1. BMC Urol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, L.; Tong, S.; Cui, Y.; Chen, J.; Huang, T.; Chen, Z.; Zu, X.-B. miR-128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF-C upregulation. Oncol. Lett. 2015, 10, 3183–3190. [Google Scholar] [CrossRef]
- Mylona, E.; Magkou, C.; Gorantonakis, G.; Giannopoulou, I.; Nomikos, A.; Zarogiannos, A.; Zervas, A.; Nakopoulou, L. Evaluation of the vascular endothelial growth factor (VEGF)-C role in urothelial carcinomas of the bladder. Anticancer Res. 2006, 26, 3567–3571. [Google Scholar]
- Kim, S.M.; Kang, H.W.; Kim, W.T.; Kim, Y.-J.; Yun, S.J.; Lee, S.-C.; Kim, W.-J. Cell-Free microRNA-214 From Urine as a Biomarker for Non-Muscle-Invasive Bladder Cancer. Korean J. Urol. 2013, 54, 791. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, L.; Zhao, S.; Zhu, X. The functions of microRNA-124 on bladder cancer. OTT 2019, 12, 3429–3439. [Google Scholar] [CrossRef]
- Wu, S.-Q.; He, H.-Q.; Kang, Y.; Xu, R.; Zhang, L.; Zhao, X.-K.; Zhu, X. MicroRNA-200c affects bladder cancer angiogenesis by regulating the Akt2/mTOR/HIF-1 axis. Transl. Cancer Res. TCR 2019, 8, 2713–2724. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, S.; Shi, D.; Zhang, J.; Zhang, Z.; Guo, Y.; Wu, Y.; Wang, R.; Wang, L.; Huang, Y.; et al. MicroRNA-153 Decreases Tryptophan Catabolism and Inhibits Angiogenesis in Bladder Cancer by Targeting Indoleamine 2,3-Dioxygenase 1. Front. Oncol. 2019, 9, 619. [Google Scholar] [CrossRef]
- Fendler, A.; Stephan, C.; Yousef, G.M.; Kristiansen, G.; Jung, K. The translational potential of microRNAs as biofluid markers of urological tumours. Nat. Rev. Urol. 2016, 13, 734–752. [Google Scholar] [CrossRef]
- Lodewijk, I.; Dueñas, M.; Rubio, C.; Munera-Maravilla, E.; Segovia, C.; Bernardini, A.; Teijeira, A.; Paramio, J.M.; Suárez-Cabrera, C. Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring. IJMS 2018, 19, 2514. [Google Scholar] [CrossRef]
- Piao, X.-M.; Cha, E.-J.; Yun, S.J.; Kim, W.-J. Role of Exosomal miRNA in Bladder Cancer: A Promising Liquid Biopsy Biomarker. IJMS 2021, 22, 1713. [Google Scholar] [CrossRef]
- Zhang, D.-Z.; Lau, K.-M.; Chan, E.S.Y.; Wang, G.; Szeto, C.-C.; Wong, K.; Choy, R.K.W.; Ng, C.-F. Cell-Free Urinary MicroRNA-99a and MicroRNA-125b Are Diagnostic Markers for the Non-Invasive Screening of Bladder Cancer. PLoS ONE 2014, 9, e100793. [Google Scholar] [CrossRef]
- Eissa, S.; Habib, H.; Ali, E.; Kotb, Y. Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med. Oncol. 2015, 32, 413. [Google Scholar] [CrossRef] [PubMed]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016, 7, 86290–86299. [Google Scholar] [CrossRef]
- Franzen, C.A.; Blackwell, R.H.; Foreman, K.E.; Kuo, P.C.; Flanigan, R.C.; Gupta, G.N. Urinary Exosomes: The Potential for Biomarker Utility, Intercellular Signaling and Therapeutics in Urological Malignancy. J. Urol. 2016, 195, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, S.; Hölters, S.; Ohlmann, C.-H.; Bohle, R.; Stöckle, M.; Ostenfeld, M.S.; Dyrskjøt, L.; Junker, K.; Heinzelmann, J. Exosomes of invasive urothelial carcinoma cells are characterized by a specific miRNA expression signature. Oncotarget 2017, 8, 58278–58291. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Jeppesen, D.K.; Laurberg, J.R.; Boysen, A.T.; Bramsen, J.B.; Primdal-Bengtson, B.; Hendrix, A.; Lamy, P.; Dagnaes-Hansen, F.; Rasmussen, M.H.; et al. Cellular Disposal of miR23b by RAB27-Dependent Exosome Release Is Linked to Acquisition of Metastatic Properties. Cancer Res. 2014, 74, 5758–5771. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Green, B.B.; Seigne, J.D.; Schned, A.R.; Marsit, C.J. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol. Cancer 2015, 14, 194. [Google Scholar] [CrossRef]
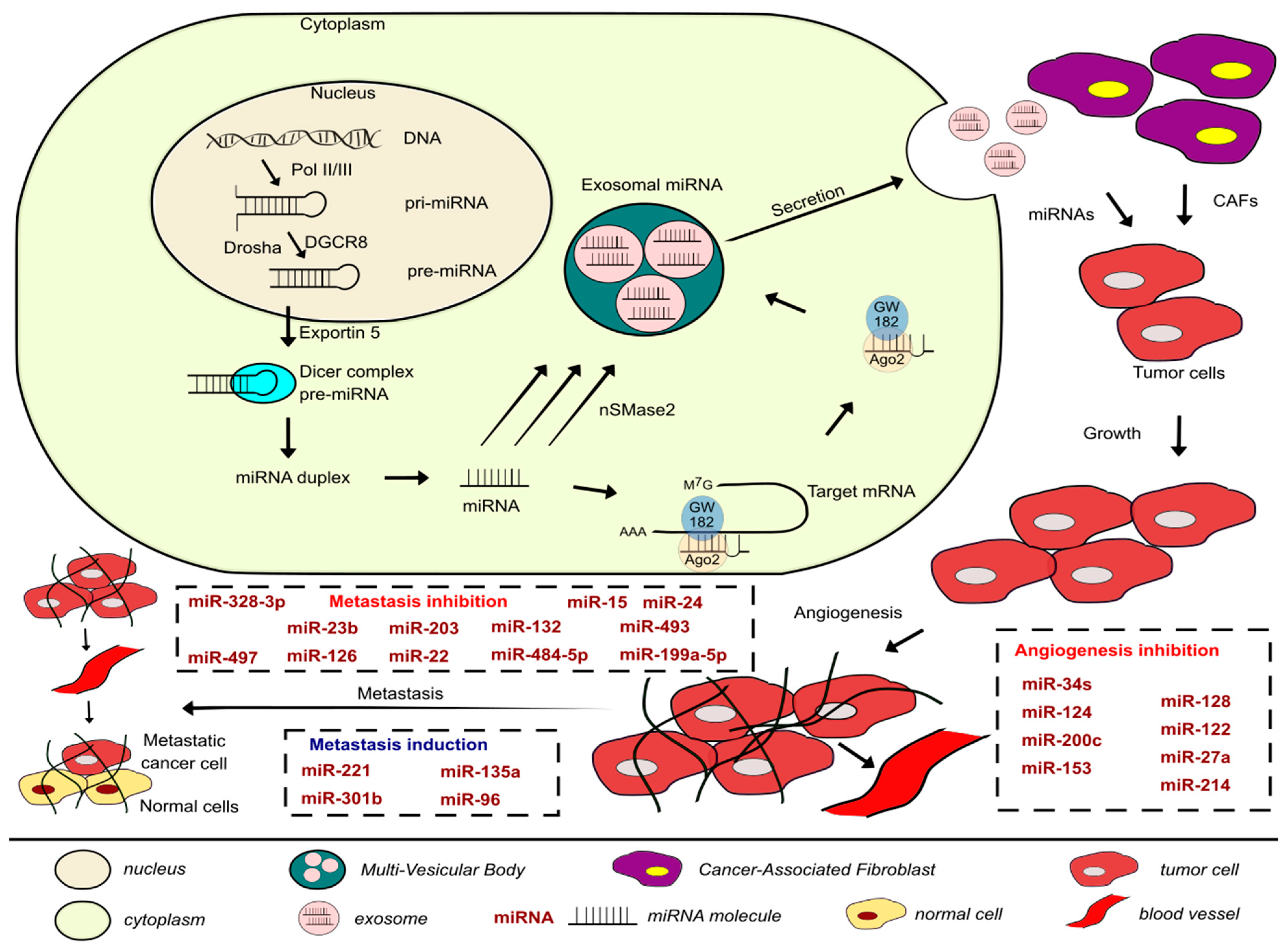
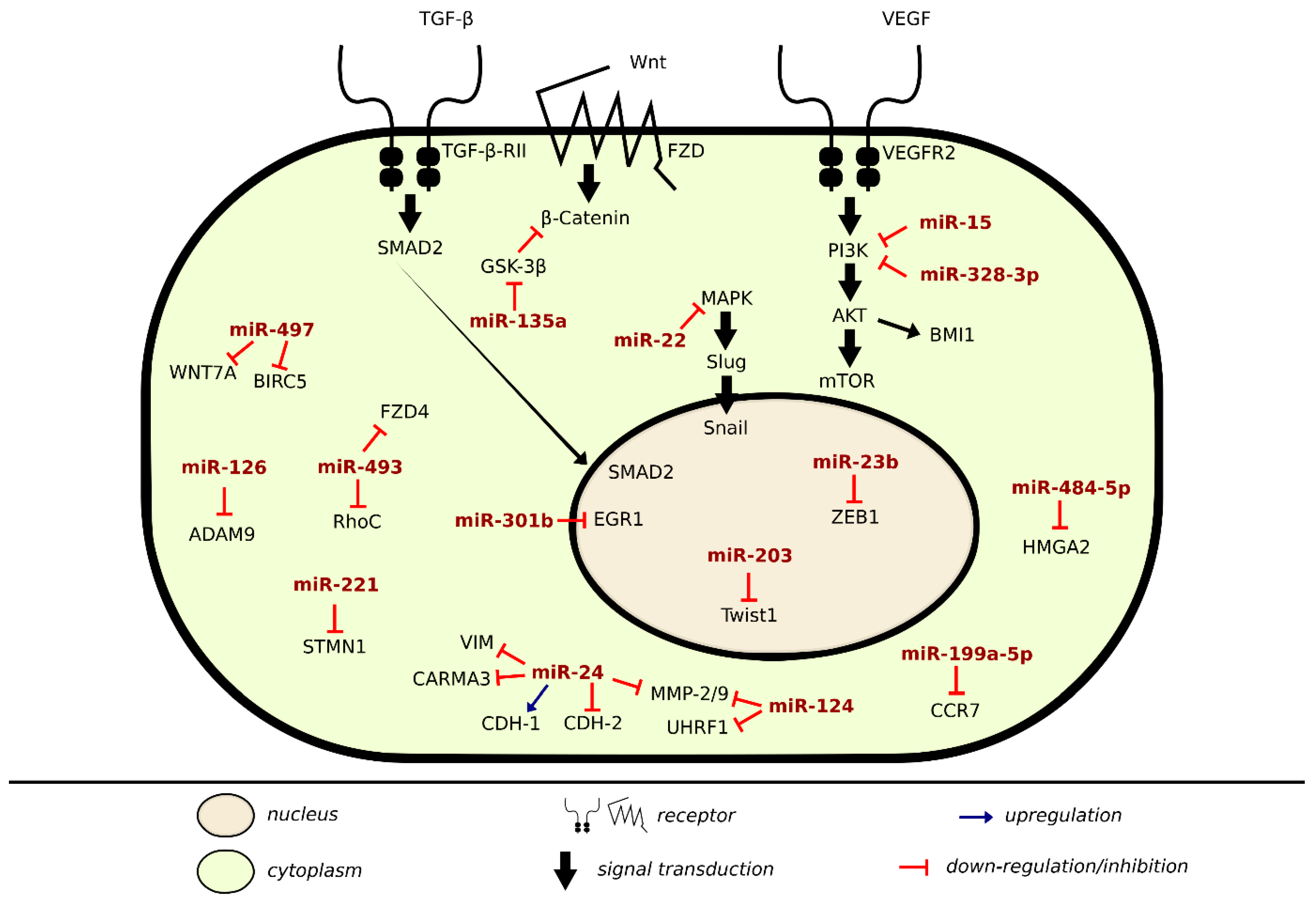
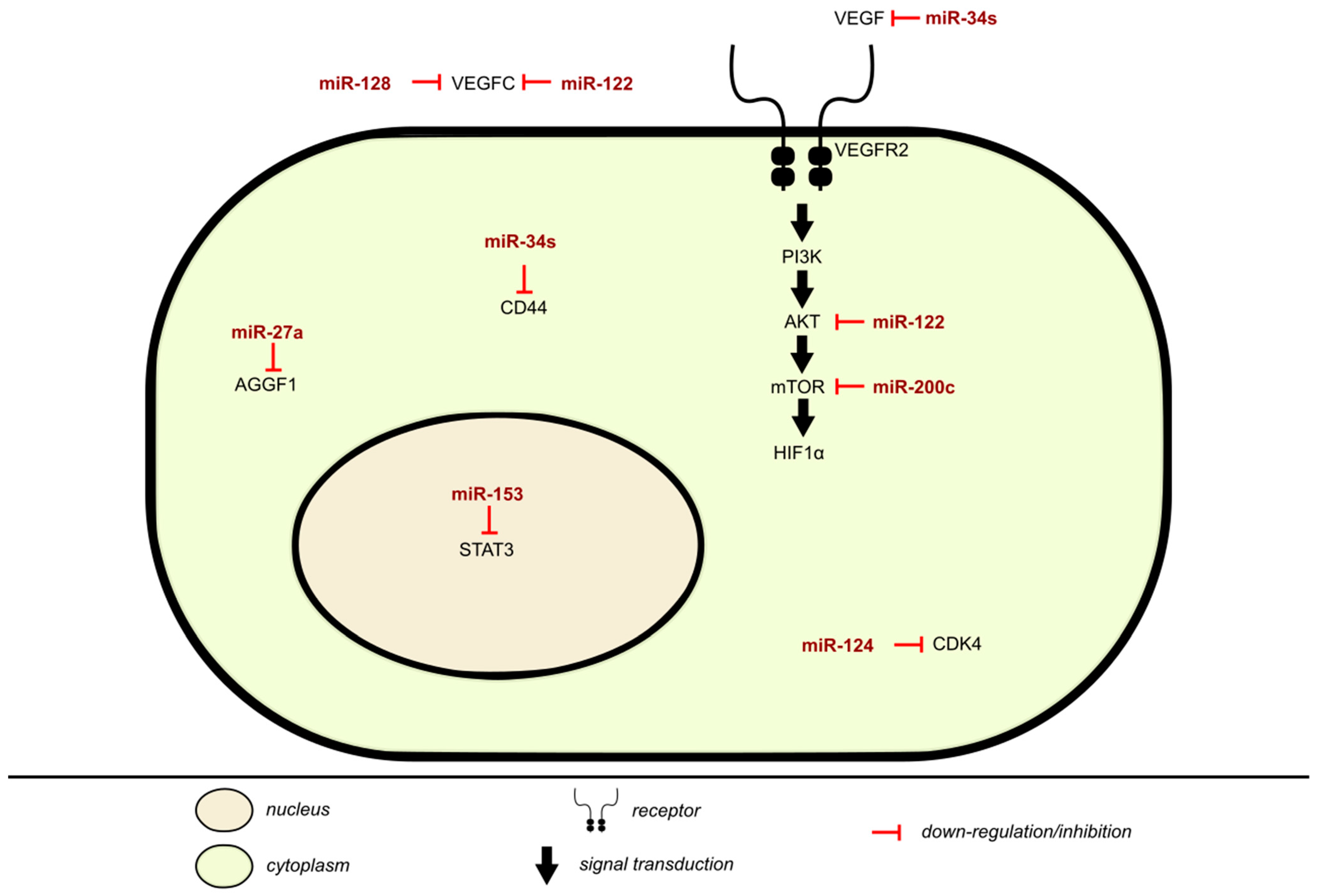
| miRNA | Signal Transduction Molecules | Target Cytoskeletal Protein | Cell–Cell Adhesion Molecules | Cell–Matrix Adhesion Molecules | Function | Notes | References |
|---|---|---|---|---|---|---|---|
| miR-373 | It inhibits expression of E-cadherin downstream genes: cyclinD1, c-myc, MMP2 mRNA levels | Its expression positively associates with | Inhibits cell proliferation and metastasis by activating E-cadherin expression. Acted as a tumor suppressor | Expression significantly correlated with tumor stage, grade, and lymph node metastasis | [34] | ||
| E-cadherin, activating its expression by interacting with E-cadherin promoter | |||||||
| miR-141 and miR-200b | Activity of MMP-2, MMP-9 enzymes was inversely proportional to that of miRNAs | Vimentin levels were downregulated following miRNA overexpression | E-cadherins levels increased, while | Loss of expression increased invasion and migration capacity by up-regulating N-cadherin and downregulating | Urine analysis distinguished presence of or lack thereof patient lymph node metastasis | [35] | |
| N-cadherin levels decreased when both miRNAs were overexpressed | E-cadherin | ||||||
| miR-145 | MMP-9 | N-cadherin | Suppresses migration and invasion by targeting and regulating N-cadherin and MMP-9 expression | [36] | |||
| miR-135a | Increased expression of β-catenin, cyclinD1, downregulation of GSK3β | Increases expression of vimentin | Decreases expression of E-cadherins | It is an oncogene that accelerates EMT, migration, and invasion via activation of Wnt/β-catenin signaling pathway and inhibiting GSK3β | [37] | ||
| miR-200b and miR-200c | Decreases ZEB1, ZEB2, EGFR, ERRFI-1 expression | Increases E-cadherin expression | Control the EMT process, decreasing cell migration, and via E-cadherin affects response to EGFR therapy | [38] | |||
| miR-3619 | It interacts with p21 promoter, targeting β-catenin and CDK2. Downregulates Snail protein levels | Decreases vimentin expression | Increases E-cadherins and decreased N-cadherin expression | Inhibits Wnt-β-catenin signal pathway and EMT progression | Low expression of p21 and miR-3619 linked to poor OS. | [39] | |
| β-catenin and CDK2 are direct downstream targets of miR-3619 | |||||||
| miR-31 | Integrin α5 is a direct target of miR-31 | miR-31 acted as a tumor suppressor deactivating Akt and ERK. Activation of miR-31/ITGA5 axis increased sensitivity of UBC to mitomycin-C | Downregulation of miR-31 correlates with higher risk of recurrence and progression of noninvasive UBC cases | [40] | |||
| miR-124-3p | FAK/PI3K/AKT and FAK/Src signalling pathways | Integrin α3 is a direct target of miR-124-3p | By targeting ITGA3 and downstream FAK/PI3K/AKT and FAK/Src signaling pathways, miR-124-3p suppresses cell migration and invasion | [41] | |||
| miR-34a | CD44 a target gene, inhibits β-catenin expression | Inhibited vimentin expression | Promotes E-cadherins and inhibited N-cadherin expression | Anti-metastatic and suppresses angiogenesis by directly targeting CD44 | [42] |
| miRNA | Target | Function | References |
|---|---|---|---|
| miR-142-3p | ADAM9 (direct) | ADAM9 expression was regulated in wing and leg mesenchymal cells and contributed to the modulation of position-dependent chondrogenesis. | [44] |
| miR-200b | MMP-16 (direct) | miR-200b overexpression inhibited TGF-β1-induced MMP-16 upregulation and BLCA migration | [54] |
| miR-370-3p | Wnt7a | Wnt7a overexpression up-regulated MMP1/10 to degrade the extracellular matrix and to facilitate UBC cell invasion | [42] |
| miR-139-5p and miR-139-3p | MMP11 | MMP11 contributed to migration and invasion through its regulation of many oncogenic genes. Several genes known to contribute to cancer cell aggressiveness were downstream from MMP11, such as CXCL1 and CXCL3 | [55] |
| microRNA | Samples/Cell Culture | Targets/ Regulators | Function | Patient’s Prognosis | References |
|---|---|---|---|---|---|
| miR-124 | Human bladder transitional cancer cell lines J82 and T24 | UHRF1 | miR-124 can impair the proliferation or metastasis of human BLCA cells by down-regulation of UHRF1 | [56] | |
| miRNA-139-5p and miRNA-139-3p | human BLCA cell lines: T24 and BOY | MMP11 | Downregulation of both miRNAs enhanced BLCA cell migration and invasion | Higher expression of MMP11 predicted shorter survival of BLCA patients (p = 0.029) | [55] |
| pre-miR-145 and miR-145-5p | Clinical tissue: 62 BLCA patients at Kagoshima University Hospital between 2003 and 2013 | UHRF1 | Overexpression of miR-124 in vitro, attenuated cellular proliferation, migration, invasion, and angiogenesis by downregulating UHRF1 | [55] | |
| miR-328-3p | Cell lines: Urinary epithelial SV-HUC-1 and 5637, T24, J82 BC cell lines Clinical tissue:28 pairs of BLCA tissues and adjacent normal samples were acquired from the Yinzhou Hospital | ITGA5 | miR-328-3p inhibited the development of BLCA by targeting ITGA5 and inactivating the PI3K/AKT pathway | Downregulation of miRNA predicted poor prognosis in BLCA patients | [57] |
| miR-15 | T24, BIU87, HT1376 BLCA cell lines, and normal uroepithelial cell lines SV-HUV-1 | BMI1 | Overexpression of miR-15 inhibited EMT and PI3K/AKT pathway | [58] | |
| miR-24 | Human BLCA cell lines T24, UMUC-3, J82, 5637 and normal transitional epithelial cell line SV-HUC-1 | CARMA3 | Upregulation of miR-24 inhibited proliferation, invasion, EMT, and induced apoptosis of T24 and UMUC-3 cells. Additionally, upregulation of miR-24 decreased the protein levels of cyclin D1, CDK4, CDK6, p-Rb, and Bcl-2 | [59] | |
| miR-23b | T24 and J82, and normal SV-HUC-1 | 3′UTR of Zeb1 | Overexpression of miR-23b suppressed the oncogene ZEB1 suppressing cell proliferation, invasion apoptosis, and cell cycle arrest | Patients with higher miR-23b expression had longer OS compared to patients with low miR-23b expression. Additionally, its expression distinguished malignant from normal tissues | [60] |
| miR-203 | T24, RT4, normal urothelial cell line SV-HUC-1 | Twist1 | miR-203 mimic significantly reduced BLCA cell proliferation, migration, and invasion, and induced apoptosis by targeting Twist1 | [61] | |
| miR-22 | T24, UM-UC-3, as well as one normal bladder cell line SV-HUC-1 | MAPK1 and Snail | miR-22 was found to suppress cell proliferation/apoptosis by directly targeting MAPK1 and inhibiting cell motility by targeting both MAPK1 and Snail | Low-expression of MAPK1 or Snail is an independent prognostic factor for better OS | [62] |
| miR-132 | Human BLCA cell lines T24 and human normal urothelial cell line SV-HUC-1 Clinical tissue:32 patient samples | SMAD2 | miR-132 may play a suppressive role in the metastasis of BLCA cells via TGFβ1/Smad2 signaling pathway. Overexpression of miR-132 suppressed expression of mesenchymal cell markers (N-Cadherin, Zeb1, Snail, and Vimentin) | [63] | |
| miR-484-5p | Cell lines: Human bladder cancer cell lines SW780, T24, HT1376, and HT5637 and human bladder epithelial cell lines HU609 and HEK293 cells Clinical tissue:15 patient samples who had undergone surgery and primary therapy | HMGA2 | miR-485-5p exerts a suppressive effect, partly through the suppression of HMGA2 | [64] | |
| miR-126 | Cell lines: HUC, EJ138, MCF10A, TCCSUP, J82, and 293FT cells Clinical samples: TaLG (n = 3), TaHG (n = 3), CIS (n = 3), T1LG (n = 3), T1HG (n = 3), and T2HG (n = 6) | ADAM9 | miR-126 exerts its tumor suppressive role by targeting ADAM9 to inhibit cell invasion | [65] | |
| miR-199a-5p | T24 and SV-HUC-1 cell lines Clinical samples:40 BLCA tissue samples and adjacent normal tissue from patients who underwent transurethral bladder tumor resection or radical cystectomy | 3′UTR of CCR7 | miR-199a-5p was confirmed to be able to target the 3′ UTR of CCR7 and regulate the expression of CCR7, MMP-9, and vimentin and E-cadherin | [66] | |
| miR-493 | SV-HUC-1, T24, J82, and TCCSUP cells Clinical samples: human bladder cancer tissue array from US Biomax | RhoC and FZD4 | miR-493 possibly a tumor suppressive, inhibiting cell invasion and migration by blocking FZD4 and RhoC, implicating the Wnt-PCP pathway in bladder carcinogenesis | [67] | |
| miR-497 | BOY and T24 cell lines Clinical samples: 5 BLCA patients and five normal epithelial samples of patients who underwent cystectomy or transurethral resection. | BIRC5 and WNT7A | Downregulation of miR-195/497 contributed to BLCA progression and metastasis | [68] | |
| Induction | |||||
| miRNA-135a | Normal human SVHUC-1 epithelial cells, EJ, T24, BIU87, ScaBER, and 5637165 Clinical samples: paired BLCA tissues and adjacent normal tissues were obtained from patients with BLCA who had undergone a bladder resection | GSK3β | miR-135a accelerates the EMT, invasion, and migration of BLCA cells by activating the Wnt/β-catenin signaling pathway through the downregulation of GSK3β expression. | [37] | |
| miR-96 | HT1376 | FOXQ1 | TGF-β1 could change the expression of FOXQ1 induced by miR-96, which revealed that TGF-β1 regulates miR-96/FOXQ1 signaling | [69] | |
| miR-221 | RT4 and T24 | 3′UTR of STMN1 | miR-221 can facilitate the TGFβ1-induced EMT process in human BLCA cells by suppressing STMN1 | [70] | |
| miR-301b | J82, UM-UC-3, T24, 5637 BLCA cell line and normal SVHUC-1 cell line Clinical tissue: 31 paired BLCA and normal tissue obtained from patients who underwent radical cystectomy. | EGR1 | miR-301b promotes the proliferation, migration, and aggressiveness of human BLCA cells by inhibiting the expression of EGR1. | [70] | |
| microRNA | Samples/Cell Culture | Targets/Regulators | Function | References |
|---|---|---|---|---|
| miR-128 | Human bladder epithelial cell line SV-HUC-1, and the BLCA cell lines T24, 5637, 3-UM-UC-3, and RT4 | VEGF-C | Overexpression of miR-128 inhibited growth rate, proliferation, migration, and invasion capacities. | [71,72] |
| miR-122 | BLCA cells BIU-87, T24, SW780, HT1376, 5637, RT4, and normal bladder epithelial cell line SV-HUC-1 | 3′-UTR of VEGF-C | miR-122 regulated cell proliferation through the VEGFC/AKT/mTOR signaling pathway. | [12] |
| miR-27a | Clinical samples: Urothelial carcinoma and normal urothelial tissue samples were collected from 59 patients undergoing surgery | AGGF1 | Down-regulation of AGGF1 expression by hypoxia-induced miR-27a expression represents a signaling network for development of high-grade UBC. | [7] |
| miR-214 | 138 patients with primary urothelial carcinoma of the urinary bladder and 144 healthy controls | The urinary levels of cell-free miR-214 were significantly higher in the NMIBC patients than in the controls. Thus, they could be used as a prognostic marker for NMIBC. | [73] | |
| miR-34s | Cell lines: 5637, T24, HT-1376, J82, SCABER, and EJ Clinical samples: BLCA tissue and adjacent normal tissue specimens | CD44 | miR-34a overexpression can inhibit bladder cell migration, invasion, tube formation in vitro, and metastasis and angiogenesis in vivo. Additionally, CD44-mediated functions can be reversed by miR-34a in bladder cells. | [52] |
| miR-124 | Hek293, human normal cell SV-HUC-1 and BLCA T24, 5637, J82, and UM-UC-3 Clinical tissues: 83 bladder tissues and their adjacent non-tumor tissues | CDK4 | Overexpression of miR-124 induced by mimic transfection was observed to inhibit the cells viability, angiogenesis, and proliferation. | [74] |
| miR-200c | Human bladder epithelial cell line, SV-HUC-1, BLCA cell lines 5637 and T24 | Akt2 | miR-200c could suppress HIF-1α/VEGF expression in BLCA cells and inhibit angiogenesis, and these regulations were achieved by targeting Akt2/mTOR. | [75] |
| miR-153 | T24, UMUC3, 5637, and J82 cell lines and an immortalized human normal bladder epithelial cell line SV-HUC-1 Clinical tissue: normal and cancerous tissue specimens from 45 BLCA patients | IDO1 | IDO1 mediated miR-153 anti-tumor activity in BLCA via inactivating the IL6/STAT3/VEGF pathway. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammouz, R.Y.; Kołat, D.; Kałuzińska, Ż.; Płuciennik, E.; Bednarek, A.K. MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas. Cancers 2021, 13, 891. https://doi.org/10.3390/cancers13040891
Hammouz RY, Kołat D, Kałuzińska Ż, Płuciennik E, Bednarek AK. MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas. Cancers. 2021; 13(4):891. https://doi.org/10.3390/cancers13040891
Chicago/Turabian StyleHammouz, Raneem Y., Damian Kołat, Żaneta Kałuzińska, Elżbieta Płuciennik, and Andrzej K. Bednarek. 2021. "MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas" Cancers 13, no. 4: 891. https://doi.org/10.3390/cancers13040891
APA StyleHammouz, R. Y., Kołat, D., Kałuzińska, Ż., Płuciennik, E., & Bednarek, A. K. (2021). MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas. Cancers, 13(4), 891. https://doi.org/10.3390/cancers13040891









