Epithelial–Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma
Abstract
Simple Summary
Abstract
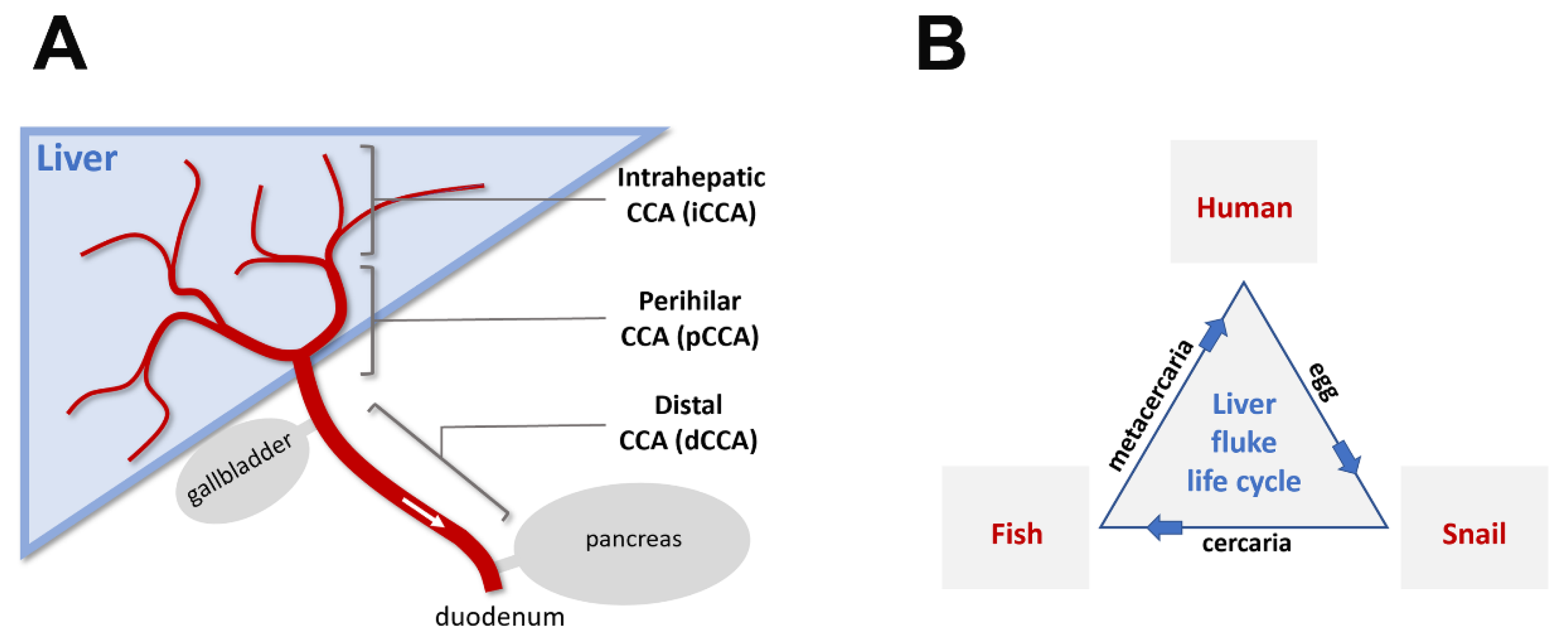
1. What Is Cholangiocarcinoma?
2. What Are Flukes
3. Flukes and Cholangiocarcinoma
4. Cholangiocarcinoma and EMT
5. Fluke-Associated Cholangiocarcinoma and EMT
6. Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, M.; Leftley, Z.; Barbera, T.A.; Sithithaworn, P.; Khuntikeo, N.; Loilome, W.; Yongvanit, P.; Cox, I.J.; Chamodol, N.; Syms, R.R.; et al. Cholangiocarcinoma: A guide for the nonspecialist. Int. J. Gen. Med. 2019, 12, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; Allen, G.E.; Ng, C.C.; Wong, B.H.; et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kaewpitoon, N.; Kaewpitoon, S.J.; Pengsaa, P. Opisthorchiasis in Thailand: Review and current status. World J. Gastroenterol. 2008, 14, 2297–2302. [Google Scholar] [CrossRef]
- Moller, H.; Heseltine, E.; Vainio, H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int. J. Cancer 1995, 60, 587–589. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Tang, Z.L.; Huang, Y.; Yu, X.B. Current status and perspectives of Clonorchis sinensis and clonorchiasis: Epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty 2016, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Kaewpitoon, N.; Kaewpitoon, S.J.; Pengsaa, P.; Sripa, B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J. Gastroenterol. 2008, 14, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jimenez, F.; Muinos, F.; Sentis, I.; Deu-Pons, J.; Reyes-Salazar, I.; Arnedo-Pac, C.; Mularoni, L.; Pich, O.; Bonet, J.; Kranas, H.; et al. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 2020, 20, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Geng, Z.H.; Chi, S.; Zhang, W.; Niu, X.F.; Lan, S.J.; Ma, L.; Yang, X.; Wang, L.J.; Ding, Y.Q.; et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011, 21, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.V.; Van Bulck, M.; Chantrill, L.; Arshi, M.; Sklyarova, T.; Herrmann, D.; Vennin, C.; Gallego-Ortega, D.; Mawson, A.; Giry-Laterriere, M.; et al. ROBO2 is a stroma suppressor gene in the pancreas and acts via TGF-beta signalling. Nat. Commun. 2018, 9, 5083. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Ozark, P.A.; Fantini, D.; Marshall, S.A.; Rendleman, E.J.; Cozzolino, K.A.; Louis, N.; He, X.; Morgan, M.A.; et al. Resetting the epigenetic balance of Polycomb and COMPASS function at enhancers for cancer therapy. Nat. Med. 2018, 24, 758–769. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Gao, X.; Guo, A.; Diao, Y.; Zhao, Y. RNF43 ubiquitinates and degrades phosphorylated E-cadherin by c-Src to facilitate epithelial-mesenchymal transition in lung adenocarcinoma. BMC Cancer 2019, 19, 670. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Ohm, J.E.; McGarvey, K.M.; Yu, X.; Cheng, L.; Schuebel, K.E.; Cope, L.; Mohammad, H.P.; Chen, W.; Daniel, V.C.; Yu, W.; et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat. Genet. 2007, 39, 237–242. [Google Scholar] [CrossRef]
- Schlesinger, Y.; Straussman, R.; Keshet, I.; Farkash, S.; Hecht, M.; Zimmerman, J.; Eden, E.; Yakhini, Z.; Ben-Shushan, E.; Reubinoff, B.E.; et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007, 39, 232–236. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Nakaya, Y.; Sheng, G. EMT in developmental morphogenesis. Cancer Lett. 2013, 341, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Vaquero, J.; Guedj, N.; Claperon, A.; Nguyen Ho-Bouldoires, T.H.; Paradis, V.; Fouassier, L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J. Hepatol. 2017, 66, 424–441. [Google Scholar] [CrossRef]
- Nitta, T.; Mitsuhashi, T.; Hatanaka, Y.; Miyamoto, M.; Oba, K.; Tsuchikawa, T.; Suzuki, Y.; Hatanaka, K.C.; Hirano, S.; Matsuno, Y. Prognostic significance of epithelial-mesenchymal transition-related markers in extrahepatic cholangiocarcinoma: Comprehensive immunohistochemical study using a tissue microarray. Br. J. Cancer 2014, 111, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Indramanee, S.; Sawanyawisuth, K.; Silsirivanit, A.; Dana, P.; Phoomak, C.; Kariya, R.; Klinhom-On, N.; Sorin, S.; Wongkham, C.; Okada, S.; et al. Terminal fucose mediates progression of human cholangiocarcinoma through EGF/EGFR activation and the Akt/Erk signaling pathway. Sci. Rep. 2019, 9, 17266. [Google Scholar] [CrossRef]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef]
- Techasen, A.; Namwat, N.; Loilome, W.; Duangkumpha, K.; Puapairoj, A.; Saya, H.; Yongvanit, P. Tumor necrosis factor-alpha modulates epithelial mesenchymal transition mediators ZEB2 and S100A4 to promote cholangiocarcinoma progression. J. Hepatobiliary Pancreat. Sci. 2014, 21, 703–711. [Google Scholar] [CrossRef]
- Kimawaha, P.; Jusakul, A.; Junsawang, P.; Loilome, W.; Khuntikeo, N.; Techasen, A. Circulating TGF-beta1 as the potential epithelial mesenchymal transition-biomarker for diagnosis of cholangiocarcinoma. J. Gastrointest. Oncol. 2020, 11, 304–318. [Google Scholar] [CrossRef]
- Myint, K.Z.; Kongpracha, P.; Rattanasinganchan, P.; Leelawat, K.; Moolthiya, P.; Chaiyabutr, K.; Tohtong, R. Gadd45beta silencing impaired viability and metastatic phenotypes in cholangiocarcinoma cells by modulating the EMT pathway. Oncol. Lett. 2018, 15, 3031–3041. [Google Scholar] [CrossRef]
- Saensa-Ard, S.; Leuangwattanawanit, S.; Senggunprai, L.; Namwat, N.; Kongpetch, S.; Chamgramol, Y.; Loilome, W.; Khansaard, W.; Jusakul, A.; Prawan, A.; et al. Establishment of cholangiocarcinoma cell lines from patients in the endemic area of liver fluke infection in Thailand. Tumour Biol. 2017, 39, 1010428317725925. [Google Scholar] [CrossRef] [PubMed]
- Arunsan, P.; Chaidee, A.; Cochran, C.J.; Mann, V.H.; Tanno, T.; Kumkhaek, C.; Smout, M.J.; Karinshak, S.E.; Rodpai, R.; Sotillo, J.; et al. Liver fluke granulin promotes extracellular vesicle-mediated crosstalk and cellular microenvironment conducive to cholangiocarcinoma. Neoplasia 2020, 22, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Laha, T.; Mulvenna, J.; Sripa, B.; Suttiprapa, S.; Jones, A.; Brindley, P.J.; Loukas, A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009, 5, e1000611. [Google Scholar] [CrossRef]
- Tanimoto, R.; Lu, K.G.; Xu, S.Q.; Buraschi, S.; Belfiore, A.; Iozzo, R.V.; Morrione, A. Mechanisms of Progranulin Action and Regulation in Genitourinary Cancers. Front. Endocrinol. 2016, 7, 100. [Google Scholar] [CrossRef]
- Daorueang, D.; Thuwajit, P.; Roitrakul, S.; Laha, T.; Kaewkes, S.; Endo, Y.; Thuwajit, C. Secreted Opisthorchis viverrini glutathione S-transferase regulates cell proliferation through AKT and ERK pathways in cholangiocarcinoma. Parasitol. Int. 2012, 61, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Arechavaleta-Velasco, F.; Perez-Juarez, C.E.; Gerton, G.L.; Diaz-Cueto, L. Progranulin and its biological effects in cancer. Med. Oncol. 2017, 34, 194. [Google Scholar] [CrossRef]
- Phoomak, C.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Seubwai, W.; Wongkham, C.; Silsirivanit, A.; Wongkham, S. Mechanistic insights of O-GlcNAcylation that promote progression of cholangiocarcinoma cells via nuclear translocation of NF-kappaB. Sci. Rep. 2016, 6, 27853. [Google Scholar] [CrossRef]
- Phoomak, C.; Vaeteewoottacharn, K.; Silsirivanit, A.; Saengboonmee, C.; Seubwai, W.; Sawanyawisuth, K.; Wongkham, C.; Wongkham, S. High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via O-GlcNAcylation. Sci. Rep. 2017, 7, 43842. [Google Scholar] [CrossRef] [PubMed]
- Phoomak, C.; Park, D.; Silsirivanit, A.; Sawanyawisuth, K.; Vaeteewoottacharn, K.; Detarya, M.; Wongkham, C.; Lebrilla, C.B.; Wongkham, S. O-GlcNAc-induced nuclear translocation of hnRNP-K is associated with progression and metastasis of cholangiocarcinoma. Mol. Oncol. 2019, 13, 338–357. [Google Scholar] [CrossRef]
- Pastushenko, I.; Mauri, F.; Song, Y.; de Cock, F.; Meeusen, B.; Swedlund, B.; Impens, F.; Van Haver, D.; Opitz, M.; Thery, M.; et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 2020, 589, 448–455. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Arunsan, P.; Ittiprasert, W.; Smout, M.J.; Cochran, C.J.; Mann, V.H.; Chaiyadet, S.; Karinshak, S.E.; Sripa, B.; Young, N.D.; Sotillo, J.; et al. Programmed knockout mutation of liver fluke granulin attenuates virulence of infection-induced hepatobiliary morbidity. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Rizvi, S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.W.; Spofford, E.M.; Price, C.; Wright, H.L.; Salao, K.; Suttiprapa, S.; Sripa, B. Opisthorchiasis-Induced Cholangiocarcinoma: How Innate Immunity May Cause Cancer. Adv. Parasitol. 2018, 101, 149–176. [Google Scholar] [CrossRef]
- Sripa, B.; Jumnainsong, A.; Tangkawattana, S.; Haswell, M.R. Immune Response to Opisthorchis viverrini Infection and Its Role in Pathology. Adv. Parasitol. 2018, 102, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Bowlus, C.L.; Yang, G.; Leung, P.S.C.; Gershwin, M.E. Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis (PSC): A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 134–149. [Google Scholar] [CrossRef]
- Burak, K.; Angulo, P.; Pasha, T.M.; Egan, K.; Petz, J.; Lindor, K.D. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2004, 99, 523–526. [Google Scholar] [CrossRef]


| Molecular/Cellular Evidence Linking EMT and CCA (Based on Banales et al. [1], 2020; Nitta et al [25]., 2014; Vaquero et al. [24], 2017) | Molecular/Cellular Evidence Linking EMT and Fluke-Associated CCA |
|---|---|
| TGFb1,2,3; TGFb receptor 1,2; SMAD4 | SMAD4; MLL3; ROBO2; RNF43 (Ong et al., 2012 [5]) |
| HMGB1 | FUT1 (Fucosyltransferase 1) (Indramanee et al., 2019 [26]) |
| BMPR; EGFR; CXCR; EPH; H4HR; CXCR4; NOTCH1; HH pathways | GALNT5 (GalNAc transferase 5) (Detarya et al., 2020 [27]) |
| TNFa | TNFa (Techasen et al., 2014 [28]) |
| IL-6; SOCS3 | Anti-parasitic drugs Xanthohumol and Praziquantel (Kimawaha et al., 2020 [29]) |
| microRNAs (miR-221, miR-200c, miR204, miR214, miR34a, miR21) | GADD45B (Myint et al., 2018 [30]) |
| Altered expression/distribution of E-cad, N-cad, b-catenin, Cytokeratin 19, vimentin, and S100A4 | Fluke-associated CCA cell lines with different E/M characteristics show different metastatic capacities (Saensa-Ard et al., 2017 [31]) |
| Altered expression/distribution of SNAI1, SNAI2, TWIST1, ZEB1, and ZEB2 | Fluke-secreted granulin and GST (Arunsan et al., 2020 [32]; Smout et al., 2009 [33]; Tanimoto et al. [34], 2016; Daorueang et al. [35], 2012; Arechavaleta-Velascro et al., 2017 [36]) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawanyawisuth, K.; Sashida, G.; Sheng, G. Epithelial–Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma. Cancers 2021, 13, 791. https://doi.org/10.3390/cancers13040791
Sawanyawisuth K, Sashida G, Sheng G. Epithelial–Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma. Cancers. 2021; 13(4):791. https://doi.org/10.3390/cancers13040791
Chicago/Turabian StyleSawanyawisuth, Kanlayanee, Goro Sashida, and Guojun Sheng. 2021. "Epithelial–Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma" Cancers 13, no. 4: 791. https://doi.org/10.3390/cancers13040791
APA StyleSawanyawisuth, K., Sashida, G., & Sheng, G. (2021). Epithelial–Mesenchymal Transition in Liver Fluke-Induced Cholangiocarcinoma. Cancers, 13(4), 791. https://doi.org/10.3390/cancers13040791







