Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
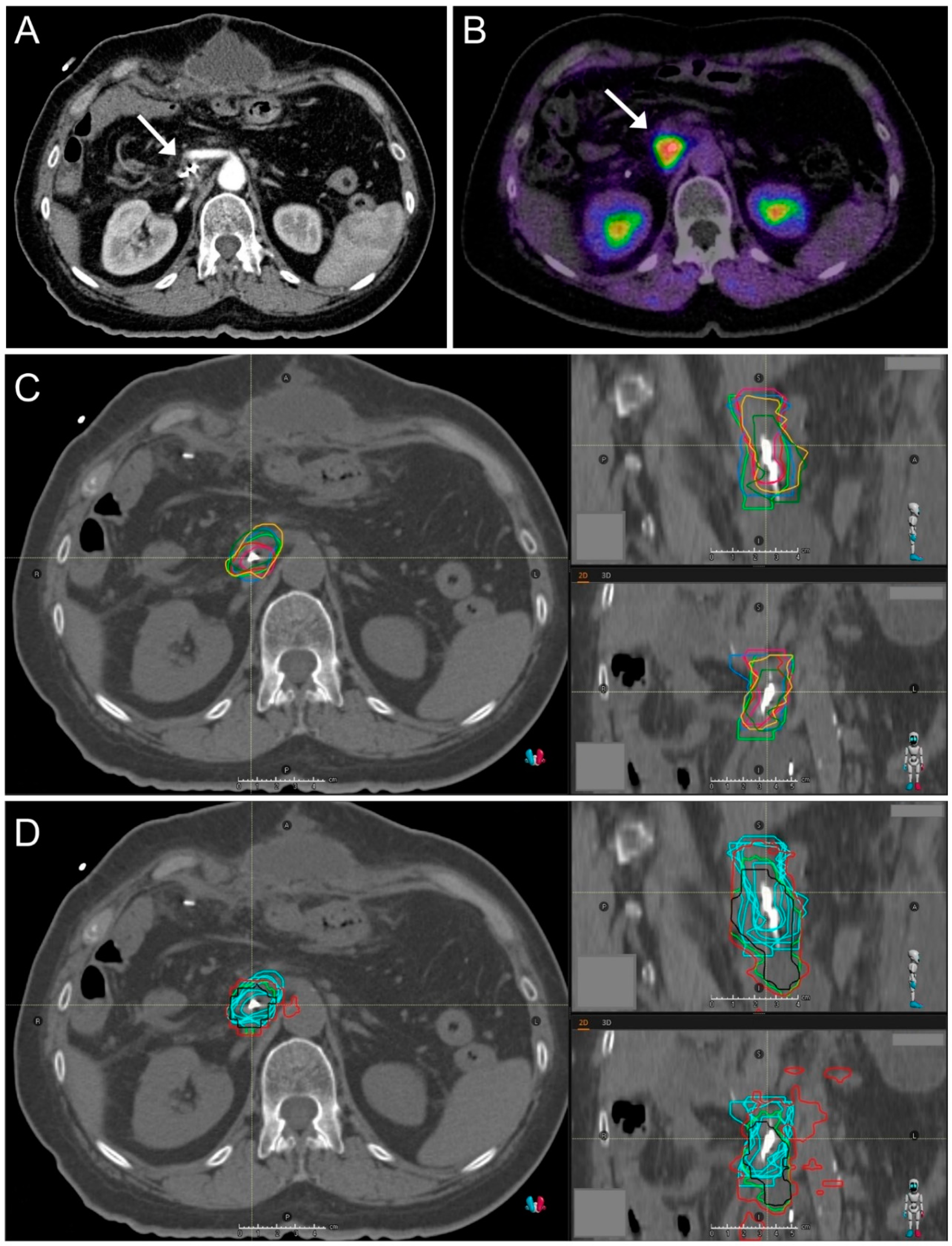
2.1. CT Imaging and Target Volume Definition by Radiation Oncologists
2.2. FAPI-PET/CT Imaging
2.3. Automated Target Volume Definition in FAPI-PET/CT-Scans
2.4. Image Registration
2.5. Interobserver Variability
2.6. Comparison of Volume Geometries
2.7. GTV Size Comparison
2.8. Statistics
3. Results
3.1. Patient and Tumor Characteristics
3.2. Target Volume Definition by Radiation Oncologists
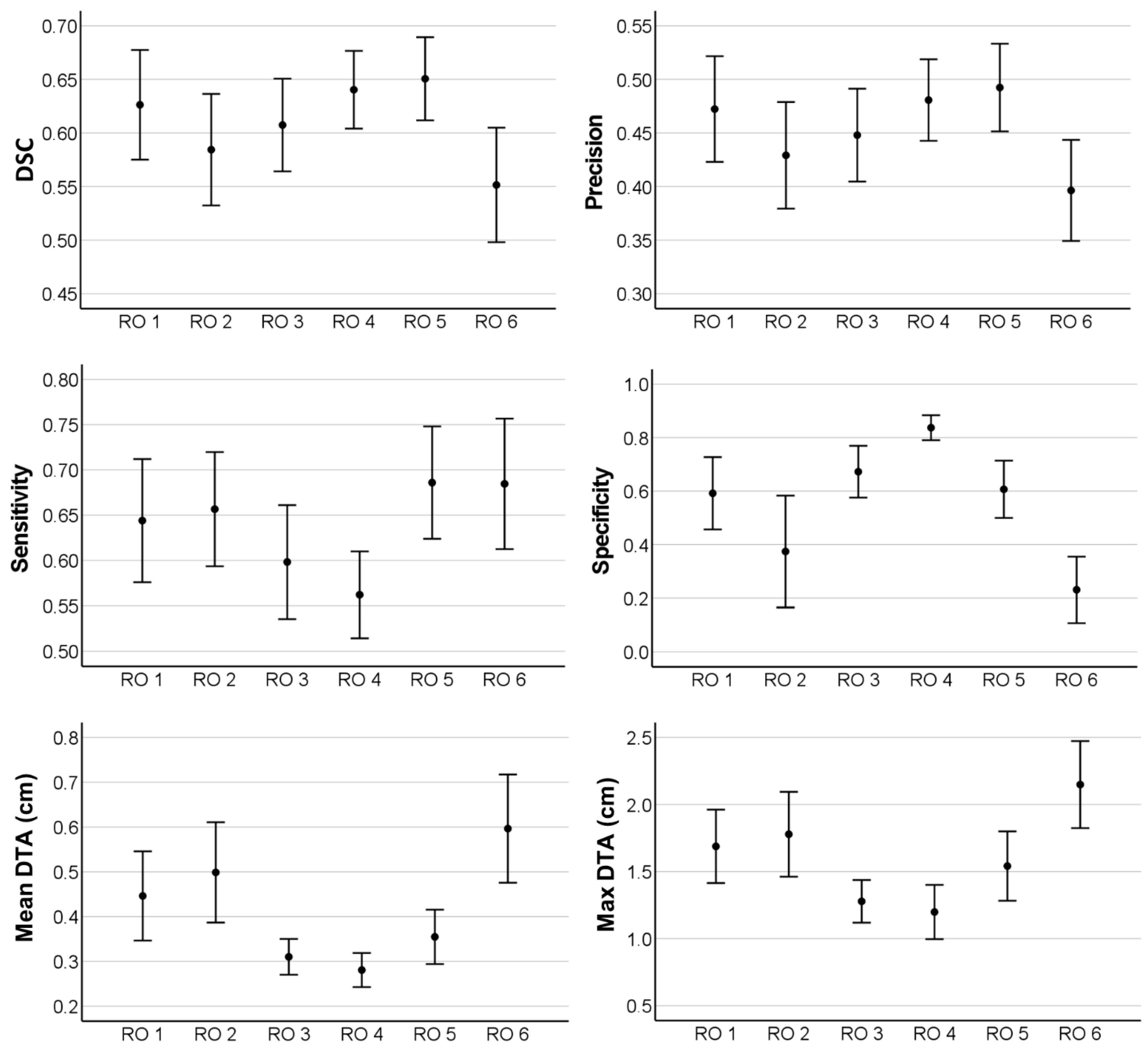
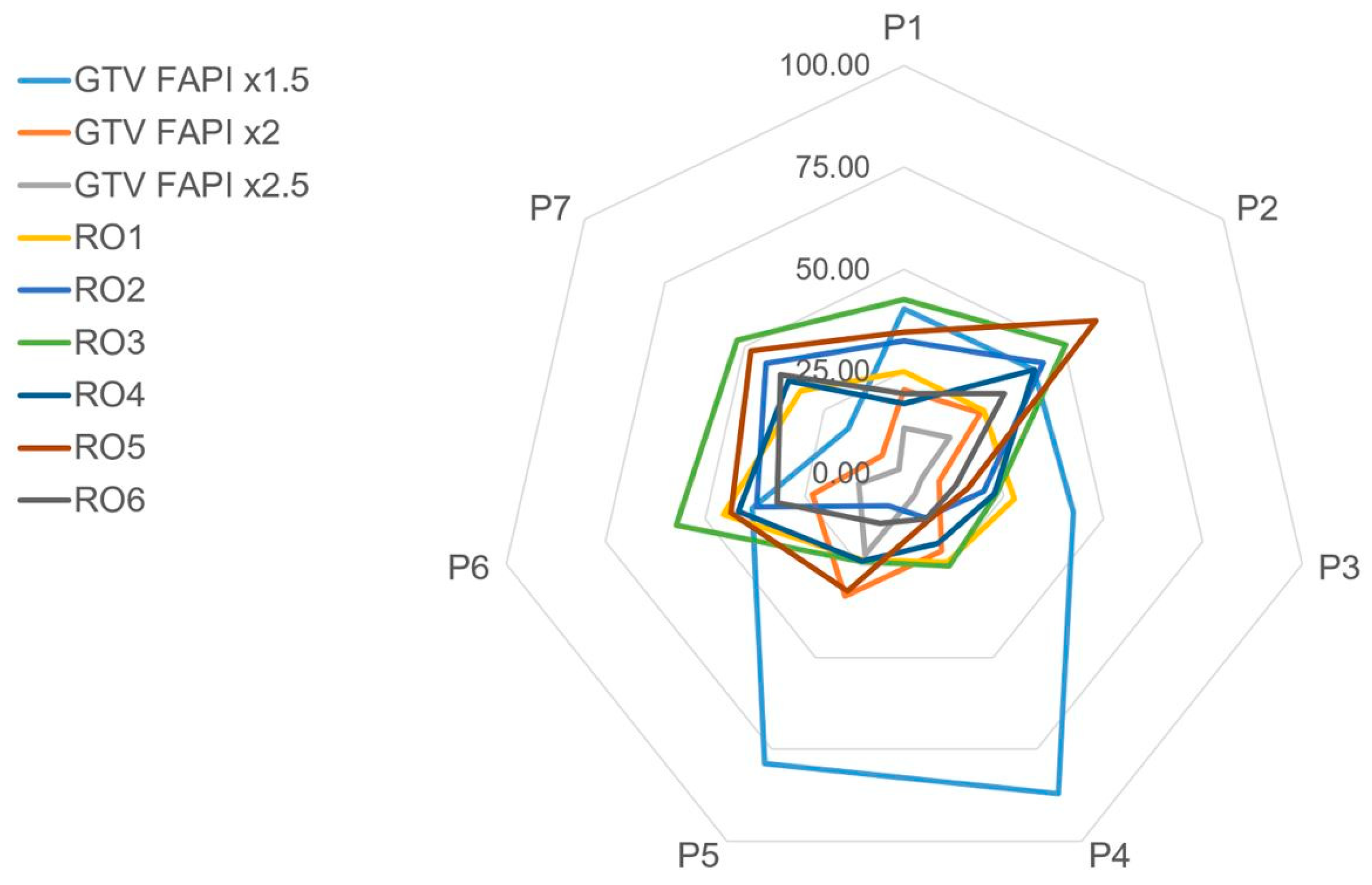
3.3. Interobserver Variability
3.4. GTV Size Comparison
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Iacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 Gene Status of the Primary Carcinoma Correlates With Patterns of Failure in Patients With Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, A.; Wu, L.; Si, X.; Li, Y. Second pancreatectomy for recurrent pancreatic ductal adenocarcinoma in the remnant pancreas: A pooled analysis. Pancreatology 2016, 16, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Itasaka, S.; Takaori, K.; Kawaguchi, Y.; Shibuya, K.; Yoshimura, M.; Matsuo, Y.; Mizowaki, T.; Uemoto, S.; Hiraoka, M. Radiotherapy for patients with isolated local recurrence of primary resected pancreatic cancer. Strahlenther. Onkol. 2014, 190, 485–490. [Google Scholar] [CrossRef]
- Habermehl, D.; Brecht, I.C.; Bergmann, F.; Welzel, T.; Rieken, S.; Werner, J.; Schirmacher, P.; Büchler, M.W.; Debus, J.; Combs, S. Chemoradiation in patients with isolated recurrent pancreatic cancer-therapeutical efficacy and probability of re-resection. Radiat. Oncol. 2013, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Kawashiro, S.; Yamada, S.; Isozaki, Y.; Nemoto, K.; Tsuji, H.; Kamada, T. Carbon-ion radiotherapy for locoregional recurrence after primary surgery for pancreatic cancer. Radiother. Oncol. 2018, 129, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Koong, A.C.; Christofferson, E.; Le, Q.T.; Goodman, K.A.; Ho, A.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Greco, R.; Norton, J.; et al. Phase Ii Study to Assess the Efficacy of Conventionally Fractionated Radiotherapy Followed by a Stereotactic Radiosurgery Boost in Patients with Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Balaj, C.; Ayav, A.; Oliver, A.; Jausset, F.; Sellal, C.; Claudon, M.; Laurent, V. CT imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom. Radiol. 2016, 41, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zins, M.; Matos, C.; Cassinotto, C. Pancreatic Adenocarcinoma Staging in the Era of Preoperative Chemotherapy and Radiation Therapy. Radiology 2018, 287, 374–390. [Google Scholar] [CrossRef]
- Daamen, L.A.; Groot, V.P.; Goense, L.; Wessels, F.J.; Rinkes, I.H.B.; Intven, M.P.; Van Santvoort, H.C.; Molenaar, I.Q. The diagnostic performance of CT versus FDG PET-CT for the detection of recurrent pancreatic cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2018, 106, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Bijan, B. PET/CT for Pancreatic Malignancy: Potential and Pitfalls. J. Nucl. Med. Technol. 2015, 43, 92–97. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Awaji, M.; Singh, R.K. Cancer-Associated Fibroblasts’ Functional Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of (68)Ga-Fapi-Pet/Ct Im-aging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2020, 62. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast Activation Protein Inhibitor (Fapi) Pet for Diagnostics and Ad-vanced Targeted Radiotherapy in Head and Neck Cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Dice, L.R. Measures of the Amount of Ecologic Association between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Udupa, J.K.; Leblanc, V.R.; Zhuge, Y.; Imielinska, C.; Schmidt, H.; Currie, L.M.; Hirsch, B.E.; Woodburn, J. A framework for evaluating image segmentation algorithms. Comput. Med. Imaging Graph. 2006, 30, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Caravatta, L.; Cellini, F.; Simoni, N.; Rosa, C.; Niespolo, R.M.; Lupattelli, M.; Picardi, V.; Macchia, G.; Sainato, A.; Mantello, G.; et al. Magnetic resonance imaging (MRI) compared with computed tomography (CT) for interobserver agreement of gross tumor volume delineation in pancreatic cancer: A multi-institutional contouring study on behalf of the AIRO group for gastrointestinal cancers. Acta Oncol. 2019, 58, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veen, J.; Gulyban, A.; Nuyts, S. Interobserver variability in delineation of target volumes in head and neck cancer. Radiother. Oncol. 2019, 137, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Fong, A.; McVicar, N.; Smith, S.; Giambattista, J.; Wells, D.; Kolbeck, C.; Giambattista, J.; Gondara, L.; Alexan-der, A. Comparing Deep Learning-Based Auto-Segmentation of Organs at Risk and Clinical Target Volumes to Expert In-ter-Observer Variability in Radiotherapy Planning. Radiother. Oncol. 2020, 144, 152–158. [Google Scholar] [CrossRef]
- Liang, S.; Tang, F.; Huang, X.; Yang, K.; Zhong, T.; Hu, R.; Liu, S.; Yuan, X.; Zhang, Y. Deep-Learning-Based Detection and Segmentation of Organs at Risk in Nasopharyngeal Carcinoma Computed Tomographic Images for Radiotherapy Planning. Eur. Radiol. 2019, 29, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [(68)Ga]Ga-Dota-Fapi-04 and [(18)F] Fdg Pet/Ct for the Diagnosis of Primary and Metastatic Lesions in Pa-tients with Various Types of Cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef]
- Norton, J.; Foster, D.; Chinta, M.; Titan, A.; Longaker, M. Pancreatic Cancer Associated Fibroblasts (CAF): Under-Explored Target for Pancreatic Cancer Treatment. Cancers 2020, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Piper, M.; Mueller, A.C.; Karam, S.D. The interplay between cancer associated fibroblasts and immune cells in the context of radiation therapy. Mol. Carcinog. 2020, 59, 754–765. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Ansems, M.; Span, P.N. The Tumor Microenvironment and Radiotherapy Response; a Central Role for Cancer-Associated Fibroblasts. Clin. Transl. Radiat. Oncol. 2020, 22, 90–97. [Google Scholar] [CrossRef] [PubMed]


| Demographics and Initial Tumor Stage and Treatment | n | (%) | |
|---|---|---|---|
| Sex | |||
| Male | 1 | (14) | |
| Female | 6 | (86) | |
| Age at performed planning CT imaging (median, range; in years) | 66 (55–77) | ||
| Localization of initial pancreatic cancer | |||
| Pancreatic head | 5 | (72) | |
| Pancreatic body | 1 | (14) | |
| Pancreatic tail | 1 | (14) | |
| Histology | |||
| Ductal adenocarcinoma | 7 | (100) | |
| Grading | |||
| G2 | 6 | ||
| G3 | 1 | ||
| Initial surgery | |||
| Whipple procedure | 3 | (43) | |
| Total pancreatectomy | 3 | (43) | |
| Distal pancreatectomy | 1 | (14) | |
| Resection status | |||
| R1/RX | 6 | (86) | |
| R0 | 1 | (14) | |
| Initial AJCC * stage | |||
| IA | 1 | (14) | |
| IIA | 1 | (14) | |
| IIB | 3 | (43) | |
| III | 2 | (29) | |
| Pre-radiotherapy AJCC * stage | |||
| III | 7 | (100) | |
| Pre-radiotherapy TNM † stage | |||
| rT4 cN0 cM0 | 6 | (86) | |
| rT4 cN1 cM0 | 1 | (14) | |
| Radiotherapy | |||
| Time from resection (median, range; in months) | 23 (8–50) | ||
| Technique | |||
| Carbon ions, active raster-scanning, 2 beams, supine position | 7 | (100) | |
| Prescribed dose | |||
| 48 Gy (RBE) in 12 fractions (2 weeks) | 7 | (100) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liermann, J.; Syed, M.; Ben-Josef, E.; Schubert, K.; Schlampp, I.; Sprengel, S.D.; Ristau, J.; Weykamp, F.; Röhrich, M.; Koerber, S.A.; et al. Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers 2021, 13, 796. https://doi.org/10.3390/cancers13040796
Liermann J, Syed M, Ben-Josef E, Schubert K, Schlampp I, Sprengel SD, Ristau J, Weykamp F, Röhrich M, Koerber SA, et al. Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers. 2021; 13(4):796. https://doi.org/10.3390/cancers13040796
Chicago/Turabian StyleLiermann, Jakob, Mustafa Syed, Edgar Ben-Josef, Kai Schubert, Ingmar Schlampp, Simon David Sprengel, Jonas Ristau, Fabian Weykamp, Manuel Röhrich, Stefan A. Koerber, and et al. 2021. "Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer" Cancers 13, no. 4: 796. https://doi.org/10.3390/cancers13040796
APA StyleLiermann, J., Syed, M., Ben-Josef, E., Schubert, K., Schlampp, I., Sprengel, S. D., Ristau, J., Weykamp, F., Röhrich, M., Koerber, S. A., Haberkorn, U., Debus, J., Herfarth, K., Giesel, F. L., & Naumann, P. (2021). Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers, 13(4), 796. https://doi.org/10.3390/cancers13040796






