The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
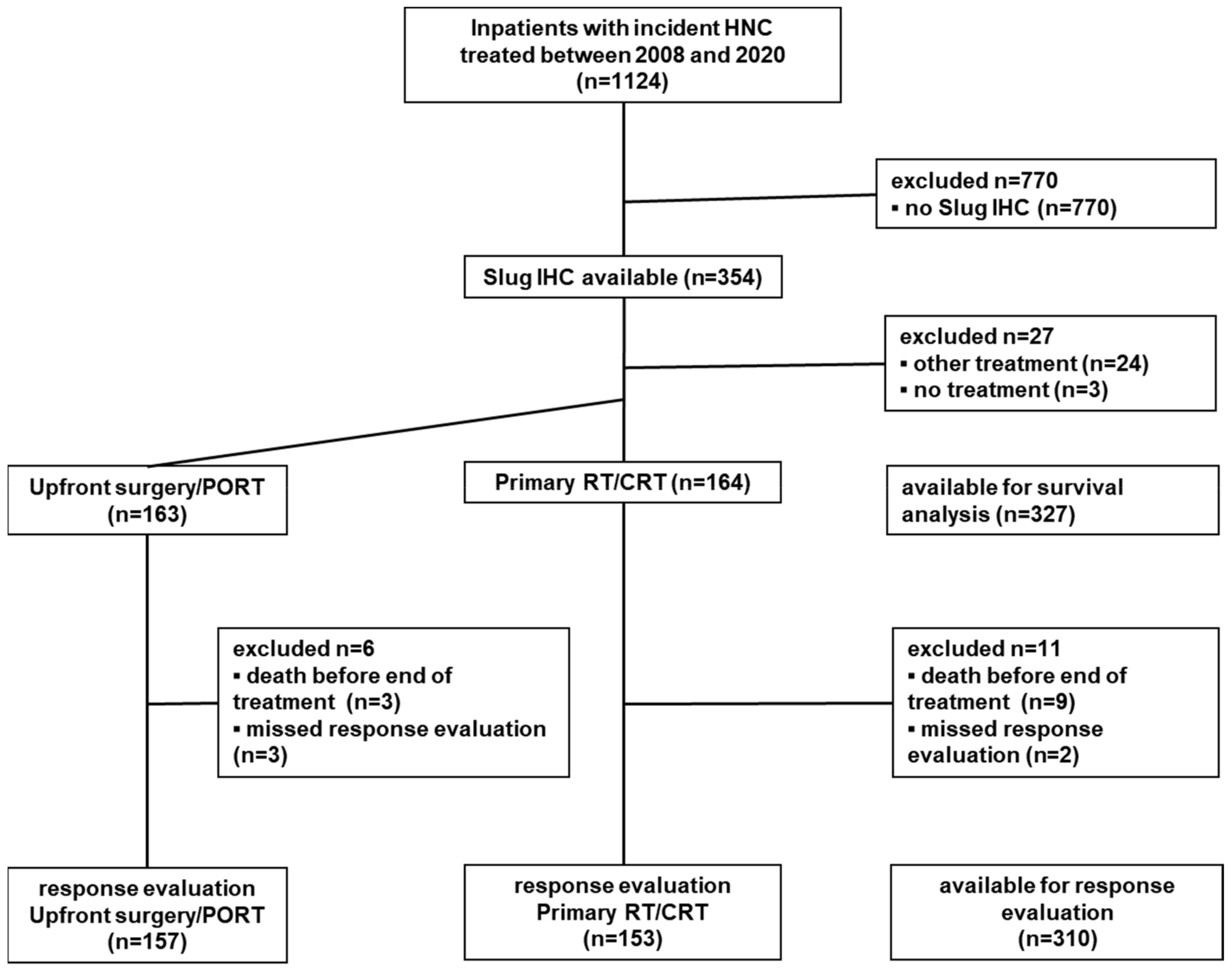
2.1. Study Population
2.2. Host Factors, Disease Factors, and First-Line Treatment Modality
2.3. Treatment Response Evaluation and Follow-Up
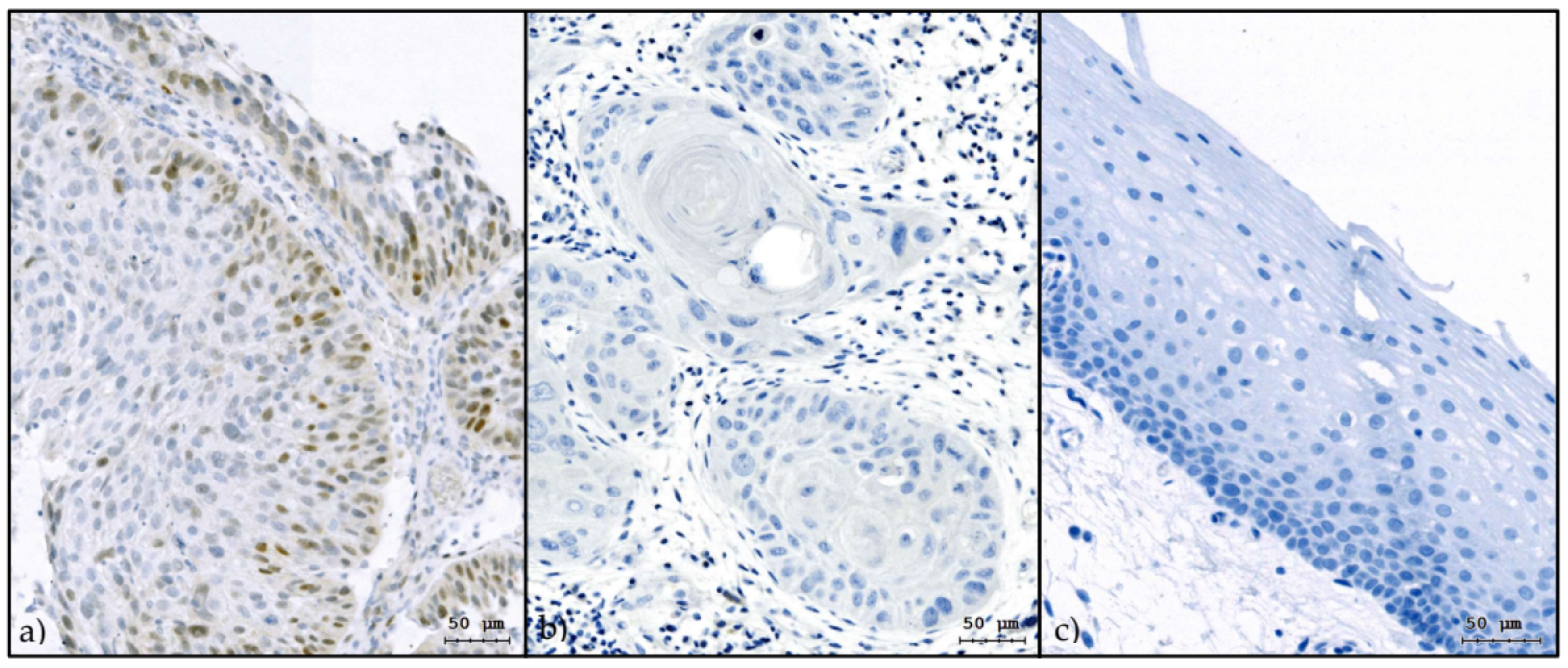
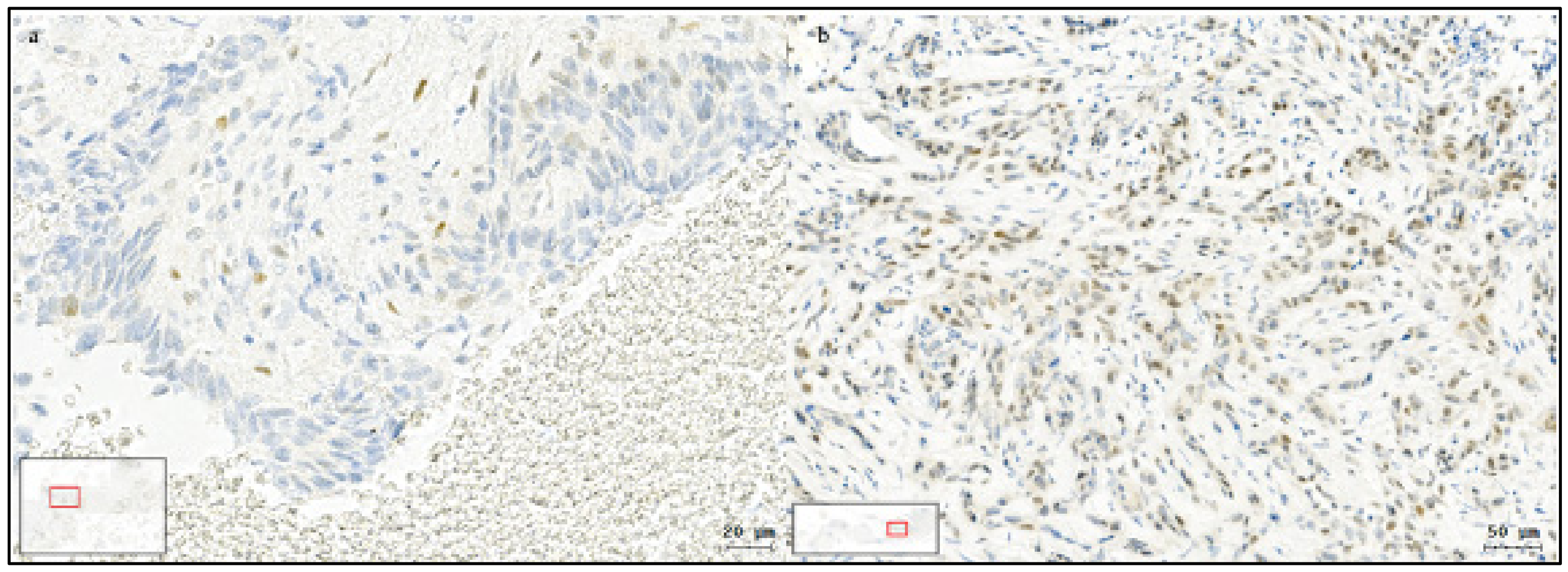
2.4. Immunohistochemistry
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Slug IHC
3.3. Upfront Surgery/PORT vs. Primary RT/CRT
3.4. Slug IHC and Treatment Response to Primary RT/CRT
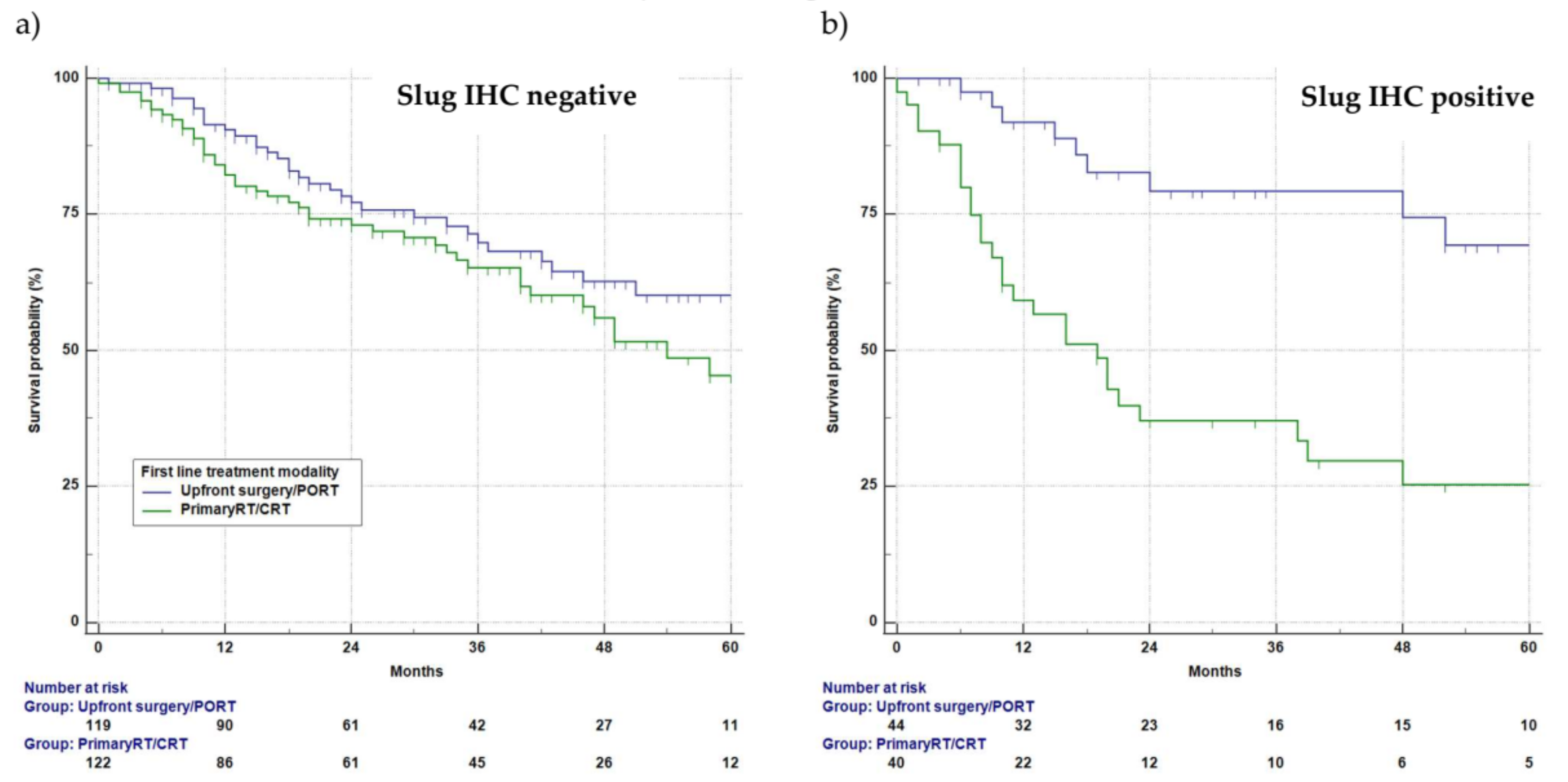
3.5. Slug IHC and Survival
3.6. Univariate Survival Analysis
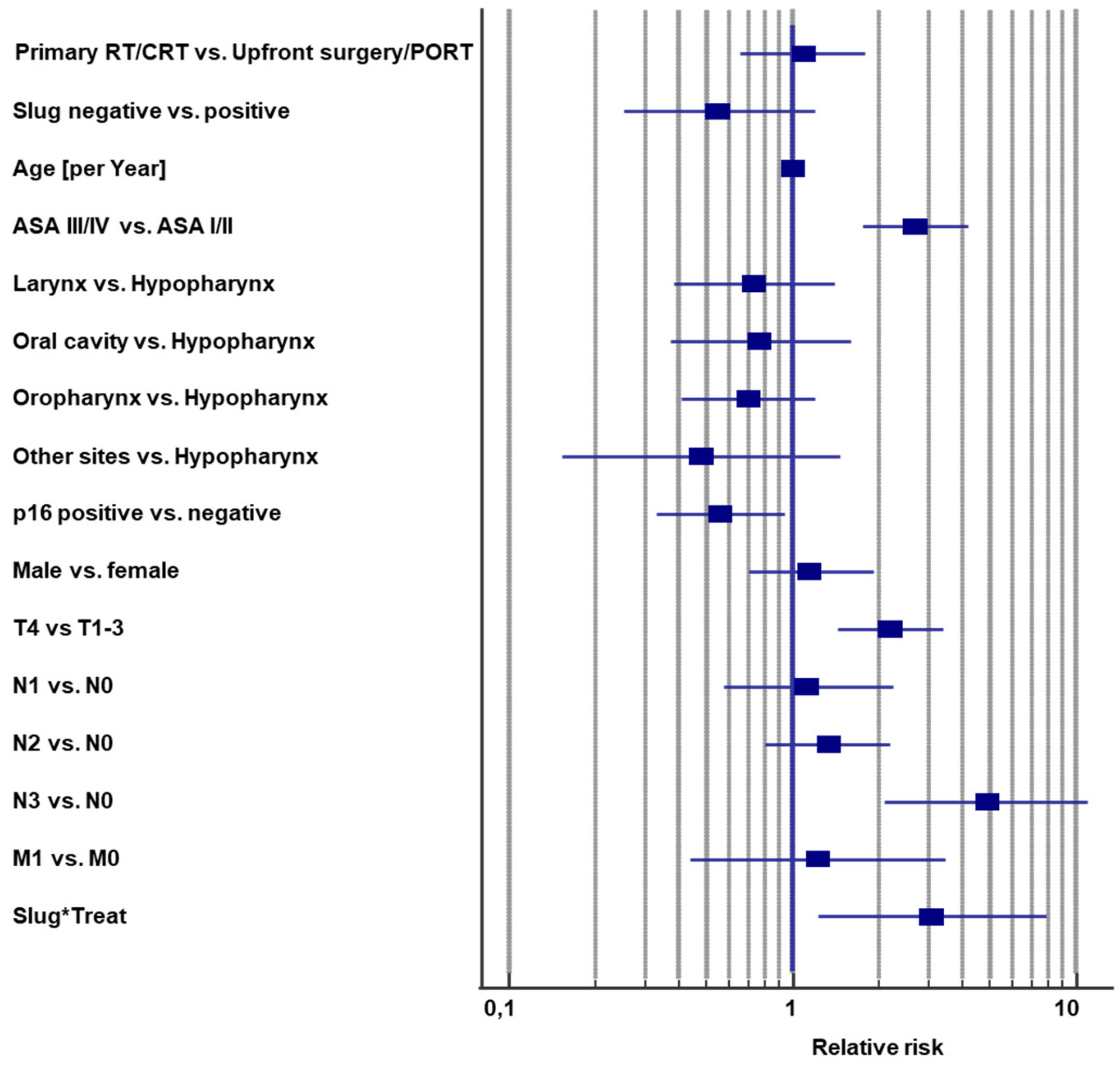
3.7. Multivariable Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, L.Q.M. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Gillison, M.L.; Nguyen-Tân, P.F.; Rosenthal, D.I.; Weber, R.S.; Lambert, L.; Trotti, A.M., 3rd; Barrett, W.L.; Thorstad, W.L.; et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer 2019, 125, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, B.J.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol 2012, 23 (Suppl. 10), x173–x177. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Lichtenecker, M.; Anegg, M.; Dejaco, D.; Kofler, B.; Schartinger, V.H.; Kasseroler, M.T.; Forthuber, B.; Posch, A.; Riechelmann, H. Persistent head and neck cancer following lirst-line treatment. Cancers 2018, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Golm, L.; Dejaco, D.; Riedl, D.; Kofler, B.; Url, C.; Wolfram, D.; Riechelmann, H. Surgical rescue for persistent head and neck cancer after first-line treatment. Eur. Arch. Otorhinolaryngol. 2020, 1437–1448. [Google Scholar] [CrossRef]
- Dragovic, A.F.; Caudell, J.J.; Spencer, S.A.; Carroll, W.R.; Nabell, L.A.; Bonner, J.A. Locoregional failure and the risk of distant metastasis after modern radiotherapy for head and neck cancer. Head Neck 2013, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, K.K.; Cesano, A.; Alvarez, J.; Hawtin, R.; Janetzki, S.; Kirsch, I.; Masucci, G.V.; Robbins, P.B.; Selvan, S.R.; Streicher, H.Z.; et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume II—Clinical validation and regulatory considerations. J. Immunother. Cancer 2016, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef]
- Yamamoto, V.N.; Thylur, D.S.; Bauschard, M.; Schmale, I.; Sinha, U.K. Overcoming radioresistance in head and neck squamous cell carcinoma. Oral Oncol. 2016, 63, 44–51. [Google Scholar] [CrossRef]
- Hong, A.M.; Dobbins, T.A.; Lee, C.S.; Jones, D.; Harnett, G.B.; Armstrong, B.K.; Clark, J.R.; Milross, C.G.; Kim, J.; O’Brien, C.J.; et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br. J. Cancer 2010, 103, 1510–1517. [Google Scholar] [CrossRef]
- Fischer, C.A.; Zlobec, I.; Green, E.; Probst, S.; Storck, C.; Lugli, A.; Tornillo, L.; Wolfensberger, M.; Terracciano, L.M. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int. J. Cancer 2010, 126, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Perrone, F.; Bossi, P.; Suardi, S.; Mariani, L.; Artusi, R.; Oggionni, M.; Rossini, C.; Cantu, G.; Squadrelli, M.; et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2006, 24, 5630–5636. [Google Scholar] [CrossRef]
- Skinner, H.D.; Sandulache, V.C.; Ow, T.J.; Meyn, R.E.; Yordy, J.S.; Beadle, B.M.; Fitzgerald, A.L.; Giri, U.; Ang, K.K.; Myers, J.N. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin. Cancer Res. 2012, 18, 290–300. [Google Scholar] [CrossRef]
- Alsner, J.; Sorensen, S.B.; Overgaard, J. TP53 mutation is related to poor prognosis after radiotherapy, but not surgery, in squamous cell carcinoma of the head and neck. Radiother. Oncol. 2001, 59, 179–185. [Google Scholar] [CrossRef]
- Bossi, P.; Resteghini, C.; Paielli, N.; Licitra, L.; Pilotti, S.; Perrone, F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 74362–74379. [Google Scholar] [CrossRef]
- Langer, C.J. Exploring biomarkers in head and neck cancer. Cancer 2012, 118, 3882–3892. [Google Scholar] [CrossRef] [PubMed]
- Noordhuis, M.G.; Kop, E.A.; van der Vegt, B.; Langendijk, J.A.; van der Laan, B.; Schuuring, E.; de Bock, G.H. Biological tumor markers associated with local control after primary radiotherapy in laryngeal cancer: A systematic review. Clin. Otolaryngol. 2020, 45, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Simeone, P.; Trerotola, M.; Franck, J.; Cardon, T.; Marchisio, M.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. The multiverse nature of epithelial to mesenchymal transition. Semin. Cancer Biol. 2019, 58, 1–10. [Google Scholar] [CrossRef]
- Wilson, M.M.; Weinberg, R.A.; Lees, J.A.; Guen, V.J. Emerging mechanisms by which EMT programs control stemness. Trends Cancer 2020, 9, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Abdelmoez, A.; Metzler, V.M.; Dejaco, D.; Riechelmann, H.; Dudas, J.; Skvortsova, I. Epithelial-mesenchymal crosstalk induces radioresistance in HNSCC cells. Oncotarget 2018, 9, 3641–3652. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Metzler, V.; Pritz, C.; Riechelmann, H.; Dudas, J. Tumor-associated fibroblast-conditioned medium induces CDDP resistance in HNSCC cells. Oncotarget 2016, 7, 2508–2518. [Google Scholar] [CrossRef]
- van der Heijden, M.; Essers, P.B.M.; Verhagen, C.V.M.; Willems, S.M.; Sanders, J.; de Roest, R.H.; Vossen, D.M.; Leemans, C.R.; Verheij, M.; Brakenhoff, R.H.; et al. Epithelial-to-mesenchymal transition is a prognostic marker for patient outcome in advanced stage HNSCC patients treated with chemoradiotherapy. Radiother. Oncol. 2020, 147, 186–194. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudas, J.; Ingruber, J.; Glueckert, R.; Sprung, S.; Fleischer, F.; Cidlinsky, N.; Dejaco, D.; Kofler, B.; Giotakis, A.I.; et al. Slug Is A Surrogate Marker of Epithelial to Mesenchymal Transition (EMT) in Head and Neck Cancer. J. Clin. Med. 2020, 9, 2061. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. BMC Med. 2012, 10, 51. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Mandrekar, S.J.; Sargent, D.J. Clinical trial designs for predictive biomarker validation: Theoretical considerations and practical challenges. J. Clin. Oncol. 2009, 27, 4027–4034. [Google Scholar] [CrossRef]
- Reid, B.C.; Alberg, A.J.; Klassen, A.C.; Koch, W.M.; Samet, J.M. The American Society of Anesthesiologists’ class as a comorbidity index in a cohort of head and neck cancer surgical patients. Head Neck 2001, 23, 985–994. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Hinni, M.L.; Zarka, M.A.; Hoxworth, J.M. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope 2013, 123, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Maihoefer, C.; Schuttrumpf, L.; Macht, C.; Pflugradt, U.; Hess, J.; Schneider, L.; Woischke, C.; Walch, A.; Baumeister, P.; Kirchner, T.; et al. Postoperative (chemo) radiation in patients with squamous cell cancers of the head and neck—Clinical results from the cohort of the clinical cooperation group "Personalized Radiotherapy in Head and Neck Cancer". Radiat. Oncol. 2018, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Schuttrumpf, L.; Marschner, S.; Scheu, K.; Hess, J.; Rietzler, S.; Walch, A.; Baumeister, P.; Kirchner, T.; Ganswindt, U.; Zitzelsberger, H.; et al. Definitive chemoradiotherapy in patients with squamous cell cancers of the head and neck—Results from an unselected cohort of the clinical cooperation group "Personalized Radiotherapy in Head and Neck Cancer". Radiat. Oncol. 2020, 15, 7. [Google Scholar] [CrossRef]
- Rades, D.; Seidl, D.; Janssen, S.; Strojan, P.; Karner, K.; Bajrovic, A.; Hakim, S.G.; Wollenberg, B.; Schild, S.E. Comparing two lower-dose cisplatin programs for radio-chemotherapy of locally advanced head-and-neck cancers. Eur. Arch. Otorhinolaryngol. 2017, 274, 1021–1027. [Google Scholar] [CrossRef]
- Budach, V.; Stromberger, C.; Poettgen, C.; Baumann, M.; Budach, W.; Grabenbauer, G.; Marnitz, S.; Olze, H.; Wernecke, K.D.; Ghadjar, P. Hyperfractionated accelerated radiation therapy (HART) of 70.6 Gy with concurrent 5-FU/Mitomycin C is superior to HART of 77.6 Gy alone in locally advanced head and neck cancer: Long-term results of the ARO 95-06 randomized phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 916–924. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Roman, B.R.; Goldenberg, D.; Givi, B. AHNS Series--Do you know your guidelines? Guideline recommended follow-up and surveillance of head and neck cancer survivors. Head Neck 2016, 38, 168–174. [Google Scholar] [CrossRef]
- Schouten, C.S.; Hoekstra, O.S.; Leemans, C.R.; Castelijns, J.A.; de Bree, R. Response evaluation after chemoradiotherapy for advanced staged oropharyngeal squamous cell carcinoma: A nationwide survey in the Netherlands. Eur. Arch. Otorhinolaryngol. 2015, 272, 3507–3513. [Google Scholar] [CrossRef]
- Pagh, A.; Grau, C.; Overgaard, J. Failure pattern and salvage treatment after radical treatment of head and neck cancer. Acta Oncol. 2016, 55, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.; Dietl, W.; Romani, A.; Reinold, S.; Glueckert, R.; Schrott-Fischer, A.; Dejaco, D.; Johnson Chacko, L.; Tuertscher, R.; Schartinger, V.H.; et al. Nerve Growth Factor (NGF)-Receptor Survival Axis in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci 2018, 19, 1771. [Google Scholar] [CrossRef]
- Reimers, N.; Kasper, H.U.; Weissenborn, S.J.; Stutzer, H.; Preuss, S.F.; Hoffmann, T.K.; Speel, E.J.; Dienes, H.P.; Pfister, H.J.; Guntinas-Lichius, O.; et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer 2007, 120, 1731–1738. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef]
- Bouchalova, P.; Nenutil, R.; Muller, P.; Hrstka, R.; Appleyard, M.V.; Murray, K.; Jordan, L.B.; Purdie, C.A.; Quinlan, P.; Thompson, A.M.; et al. Mutant p53 accumulation in human breast cancer is not an intrinsic property or dependent on structural or functional disruption but is regulated by exogenous stress and receptor status. J. Pathol. 2014, 233, 238–246. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 6 August 2020).
- Dudas, J.; Ladanyi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to mesenchymal transition: A mechanism that fuels cancer radio/chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef]
- Dudas, J.; Bitsche, M.; Schartinger, V.; Falkeis, C.; Sprinzl, G.M.; Riechelmann, H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011, 47, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kano, S.; Homma, A.; Fukuda, S. Epithelial-mesenchymal transition in human papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Oncol. Rep. 2014, 32, 2673–2679. [Google Scholar] [CrossRef][Green Version]
- Wang, S.P.; Wang, W.L.; Chang, Y.L.; Wu, C.T.; Chao, Y.C.; Kao, S.H.; Yuan, A.; Lin, C.W.; Yang, S.C.; Chan, W.K.; et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol. 2009, 11, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Danielidis, V.; Sivridis, E. Hypoxia inducible factor (HIf1alpha and HIF2alpha) and carbonic anhydrase 9 (CA9) expression and response of head-neck cancer to hypofractionated and accelerated radiotherapy. Int. J. Radiat. Biol. 2008, 84, 47–52. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, H.; Landuyt, W.; Verbeken, E.; Goethals, L.; Hermans, R.; Nuyts, S. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/− chemotherapy. BMC Cancer 2005, 5, 42. [Google Scholar] [CrossRef]
- Dudas, J.; Schartinger, V.H.; Romani, A.; Schweigl, G.; Kordsmeyer, K.; Marta, P.I.; Url, C.; Kral, F.; Riechelmann, H. Cell cycle association and hypoxia regulation of excision repair cross complementation group 1 protein (ERCC1) in tumor cells of head and neck cancer. Tumour Biol. 2014, 35, 7807–7819. [Google Scholar] [CrossRef]
- Patel, U.A.; Howell, L.K. Local response to chemoradiation in T4 larynx cancer with cartilage invasion. Laryngoscope 2011, 121, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Been, M.J.; Watkins, J.; Manz, R.M.; Gentry, L.R.; Leverson, G.E.; Harari, P.M.; Hartig, G.K. Tumor volume as a prognostic factor in oropharyngeal squamous cell carcinoma treated with primary radiotherapy. Laryngoscope 2008, 118, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, D.; Steinbichler, T.; Schartinger, V.H.; Fischer, N.; Anegg, M.; Dudas, J.; Posch, A.; Widmann, G.; Riechelmann, H. Prognostic value of tumor volume in patients with head and neck squamous cell carcinoma treated with primary surgery. Head Neck 2018, 40, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, P.; Zhang, D.; Weng, X. The prognostic implication of slug in all tumour patients—A systematic meta-analysis. Eur. J. Clin. Investig. 2016, 46, 398–407. [Google Scholar] [CrossRef]
- Chung, C.H.; Parker, J.S.; Ely, K.; Carter, J.; Yi, Y.; Murphy, B.A.; Ang, K.K.; El-Naggar, A.K.; Zanation, A.M.; Cmelak, A.J.; et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006, 66, 8210–8218. [Google Scholar] [CrossRef] [PubMed]
| Patient Factors | Slug IHC | ||||||
|---|---|---|---|---|---|---|---|
| Negative (n = 263) | Positive (n = 91) | Total (n = 354) | |||||
| Count | Column % | Count | Column % | Count | Column % | ||
| Gender | Male | 212 | 81% | 74 | 81% | 286 | 81% |
| Female | 51 | 19% | 17 | 19% | 68 | 19% | |
| ASA score 1 | ASA I/II | 155 | 59% | 43 | 47% | 198 | 56% |
| ASA III/IV | 106 | 41% | 48 | 53% | 154 | 44% | |
| Smoking | <10 pack-years | 92 | 35% | 29 | 32% | 121 | 34% |
| ≥10 pack-years | 171 | 65% | 62 | 68% | 233 | 66% | |
| Tumor site 1 | Lips and oral cavity | 35 | 13% | 16 | 18% | 51 | 14% |
| Oropharynx | 126 | 48% | 32 | 35% | 158 | 45% | |
| Hypopharynx | 27 | 10% | 21 | 23% | 48 | 14% | |
| Larynx | 62 | 24% | 17 | 19% | 79 | 22% | |
| Others | 13 | 5% | 5 | 5% | 18 | 5% | |
| T stage (I–III vs. IV) | T1–3 | 186 | 71% | 60 | 66% | 246 | 69% |
| T4 | 77 | 29% | 31 | 34% | 108 | 31% | |
| N stage | N0 | 77 | 29% | 35 | 38% | 112 | 32% |
| N1 | 55 | 21% | 17 | 19% | 72 | 20% | |
| N2 | 109 | 41% | 35 | 38% | 144 | 41% | |
| N3 | 22 | 8% | 4 | 4% | 26 | 7% | |
| Distant metastasis | M0 | 248 | 94% | 88 | 97% | 336 | 95% |
| M1 | 15 | 6% | 3 | 3% | 18 | 5% | |
| p16 status 2 | Negative | 181 | 69% | 81 | 89% | 262 | 74% |
| Positive | 81 | 31% | 10 | 11% | 91 | 26% | |
| First-line treatment modality | Upfront surgery/PORT | 119 | 49% | 44 | 52% | 163 | 50% |
| Primary RT/CRT | 123 | 51% | 41 | 48% | 164 | 50% | |
| Slug Status | CR | Failure | Total |
|---|---|---|---|
| Slug IHC negative | 89 (75%) | 29 (25%) | 118 |
| Slug IHC positive | 16 (46%) | 19 (54%) | 35 |
| Total | 105 | 48 | 157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riechelmann, H.; Steinbichler, T.B.; Sprung, S.; Santer, M.; Runge, A.; Ganswindt, U.; Gamerith, G.; Dudas, J. The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer. Cancers 2021, 13, 772. https://doi.org/10.3390/cancers13040772
Riechelmann H, Steinbichler TB, Sprung S, Santer M, Runge A, Ganswindt U, Gamerith G, Dudas J. The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer. Cancers. 2021; 13(4):772. https://doi.org/10.3390/cancers13040772
Chicago/Turabian StyleRiechelmann, Herbert, Teresa Bernadette Steinbichler, Susanne Sprung, Matthias Santer, Annette Runge, Ute Ganswindt, Gabriele Gamerith, and Jozsef Dudas. 2021. "The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer" Cancers 13, no. 4: 772. https://doi.org/10.3390/cancers13040772
APA StyleRiechelmann, H., Steinbichler, T. B., Sprung, S., Santer, M., Runge, A., Ganswindt, U., Gamerith, G., & Dudas, J. (2021). The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer. Cancers, 13(4), 772. https://doi.org/10.3390/cancers13040772








