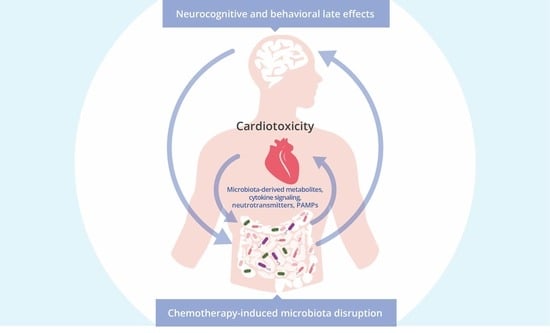
Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Treatment-Induced Cardiovascular Toxicity and Neurocognitive Disorders in Long-Term Cancer Survivors
3. Gut Microbiome, Chemotherapy, and Microbiota-Modulated Efficacy of Cancer Treatment
4. The Role of the Gut Microbiota in Brain Development, Cognitive Functioning, and Neurological Disorders
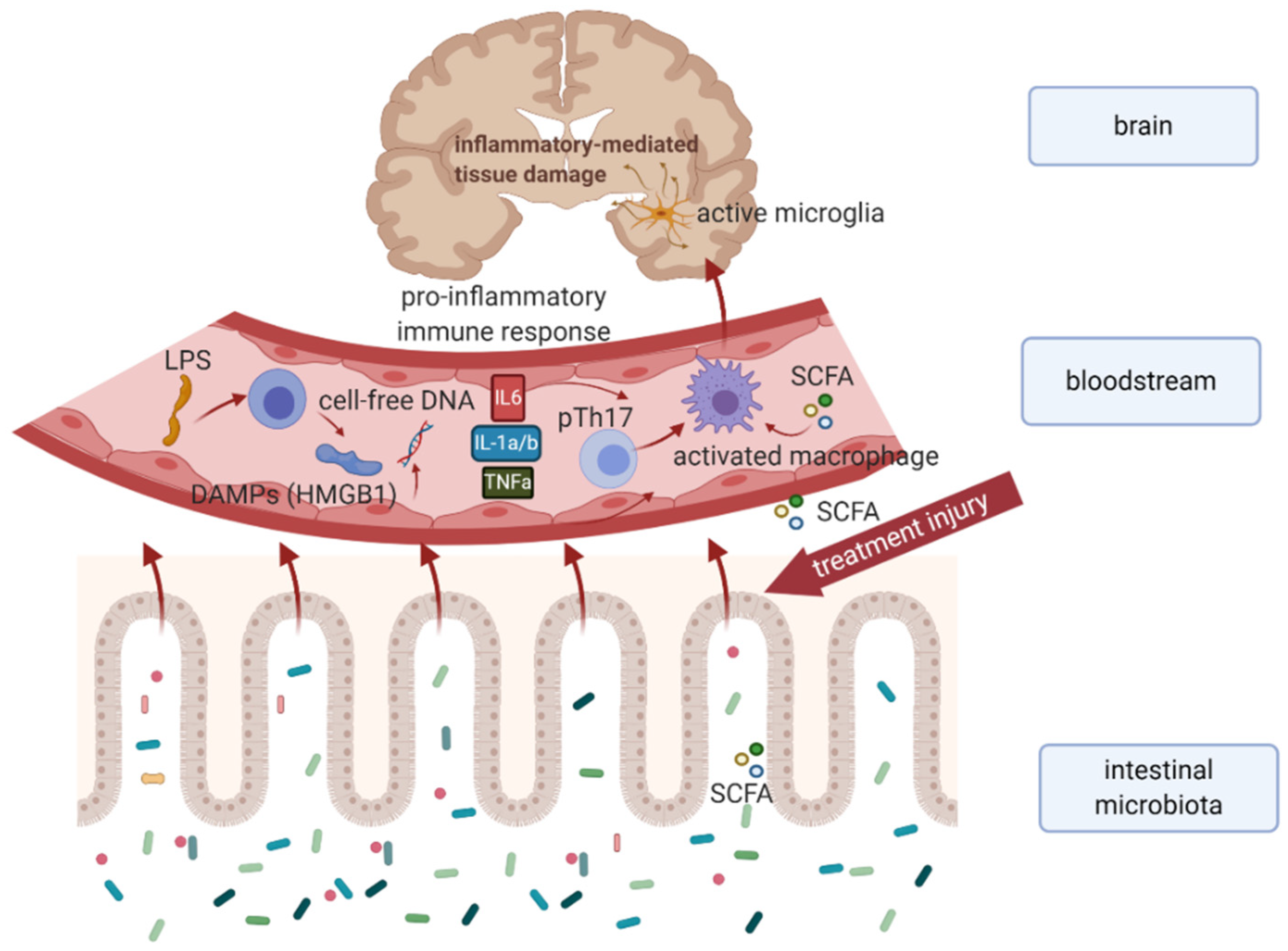
4.1. Underlying Mechanisms behind Microbiota–Gut–Brain Communication
4.2. Microbiota–Gut–Brain Axis and Neurological Disorders
5. Chemotherapy-Induced Dysbiosis Associated with Cognitive Impairment, Psychoneurological Symptoms, and Peripheral Neuropathy
5.1. Gut Microbiome and Chemotherapy-Related Cognitive Impairment
5.2. Gut Microbiome and Chemotherapy-Induced Peripheral Neuropathy
6. The Relationship between the Gut Microbiota and Cardiovascular Toxicity
7. Gut Microbiota Modulation as an Emerging Trend in Cancer Survivors
7.1. Neuro- and Cardioprotective Effect of Probiotics
7.2. Fecal Microbiota Transplantation and Improvements in Neurologic Functions and Cancer Treatment Efficacy
7.3. The Possible Impact of Diet and Physical Activity on the Gut Microbiome in Cancer Survivors
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Rowland, J.H.; Kent, E.E.; Forsythe, L.P.; Loge, J.H.; Hjorth, L.; Glaser, A.; Mattioli, V.; Fossa, S.D. Cancer survivorship research in Europe and the United States: Where have we been, where are we going, and what can we learn from each other? Cancer 2013, 119, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Reinius, M.A.; Hatcher, H.M.; Ajithkumar, T.V. Anticancer chemotherapy in teenagers and young adults: Managing long term side effects. BMJ 2016, 354, i4567. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Zaid, M.A.; Hanna, N.; El-Kouri, N.; Einhorn, L.H.; Albany, C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann. Oncol. 2017, 28, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared with Age-Matched Controls: An Analysis from a Nationwide, Multicenter, Prospective Longitudinal Study. J. Clin. Oncol. 2017, 35, 506–514. [Google Scholar] [CrossRef]
- Chovanec, M.; Vasilkova, L.; Setteyova, L.; Obertova, J.; Palacka, P.; Rejlekova, K.; Sycova-Mila, Z.; Kalavska, K.; Svetlovska, D.; Cingelova, S.; et al. Long-Term Cognitive Functioning in Testicular Germ-Cell Tumor Survivors. Oncologist 2018, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- McGinnis, G.J.; Friedman, D.; Young, K.H.; Torres, E.R.; Thomas, C.R., Jr.; Gough, M.J.; Raber, J. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget 2017, 8, 9155–9173. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.C.; Saykin, A.J. Neurocognitive dimensions of breast cancer and its treatment. Neuropsychopharmacology 2011, 36, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Saykin, A.J.; Ahles, T.A.; McDonald, B.C. Mechanisms of chemotherapy-induced cognitive disorders: Neuropsychological, pathophysiological, and neuroimaging perspectives. In Seminars in Clinical Neuropsychiatry; WB Saunders Company: Philadelphia, PA, USA, 2003; Volume 8, pp. 201–216. [Google Scholar]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Loman, B.R.; Jordan, K.R.; Haynes, B.; Bailey, M.T.; Pyter, L.M. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci. Rep. 2019, 9, 16490. [Google Scholar] [CrossRef]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Eurogast Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. The EUROGAST Study Group. Gut 1993, 34, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Zhao, L.; Zhang, X.; Nakatsu, G.; Han, J.; Xu, W.; Xiao, X.; Kwong, T.N.Y.; Tsoi, H.; Wu, W.K.K.; et al. Gavage of Fecal Samples from Patients with Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology 2017, 153, 1621–1633.e6. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Ciernikova, S.; Novisedlakova, M.; Cholujova, D.; Stevurkova, V.; Mego, M. The Emerging Role of Microbiota and Microbiome in Pancreatic Ductal Adenocarcinoma. Biomedicines 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetiere, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef]
- Bai, J.; Bruner, D.W.; Fedirko, V.; Beitler, J.J.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; Higgins, K.A.; et al. Gut Microbiome Associated with the Psychoneurological Symptom Cluster in Patients with Head and Neck Cancers. Cancers 2020, 12, 2531. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain Behav. Immun. 2020, 85, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support. Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.; Jessop, M.; Glaser, A.; Stark, D. Late effects in survivors of teenage and young adult cancer: Does age matter? Ann. Oncol. 2011, 22, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Dinh, P.C.; Fossa, S.D.; Travis, L.B. Testicular Cancer Survivorship. J. Natl. Compr. Cancer Netw. 2019, 17, 1557–1568. [Google Scholar] [CrossRef]
- Hodgson, D.C. Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: Optimizing treatment for individual patients. Clin. Adv. Hematol. Oncol. 2015, 13, 103–112. [Google Scholar]
- Shusterman, S.; Meadows, A.T. Long term survivors of childhood leukemia. Curr. Opin. Hematol. 2000, 7, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.R.; Swain, S.M. Cardiac toxicity in breast cancer survivors: Review of potential cardiac problems. Clin. Cancer Res. 2008, 14, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.A.; Vachani, C.C.; Hampshire, M.K.; Bach, C.; Arnold-Korzeniowski, K.; Metz, J.M.; Hill-Kayser, C.E. Survivorship after lower gastrointestinal cancer: Patient-reported outcomes and planning for care. Cancer 2017, 123, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H. Treatment of testicular cancer: A new and improved model. J. Clin. Oncol. 1990, 8, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J.; Hansen, M.K.; Bandak, M.; Kreiberg, M.B.; Skott, J.W.; Wagner, T.; Gundgaard Kier, M.G.; Holm, N.V.; Agerbaek, M.; Gupta, R.; et al. Cardiovascular Risk Factors and Disease after Male Germ Cell Cancer. J. Clin. Oncol. 2020, 38, 584–592. [Google Scholar] [CrossRef]
- Van den Belt-Dusebout, A.W.; Nuver, J.; de Wit, R.; Gietema, J.A.; ten Bokkel Huinink, W.W.; Rodrigus, P.T.; Schimmel, E.C.; Aleman, B.M.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J. Clin. Oncol. 2006, 24, 467–475. [Google Scholar] [CrossRef]
- Fung, C.; Fossa, S.D.; Milano, M.T.; Sahasrabudhe, D.M.; Peterson, D.R.; Travis, L.B. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. J. Clin. Oncol. 2015, 33, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.A.; Norman, A.; Shahidi, M.; Horwich, A.; Coward, D.; Nicholls, J.; Dearnaley, D.P. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J. Clin. Oncol. 2003, 21, 1513–1523. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Wethal, T.; Aass, N.; Dahl, O.; Klepp, O.; Langberg, C.W.; Wilsgaard, T.; Bremnes, R.M.; Fossa, S.D. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J. Clin. Oncol. 2010, 28, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Host, H.; Brennhovd, I.O.; Loeb, M. Postoperative radiotherapy in breast cancer—Long-term results from the Oslo study. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 727–732. [Google Scholar] [CrossRef]
- Haybittle, J.L.; Brinkley, D.; Houghton, J.; A’Hern, R.P.; Baum, M. Postoperative radiotherapy and late mortality: Evidence from the Cancer Research Campaign trial for early breast cancer. BMJ 1989, 298, 1611–1614. [Google Scholar] [CrossRef]
- Rutqvist, L.E.; Lax, I.; Fornander, T.; Johansson, H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 887–896. [Google Scholar] [CrossRef]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef]
- Zambetti, M.; Moliterni, A.; Materazzo, C.; Stefanelli, M.; Cipriani, S.; Valagussa, P.; Bonadonna, G.; Gianni, L. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J. Clin. Oncol. 2001, 19, 37–43. [Google Scholar] [CrossRef]
- Doyle, J.J.; Neugut, A.I.; Jacobson, J.S.; Grann, V.R.; Hershman, D.L. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J. Clin. Oncol. 2005, 23, 8597–8605. [Google Scholar] [CrossRef]
- Pinder, M.C.; Duan, Z.; Goodwin, J.S.; Hortobagyi, G.N.; Giordano, S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 2007, 25, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Mackenzie, J.; Tasca, G.A.; Scherling, C.; Smith, A. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. J. Int. Neuropsychol. Soc. 2014, 20, 370–379. [Google Scholar] [CrossRef]
- Wouters, H.; Baars, J.W.; Schagen, S.B. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 2016, 55, 1121–1125. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Bekele, T.; Renton, C.; Dodd, A.; Zhang, H.; Beale, P.; Clarke, S.; et al. Cognitive Function in Patients with Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. J. Clin. Oncol. 2015, 33, 4085–4092. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Kieffer, J.M.; Agelink van Rentergem, J.; Veltman, D.J.; Fruijtier, A.; Oldenburg, H.S.; Boven, E.; van der Meij, S.; Lustig, V.; et al. Cognitive Impairment in a Subset of Breast Cancer Patients after Systemic Therapy—Results from a Longitudinal Study. J. Pain Symptom Manag. 2016, 52, 560–569.e1. [Google Scholar] [CrossRef]
- Quesnel, C.; Savard, J.; Ivers, H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res. Treat. 2009, 116, 113–123. [Google Scholar] [CrossRef]
- Stouten-Kemperman, M.M.; de Ruiter, M.B.; Caan, M.W.; Boogerd, W.; Kerst, M.J.; Reneman, L.; Schagen, S.B. Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum. Brain Mapp. 2015, 36, 4638–4647. [Google Scholar] [CrossRef]
- Amidi, A.; Wu, L.M.; Pedersen, A.D.; Mehlsen, M.; Pedersen, C.G.; Rossen, P.; Agerbaek, M.; Zachariae, R. Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Support. Care Cancer 2015, 23, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Brydoy, M.; Oldenburg, J.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Hauge, E.R.; Dahl, O.; Fossa, S.D. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J. Natl. Cancer Inst. 2009, 101, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Sprauten, M.; Darrah, T.H.; Peterson, D.R.; Campbell, M.E.; Hannigan, R.E.; Cvancarova, M.; Beard, C.; Haugnes, H.S.; Fossa, S.D.; Oldenburg, J.; et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. J. Clin. Oncol. 2012, 30, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, J.L.; Barbachano, Y.; Norman, A.R.; Dearnaley, D.P.; Horwich, A.; Huddart, R.A. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 2010, 116, 2322–2331. [Google Scholar] [CrossRef]
- Mykletun, A.; Dahl, A.A.; Haaland, C.F.; Bremnes, R.; Dahl, O.; Klepp, O.; Wist, E.; Fossa, S.D. Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J. Clin. Oncol. 2005, 23, 3061–3068. [Google Scholar] [CrossRef]
- Chovanec, M.; Galikova, D.; Vasilkova, L.; Angelis, V.D.; Rejlekova, K.; Obertova, J.; Sycova-Mila, Z.; Palacka, P.; Kalavska, K.; Svetlovska, D.; et al. Chemotherapy-induced peripheral neuropathy (CIPN) as a predictor of decreased quality of life and cognitive impairment in testicular germ cell tumor survivors. J. Clin. Oncol. 2020, 38, e17063. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; Giglio, M.G.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-Ongkham, O.; Sarwoko, M.A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in gut bacterial community of school-age children in Asia. Sci. Rep. 2015, 5, 8397. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Zwielehner, J.; Lassl, C.; Hippe, B.; Pointner, A.; Switzeny, O.J.; Remely, M.; Kitzweger, E.; Ruckser, R.; Haslberger, A.G. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 2011, 6, e28654. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tobin, P.; Clarke, S.J. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005, 6, 93–102. [Google Scholar] [CrossRef]
- Wardill, H.R.; Bowen, J.M. Chemotherapy-induced mucosal barrier dysfunction: An updated review on the role of intestinal tight junctions. Curr. Opin. Support. Palliat. Care 2013, 7, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Goubet, A.G.; Daillere, R.; Routy, B.; Derosa, L.; Roberti, P.M.; Zitvogel, L. The impact of the intestinal microbiota in therapeutic responses against cancer. C. R. Biol. 2018, 341, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, W.; Liu, H.; Duan, J.; Zhang, Y.; Liu, M.; Li, H.; Hou, Z.; Wu, K.K. Effect of high-dose methotrexate chemotherapy on intestinal Bifidobacteria, Lactobacillus and Escherichia coli in children with acute lymphoblastic leukemia. Exp. Biol. Med. (Maywood) 2012, 237, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Chiba, A.; Bawaneh, A.; Velazquez, C.; Clear, K.Y.J.; Wilson, A.S.; Howard-McNatt, M.; Levine, E.A.; Levi-Polyachenko, N.; Yates-Alston, S.A.; Diggle, S.P.; et al. Neoadjuvant Chemotherapy Shifts Breast Tumor Microbiota Populations to Regulate Drug Responsiveness and the Development of Metastasis. Mol. Cancer Res. 2020, 18, 130–139. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Rivera, D.R.; Ganz, P.A.; Weyrich, M.S.; Bandos, H.; Melnikow, J. Chemotherapy-Associated Peripheral Neuropathy in Patients with Early-Stage Breast Cancer: A Systematic Review. J. Natl. Cancer Inst. 2018, 110. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Appelbaum, F.R.; Ferrans, V.J.; Deisseroth, A.; Ziegler, J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch. Intern. Med. 1981, 141, 758–763. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Cadeddu, C.; Di Lisi, D.; Femmino, S.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Penna, C.; Pepe, A.; et al. From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview. Antioxid. Redox Signal. 2019, 30, 2110–2153. [Google Scholar] [CrossRef] [PubMed]
- Mladenka, P.; Applova, L.; Patocka, J.; Costa, V.M.; Remiao, F.; Pourova, J.; Mladenka, A.; Karlickova, J.; Jahodar, L.; Voprsalova, M.; et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018, 38, 1332–1403. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.; Dockter, T.; Wolfe, E.; Le-Rademacher, J.; Wagner-Johnston, N.; Einhorn, L.; Lafky, J.M.; Smith, E.; Pachman, D.; Staff, N.; et al. Cisplatin-associated neuropathy characteristics compared with those associated with other neurotoxic chemotherapy agents (Alliance A151724). Support. Care Cancer 2021, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Saxena, S.; Williams, A.; Damurjian, C.; Auricchio, N.; Aluotto, S.; Tynan, H.; Demain, A.L. Antimicrobial spectrum of the antitumor agent, cisplatin. J. Antibiot. 2010, 63, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Gahler, A.; Hitz, F.; Hess, U.; Cerny, T. Acute pericarditis and pleural effusion complicating cytarabine chemotherapy. Onkologie 2003, 26, 348–350. [Google Scholar] [CrossRef]
- Chow, E.J.; Anderson, L.; Baker, K.S.; Bhatia, S.; Guilcher, G.M.; Huang, J.T.; Pelletier, W.; Perkins, J.L.; Rivard, L.S.; Schechter, T.; et al. Late Effects Surveillance Recommendations among Survivors of Childhood Hematopoietic Cell Transplantation: A Children’s Oncology Group Report. Biol. Blood Marrow Transplant. 2016, 22, 782–795. [Google Scholar] [CrossRef]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Ganzle, M.G. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef]
- Van der Plas, E.; Nieman, B.J.; Butcher, D.T.; Hitzler, J.K.; Weksberg, R.; Ito, S.; Schachar, R. Neurocognitive Late Effects of Chemotherapy in Survivors of Acute Lymphoblastic Leukemia: Focus on Methotrexate. J. Can. Acad. Child Adolesc. Psychiatry 2015, 24, 25–32. [Google Scholar]
- Brown, J.M.; Hazen, S.L. Targeting of microbe-derived metabolites to improve human health: The next frontier for drug discovery. J. Biol. Chem. 2017, 292, 8560–8568. [Google Scholar] [CrossRef] [PubMed]
- Bajic, J.E.; Johnston, I.N.; Howarth, G.S.; Hutchinson, M.R. From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation. Front. Behav. Neurosci. 2018, 12, 104. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2018, 172, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 1288–1295. [Google Scholar] [CrossRef]
- Fung, T.C. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Chu, A.L.; Stochl, J.; Lewis, G.; Zammit, S.; Jones, P.B.; Khandaker, G.M. Longitudinal association between inflammatory markers and specific symptoms of depression in a prospective birth cohort. Brain Behav. Immun. 2019, 76, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Zammit, S.; Burgess, S.; Lewis, G.; Jones, P.B. Association between a functional interleukin 6 receptor genetic variant and risk of depression and psychosis in a population-based birth cohort. Brain Behav. Immun. 2018, 69, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Frohlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Ceylani, T.; Jakubowska-Dogru, E.; Gurbanov, R.; Teker, H.T.; Gozen, A.G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 2018, 4, e00644. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlokken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 2018, 29, 417–425. [Google Scholar] [CrossRef]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Subramaniam, C.B.; Bowen, J.M.; Gladman, M.A.; Lustberg, M.B.; Mayo, S.J.; Wardill, H.R. The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci. Biobehav. Rev. 2020, 116, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer 2018, 124, 3990–3999. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D.; et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci. Rep. 2019, 9, 20324. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Abrey, L.E. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J. Neurooncol. 2012, 108, 285–290. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Chen, J.; Buchanan, J.B.; Sparkman, N.L.; Godbout, J.P.; Freund, G.G.; Johnson, R.W. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav. Immun. 2008, 22, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jahan, T.; Aouizerat, B.E.; Dodd, M.J.; Cooper, B.A.; Paul, S.M.; West, C.; Lee, K.; Swift, P.S.; Wara, W.; et al. Differences in symptom clusters identified using occurrence rates versus symptom severity ratings in patients at the end of radiation therapy. Cancer Nurs. 2009, 32, 429–436. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.W.; Baydoun, M.; Piedalue, K.A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef] [PubMed]
- Bai, J. Exploring the Microbiome-Gut-Brain Axis in Psychoneurological Symptoms for Children with Solid Tumors. Available online: https://grantome.com/grant/NIH/K99-NR017897-01 (accessed on 29 January 2021).
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, Z.; Liang, Y.; Cheng, X.; Li, Y.; Teng, W.; Zhao, M.; Liu, C.; Guan, M.; Zhao, C. Targeting strategies for chemotherapy-induced peripheral neuropathy: Does gut microbiota play a role? Crit. Rev. Microbiol. 2019, 45, 369–393. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E.; et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA 2020, 117, 26482–26493. [Google Scholar] [CrossRef]
- Varricchi, G.; Ameri, P.; Cadeddu, C.; Ghigo, A.; Madonna, R.; Marone, G.; Mercurio, V.; Monte, I.; Novo, G.; Parrella, P.; et al. Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 2018, 9, 167. [Google Scholar] [CrossRef]
- Amgalan, D.; Garner, T.P.; Pekson, R.; Jia, X.F.; Yanamandala, M.; Paulino, V.; Liang, F.G.; Corbalan, J.J.; Lee, J.; Chen, Y.; et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 2020, 1, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef]
- Zhang, W.; St Clair, D.; Butterfield, A.; Vore, M. Loss of Mrp1 Potentiates Doxorubicin-Induced Cytotoxicity in Neonatal Mouse Cardiomyocytes and Cardiac Fibroblasts. Toxicol. Sci. 2016, 151, 44–56. [Google Scholar] [CrossRef]
- Narikawa, M.; Umemura, M.; Tanaka, R.; Hikichi, M.; Nagasako, A.; Fujita, T.; Yokoyama, U.; Ishigami, T.; Kimura, K.; Tamura, K.; et al. Doxorubicin induces trans-differentiation and MMP1 expression in cardiac fibroblasts via cell death-independent pathways. PLoS ONE 2019, 14, e0221940. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kasahara, K.; Emoto, T.; Matsumoto, T.; Mizoguchi, T.; Kitano, N.; Sasaki, N.; Hirata, K. Intestinal Immunity and Gut Microbiota as Therapeutic Targets for Preventing Atherosclerotic Cardiovascular Diseases. Circ. J. 2015, 79, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Schiattarella, G.G.; Crotti, L.; Torchio, M.; Bertero, E.; Rodolico, D.; Forte, M.; Di Mauro, V.; Paolillo, R.; Chimenti, C.; et al. Novel Basic Science Insights to Improve the Management of Heart Failure: Review of the Working Group on Cellular and Molecular Biology of the Heart of the Italian Society of Cardiology. Int. J. Mol. Sci. 2020, 21, 1192. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Sannino, A.; Esposito, G.; Perrino, C. Diagnostics and therapeutic implications of gut microbiota alterations in cardiometabolic diseases. Trends Cardiovasc. Med. 2019, 29, 141–147. [Google Scholar] [CrossRef]
- Zollner, J.; Howe, L.G.; Edey, L.F.; O’Dea, K.P.; Takata, M.; Gordon, F.; Leiper, J.; Johnson, M.R. The response of the innate immune and cardiovascular systems to LPS in pregnant and nonpregnant mice. Biol. Reprod. 2017, 97, 258–272. [Google Scholar] [CrossRef]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Peri, F.; Granucci, F.; Weiss, J. Endotoxin, TLR4 signaling and beyond. Mol. Immunol. 2015, 63, 125–126. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-Induced Systemic Inflammation Is Driven by Upregulation of Toll-Like Receptor TLR4 and Endotoxin Leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Kaczmarek, A.; Krysko, O.; Heyndrickx, L.; Woznicki, J.; Bogaert, P.; Cauwels, A.; Takahashi, N.; Magez, S.; Bachert, C.; et al. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 2011, 18, 1316–1325. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Tang, H.; Qiu, M.; Li, C.; Duan, C.; Wang, C.; Yang, J.; Zhou, X. Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice. Front. Pharmacol. 2019, 10, 107. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.Y.; Hu, X.M.; Zhang, Y.; Zhang, S.Y. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin. Med. J. 2019, 132, 1843–1855. [Google Scholar] [CrossRef]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut microbiota and cardiovascular disease: Opportunities and challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef]
- Joly, F.; Lange, M.; Dos Santos, M.; Vaz-Luis, I.; Di Meglio, A. Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors. Cancers 2019, 11, 1896. [Google Scholar] [CrossRef]
- Ciernikova, S.; Mego, M.; Semanova, M.; Wachsmannova, L.; Adamcikova, Z.; Stevurkova, V.; Drgona, L.; Zajac, V. Probiotic Survey in Cancer Patients Treated in the Outpatient Department in a Comprehensive Cancer Center. Integr. Cancer Ther. 2017, 16, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Ebringer, L.; Drgona, L.; Mardiak, J.; Trupl, J.; Greksak, R.; Nemova, I.; Oravcova, E.; Zajac, V.; Koza, I. Prevention of febrile neutropenia in cancer patients by probiotic strain Enterococcus faecium M-74. Pilot study phase I. Neoplasma 2005, 52, 159–164. [Google Scholar] [PubMed]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Mego, M.; Hainova, K.; Adamcikova, Z.; Stevurkova, V.; Zajac, V. Modification of microflora imbalance: Future directions for prevention and treatment of colorectal cancer? Neoplasma 2015, 62, 345–352. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Park, J.H.; Lee, D.C.; Lee, J.W.; Kim, N.K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, X.; Chen, G.; Li, L.; Le, Y.; Xie, Z.; Ouyang, W.; Tong, J. Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: A randomised double-blind and placebo-controlled trial. Clin. Nutr. 2021, 40, 64–71. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Tang, S.; Huang, H.; Zhao, X.; Ning, Z.; Fu, X.; Zhang, C. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia Pac. J. Clin. Oncol. 2016, 12, e92–e96. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2020. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Distrutti, E.; O’Reilly, J.A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar] [CrossRef]
- Divyashri, G.; Krishna, G.; Prapulla, S.G. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: In vitro and in vivo evidence. J. Med. Microbiol. 2015, 64, 1527–1540. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.K.; Liu, Y.W.; Kuo, P.H.; Chung, Y.E.; Lu, M.L.; Chen, C.H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef]
- Companys, J.; Pedret, A.; Valls, R.M.; Sola, R.; Pascual, V. Fermented dairy foods rich in probiotics and cardiometabolic risk factors: A narrative review from prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Companys, J.; Pla-Paga, L.; Calderon-Perez, L.; Llaurado, E.; Sola, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P.; Hsieh, Y.M.; Kuo, W.W.; Lin, Y.M.; Yeh, Y.L.; Lin, C.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Tsai, C.C. Probiotic-fermented purple sweet potato yogurt activates compensatory IGFIR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int. J. Mol. Med. 2013, 32, 1319–1328. [Google Scholar] [CrossRef]
- Tang, T.W.H.; Chen, H.C.; Chen, C.Y.; Yen, C.Y.T.; Lin, C.J.; Prajnamitra, R.P.; Chen, L.L.; Ruan, S.C.; Lin, J.H.; Lin, P.J.; et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef]
- Sadeghzadeh, J.; Vakili, A.; Sameni, H.R.; Shadnoush, M.; Bandegi, A.R.; Zahedi Khorasani, M. The Effect of Oral Consumption of Probiotics in Prevention of Heart Injury in a Rat Myocardial Infarction Model: A Histopathological, Hemodynamic and Biochemical Evaluation. Iran. Biomed. J. 2017, 21, 174–181. [Google Scholar] [CrossRef]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simao, A.N.; Alfieri, D.F.; Lozovoy, M.A.; Mari, N.L.; de Souza, C.H.; Dichi, I.; Costa, G.N. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Chang, C.W.; Lee, H.C.; Li, L.H.; Chiang Chiau, J.S.; Wang, T.E.; Chuang, W.H.; Chen, M.J.; Wang, H.Y.; Shih, S.C.; Liu, C.Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Kaito, S.; Toya, T.; Yoshifuji, K.; Kurosawa, S.; Inamoto, K.; Takeshita, K.; Suda, W.; Kakihana, K.; Honda, K.; Hattori, M.; et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018, 2, 3097–3101. [Google Scholar] [CrossRef]
- Qi, X.; Li, X.; Zhao, Y.; Wu, X.; Chen, F.; Ma, X.; Zhang, F.; Wu, D. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease with Fecal Microbiota Transplantation: A Pilot Study. Front. Immunol. 2018, 9, 2195. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Hefazi, M.; Patnaik, M.M.; Hogan, W.J.; Litzow, M.R.; Pardi, D.S.; Khanna, S. Safety and Efficacy of Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection in Patients with Cancer Treated with Cytotoxic Chemotherapy: A Single-Institution Retrospective Case Series. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2017; Volume 92, pp. 1617–1624. [Google Scholar] [CrossRef]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T.; et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682–1688. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; McQuade, J.L. Nutrition and Cancer in the Microbiome Era. Trends Cancer 2019, 5, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed]
- George, M.A.; Lustberg, M.B.; Orchard, T.S. Psychoneurological symptom cluster in breast cancer: The role of inflammation and diet. Breast Cancer Res. Treat. 2020, 184, 1–9. [Google Scholar] [CrossRef]
- George, S.M.; Neuhouser, M.L.; Mayne, S.T.; Irwin, M.L.; Albanes, D.; Gail, M.H.; Alfano, C.M.; Bernstein, L.; McTiernan, A.; Reedy, J.; et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Prev. Biomark. 2010, 19, 2220–2228. [Google Scholar] [CrossRef]
- Wayne, S.J.; Baumgartner, K.; Baumgartner, R.N.; Bernstein, L.; Bowen, D.; Ballard-Barbash, R. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res. Treat. 2006, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, Y.; Bao, P.; Cai, H.; Hong, Z.; Ding, D.; Jackson, J.; Shu, X.O.; Dai, Q. Associations of dietary intake and supplement use with post-therapy cognitive recovery in breast cancer survivors. Breast Cancer Res. Treat. 2018, 171, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar] [PubMed]
- Villeger, R.; Lopes, A.; Carrier, G.; Veziant, J.; Billard, E.; Barnich, N.; Gagniere, J.; Vazeille, E.; Bonnet, M. Intestinal Microbiota: A Novel Target to Improve Anti-Tumor Treatment? Int. J. Mol. Sci. 2019, 20, 4584. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.B.; Desnoyers, A.; Langelier, D.M.; Amir, E. The Effect of Exercise on Quality of Life, Fatigue, Physical Function, and Safety in Advanced Solid Tumor Cancers: A Meta-Analysis of Randomized Control Trials. J. Pain Symptom Manag. 2019, 58, 899–908. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef]
- Newton, R.U.; Christophersen, C.T.; Fairman, C.M.; Hart, N.H.; Taaffe, D.R.; Broadhurst, D.; Devine, A.; Chee, R.; Tang, C.I.; Spry, N.; et al. Does exercise impact gut microbiota composition in men receiving androgen deprivation therapy for prostate cancer? A single-blinded, two-armed, randomised controlled trial. BMJ Open 2019, 9, e024872. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Disease | Purpose | Patients (n) | Intervention | Study Status |
|---|---|---|---|---|---|---|
| NCT03760653 | A prospective, randomized double-blind study | Breast cancer survivors | To determine the effects of physical exercise together with the supplementation of a probiotic on the gut microbiota balance, the gut immune system, and quality of life (intended as functional and muscular capacity, physical qualities, and emotional state) in breast cancer survivors. | 30 | Physical exercise and probiotic group vs. probiotic group vs. placebo | Suspended (the project abandonment by the research who recruited the patients) |
| NCT04088708 | A prospective, randomized, single-blind study | Breast cancer survivors | To determine exercise effects on the number, distribution, and types of bacteria in the gut of breast cancer survivors. | 126 | Aerobic exercise training vs. attention control | Ongoing |
| NCT02843425 | A prospective, randomized, open-label, cross-over study | Colorectal cancer survivors | To determine the effect of pre-cooked beans on the levels of healthy bacteria in the digestive system and reduction in obesity effect on cancer risk. | 80 | Regular diet + beans, then regular diet—beans vs. regular diet—beans, then regular diet + beans | Active, not recruiting |
| NCT04097353 | A prospective, randomized, open-label study | Pediatric cancer survivors | To examine the efficacy of Harvesting Hope for Kids (HH4K), a biobehavioral intervention delivered in the context of a university-based, cancer survivor garden to increase produce intake and physical activity in survivors and caregivers including changes in microbiome composition. | 75 | Harvesting Hope for Kids (HH4K) vs. Surviving Strong for Kids (SS4K) | Enrolling by invitation |
| NCT03781778 | A prospective, randomized, double-blind study | Stage I-III colorectal cancer survivors | To test the effect of the consumption of foods made with resistant starch compared to foods made with corn starch on biomarkers that may be related to colorectal cancer progression in stage I-III colorectal cancer survivors. | NA | Resistant starch foods vs. foods with regular corn starch | Terminated (funding expiration) |
| NCT04499950 | A non-randomized, single-arm, phase II study | Breast cancer survivors | To determine the effects of pharmacotherapy and a remote behavioral weight loss intervention on weight loss in breast cancer survivors who are overweight or obese and the impact of successful weight loss on serum biomarkers and the gut microbiome. | 55 | POWER-remote behavioral weight loss intervention | Not yet recruiting |
| NCT01929122 | A prospective, randomized, single-blind study | Colorectal cancer survivors | To explore the effects of cooked navy bean powder or rice bran consumption on the stool microbiome and metabolome of colorectal cancer survivors and healthy adults. | 29 | Cooked navy bean powder vs. rice bran vs. placebo | Completed |
| Chemotherapy Agent/Agent-Based Regimen | Cardiovascular Toxicity | Neurocognitive Toxicity | Known Effects on Gut and the Microbiome |
|---|---|---|---|
| Anthracyclines | Congestive heart failure, left ventricular dysfunction, arrhythmia, cardiomyopathies [80] | Cognitive impairment [5], peripheral neuropathy [81] | Increased intestinal permeability [73] |
| Cyclophosphamide/Ifosfamide | Congestive heart failure, left ventricular systolic dysfunction [82] | Cognitive impairment [5], peripheral neuropathy [81] | Translocation of Gram-positive bacteria into mesenteric lymph nodes and spleen [27], disrupted intestinal barrier integrity [74] |
| Taxanes | Arrhythmias, cardiac ischemia, left ventricular dysfunction [83,84] | Cognitive impairment [6], peripheral neuropathy [81,85] | Decreased abundance of Akkermansia muciniphila, disrupted intestinal barrier integrity [86] |
| Etoposide | Not significant | Occasional peripheral neuropathy [81] | Increased intestinal permeability [73] |
| Cisplatin/Carboplatin | Coronary artery disease, hypertension, myocardial infarction, Raynaud phenomenon [4] | Cognitive impairment [6], peripheral neuropathy, paresthesia, ototoxicity [4,85] | Dysbiosis, antimicrobial effect on Bacillus, Escherichia coli, disruption of intestinal mucosa [87] |
| Cytarabine | Pericarditis [88] | Neurocognitive deficits [89] | Unknown |
| 5-Fluorouracil, Capecitabine, Gemcitabine | Coronary spasms, ischemia [83] | Senzory neuropathy, paresthesia after gemcitabine [81], cognitive impairment [86] | Intestinal mucosal damage, lower abundance of Firmicutes, increase in Bacteroidetes, Actinobacteria and Verucomicrobia [90] |
| Methotrexate | Not significant | Cognitive deficits, impaired executive functions [91] | Reduction in Bifidobacterium, Lactobacillus, Escherichia coli [75], mucosal barrier disruption [73] |
| Myeloablative chemotherapy (Carmustine, Etoposide, Aracytine, Melphalan) | Hypertension, diabetes, left ventricular dysfunction, arrhythmia, stroke, myocardial infarction, heart failure [44] | Adverse psychosocial effects, mental health disorders, cognitive impairment [89] | A decrease in Firmicutes and Actinobacteria and an increase in the abundance of Proteobacteria [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciernikova, S.; Mego, M.; Chovanec, M. Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity. Cancers 2021, 13, 782. https://doi.org/10.3390/cancers13040782
Ciernikova S, Mego M, Chovanec M. Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity. Cancers. 2021; 13(4):782. https://doi.org/10.3390/cancers13040782
Chicago/Turabian StyleCiernikova, Sona, Michal Mego, and Michal Chovanec. 2021. "Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity" Cancers 13, no. 4: 782. https://doi.org/10.3390/cancers13040782
APA StyleCiernikova, S., Mego, M., & Chovanec, M. (2021). Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity. Cancers, 13(4), 782. https://doi.org/10.3390/cancers13040782