A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
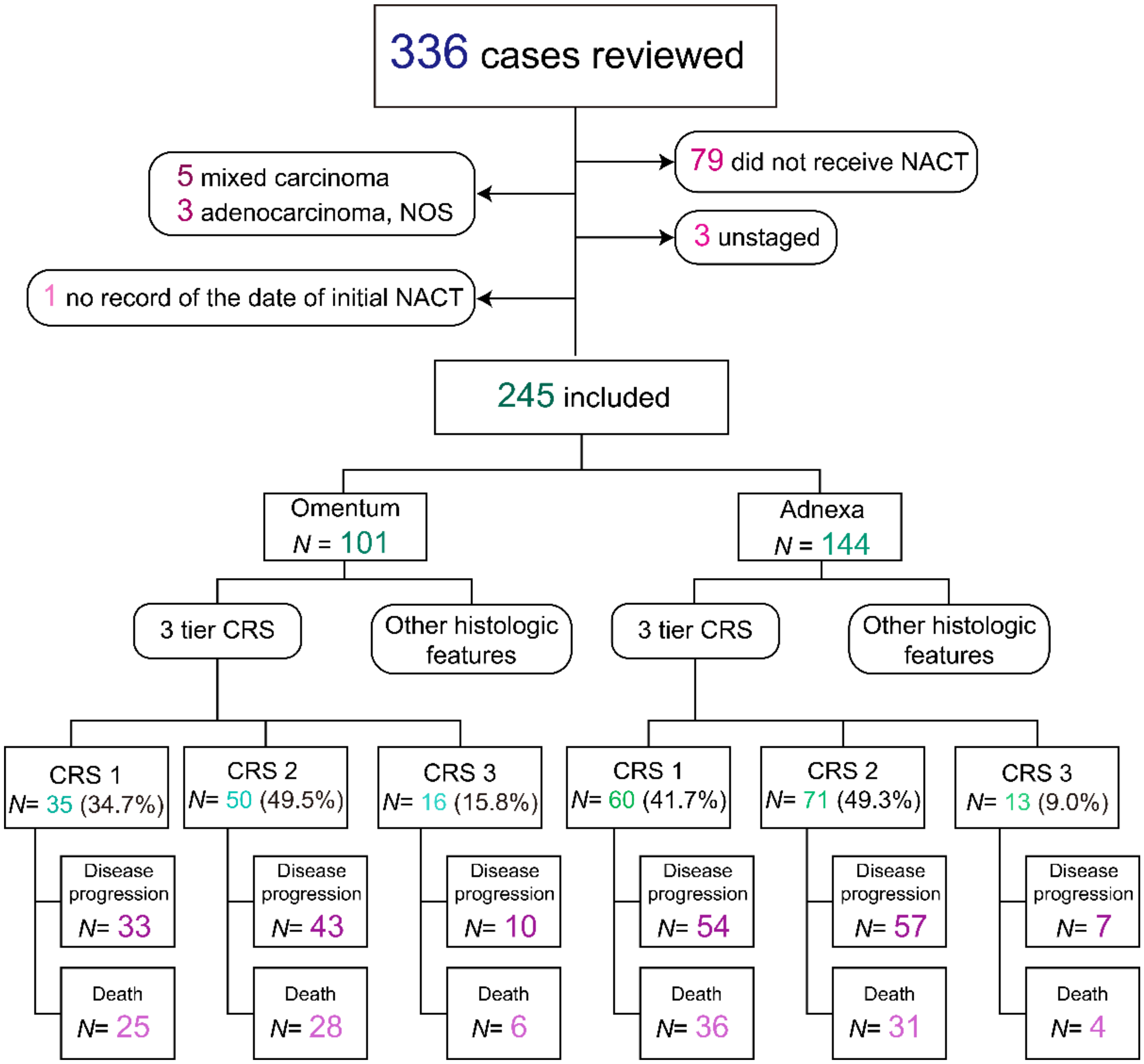
2.1. Study Population
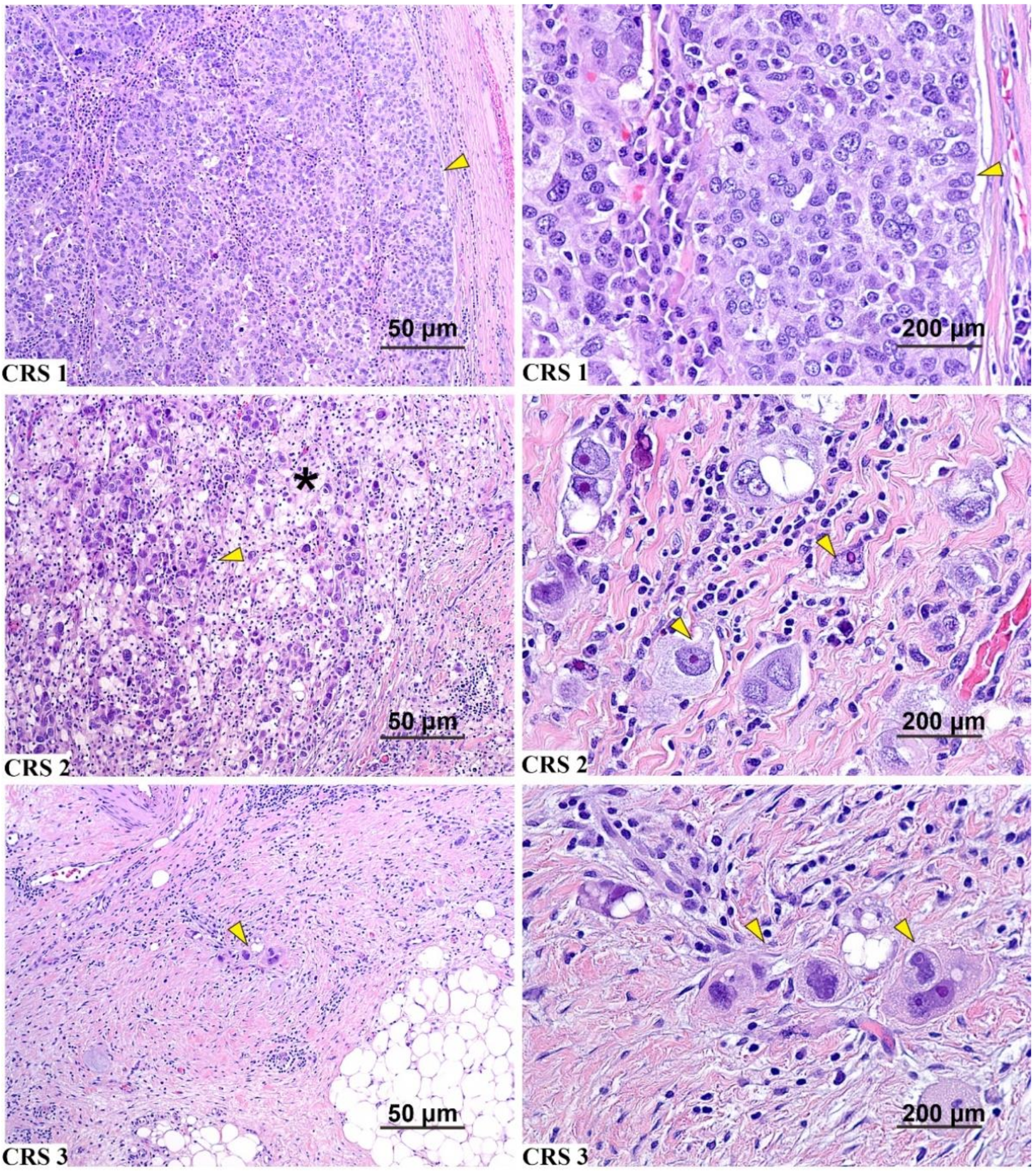
2.2. Pathology Review
2.3. Data Analyses and Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up, Survival, and Observed Event Rate
3.3. BRCA Mutation Status and Survival
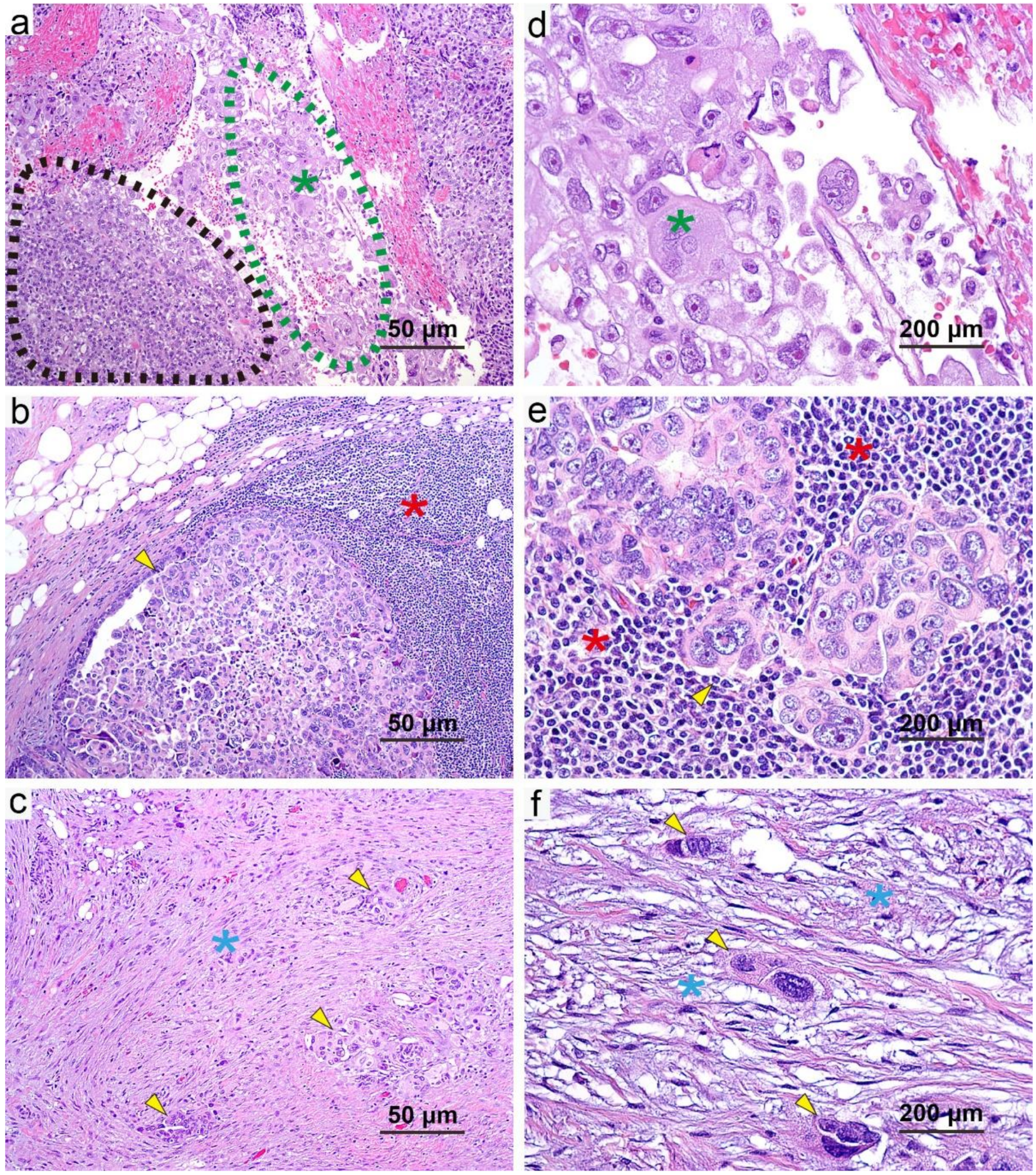
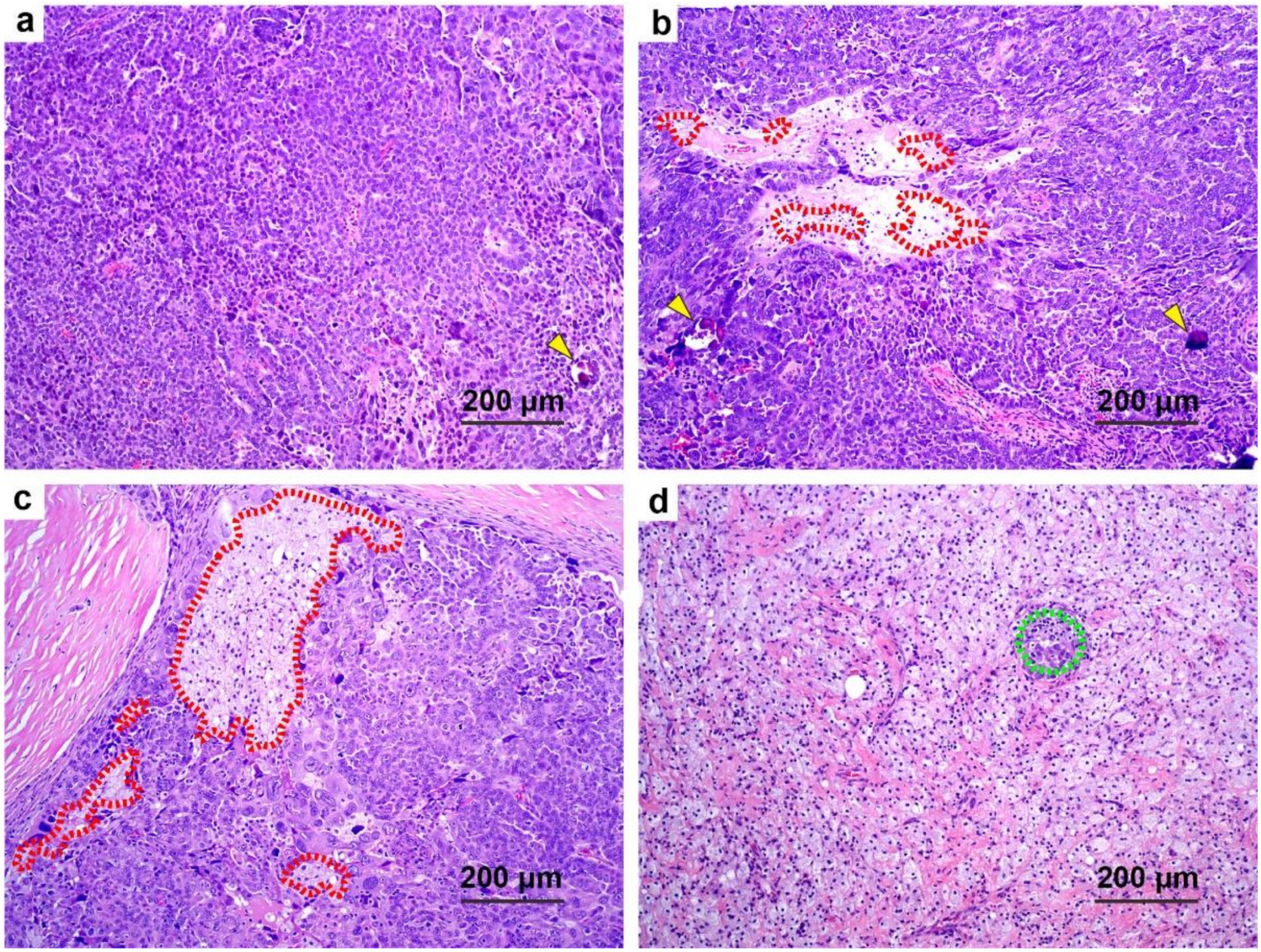
3.4. Correlation of Additional Histologic Features with CRS
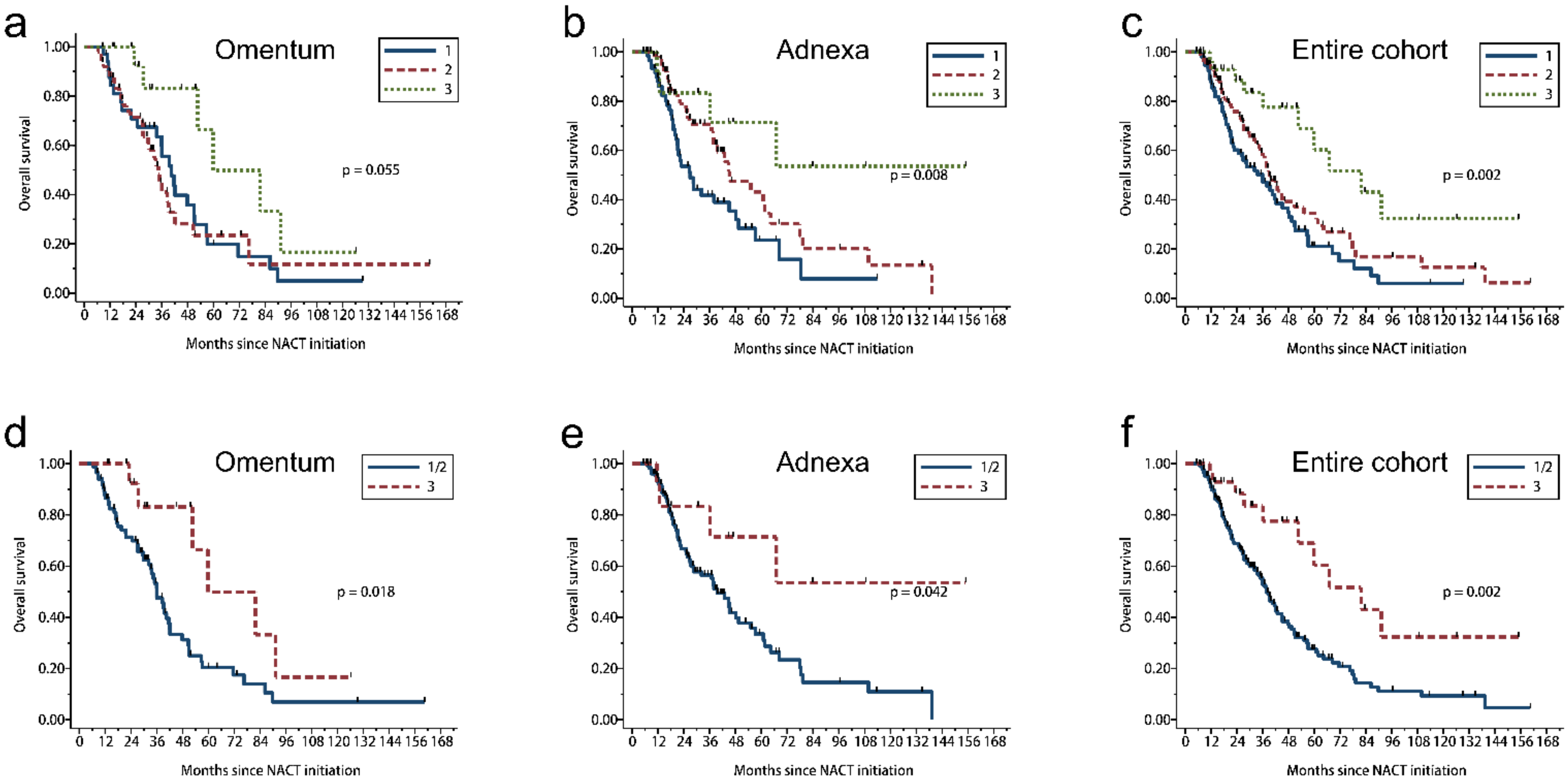
3.5. 2 Tier CRS Showed Stronger Prognostic Significance
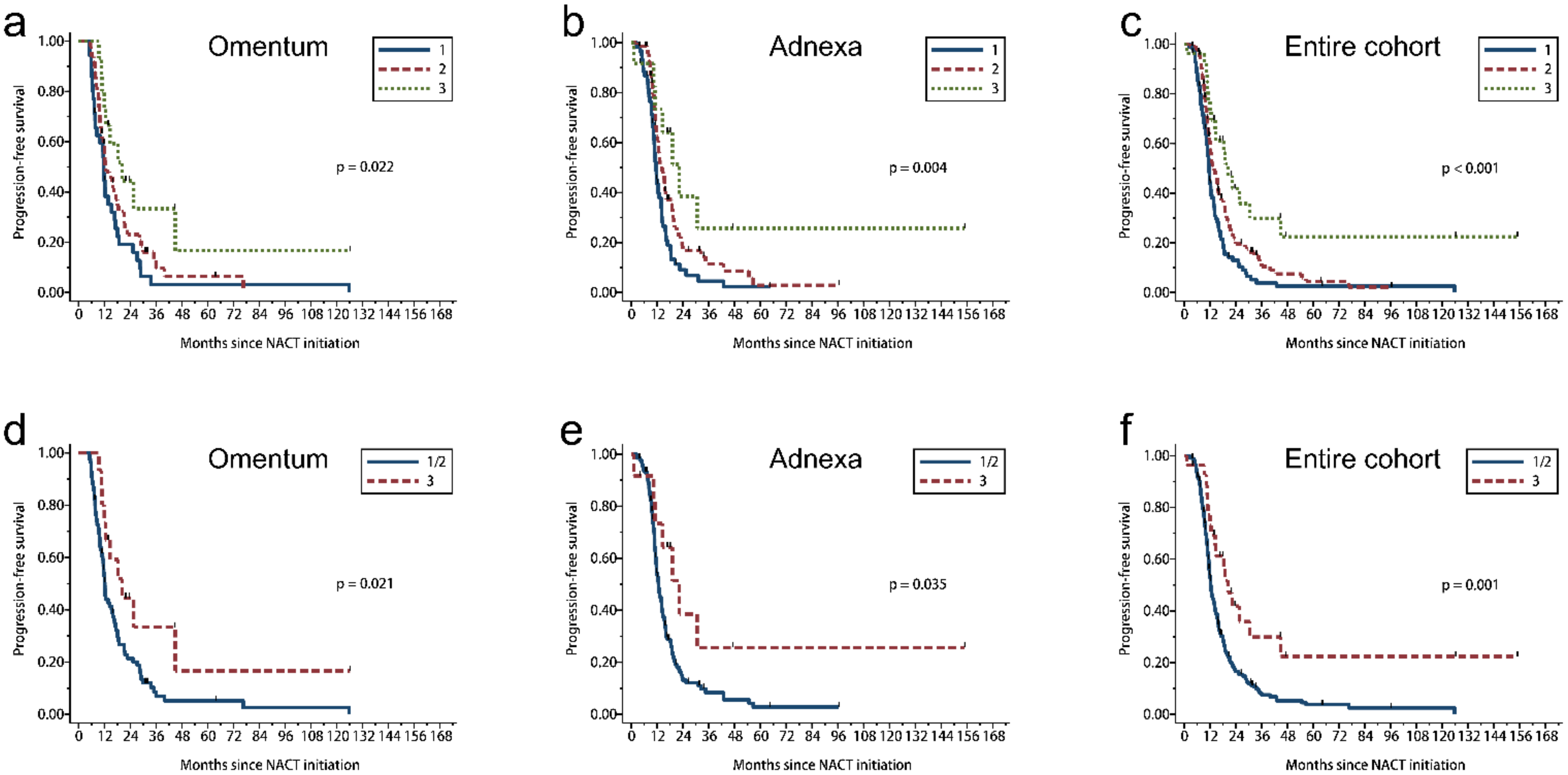
3.6. Histologic Features and Patient Survival in Univariate Analysis
3.7. Multivariate Model of Survival Based on CRS and New Histopathologic Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Bohm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef]
- Rajkumar, S.; Polson, A.; Nath, R.; Lane, G.; Sayasneh, A.; Jakes, A.; Begum, S.; Mehra, G. Prognostic implications of histological tumor regression (Bohm’s score) in patients receiving neoadjuvant chemotherapy for high grade serous tubal & ovarian carcinoma. Gynecol. Oncol. 2018, 151, 264–268. [Google Scholar] [PubMed]
- Kuerer, H.M.; Lisa, A.N.; Terry, L.S.; Fred, C.A.; Kelly, K.H. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 1999, 17, 460–469. [Google Scholar] [CrossRef]
- Langer, R.; Becker, K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Archiv. 2017, 472, 175–186. [Google Scholar] [CrossRef]
- Bohm, S.; Le, N.; Lockley, M.; Brockbank, E.; Faruqi, A.; Said, I.; Jeyarajah, A.; Wuntakal, R.; Gilks, B.; Singh, N. Histopathologic response to neoadjuvant chemo-therapy as a prognostic biomarker in tubo-ovarian high-grade serous carcinoma: Updated Chemotherapy Response Score (CRS) results. Int. J. Gynecol. Cancer 2019, 29, 353–356. [Google Scholar] [CrossRef]
- Lawson, B.C.; Euscher, E.D.; Bassett, R.L.; Liu, J.S.; Ramalingam, P.; Zhong, Y.P.; Fleming, N.D.; Malpica, A. A 3-tier chemotherapy response score for ovarian/fallopian tube/peritoneal high-grade serous carcinoma: Is it clinically relevant? Am. J. Surg. Pathol. 2020, 44, 206–213. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, Y.S.; Na, K.; Kim, H.M.; Park, C.K.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T.; Kim, H.S. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J. Gynecol. Oncol. 2017, 28, e73. [Google Scholar] [CrossRef]
- Ditzel, H.M.; Strickland, K.C.; Meserve, E.E.; Stover, E.; Konstantinopoulos, P.A.; Matulonis, U.A.; Muto, M.G.; Liu, J.F.; Feltmate, C.; Horowitz, N.; et al. Assessment of a Chemotherapy Response Score (CRS) System for Tubo-Ovarian High-Grade Serous Carcinoma (HGSC). Int. J. Gynecol. Pathol. 2019, 38, 230–240. [Google Scholar] [CrossRef]
- Coghlan, E.; Meniawy, T.M.; Munro, A.; Bulsara, M.; Stewart, C.J.; Tan, A.; Koay, M.H.E.; MaGee, D.; Codde, J.; Tan, J.; et al. Prognostic role of histological tumor regression in patients receiving neoadjuvant chemotherapy for high-grade serous tubo-ovarian carcinoma. Int. J. Gynecol. Cancer 2017, 27, 708–713. [Google Scholar] [CrossRef]
- Singh, P.; Kaushal, V.; Rai, B.; Rajwanshi, A.; Gupta, N.; Dey, P.; Garg, R.; Rohilla, M.; Suri, V.; Ghoshal, S.; et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology 2018, 72, 619–625. [Google Scholar] [CrossRef]
- Michaan, N.; Chong, W.Y.; Han, N.Y.; Lim, M.C.; Park, S.Y. Prognostic Value of Pathologic Chemotherapy Response Score in Patients With Ovarian Cancer After Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2018, 28, 1676–1682. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Piermattei, A.; Inzani, F.; Valente, M.; Arciuolo, D.; Spadola, S.; Mulè, A.; Zorzato, P.; Fagotti, A.; et al. Pathological Chemotherapy Response Score in Patients Affected by High Grade Serous Ovarian Carcinoma: The Prognostic Role of Omental and Ovarian Residual Disease. Front. Oncol. 2019, 9, 778. [Google Scholar] [CrossRef]
- Roy, P.; Serra, S.; Kennedy, E.; Chetty, R. The prognostic value of grade of regression and oncocytic change in rectal adenocarcinoma treated with neo-adjuvant chemoradiotherapy. J. Surg. Oncol. 2012, 105, 130–134. [Google Scholar] [CrossRef]
- Rouzbahman, M.; Serra, S.; Chetty, R. Rectal adenocarcinoma with oncocytic features: Possible relationship with pre-operative chemoradiotherapy. J. Clin. Pathol. 2006, 59, 1039–1043. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Salvi, F.; Betts, C.M.; Frezza, G.P.; Piemontese, A.; Del Prete, P.; Baldoni, C.; Foschini, M.P.; Viale, G. Oncocytic modifications in rectal adenocarcinomas after radio and chemotherapy. Virchows Archiv. 2005, 448, 442–448. [Google Scholar] [CrossRef]
- Rossi, M.L.; Rehman, A.A.; Gondi, C.S. Therapeutic options for the management of pancreatic cancer. World J. Gastroenterol. 2014, 20, 11142–11159. [Google Scholar] [CrossRef]
- Ueno, H.; Shinto, E.; Shimazaki, H.; Kajiwara, Y.; Sueyama, T.; Yamamoto, J.; Hase, K. Histologic categorization of desmoplastic reaction: Its relevance to the colorectal cancer microenvironment and prognosis. Ann. Surg. Oncol. 2015, 22, 1504–1512. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lympho-cytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef]
- Salmon, H.; Remark, R.; Gnjatic, S.; Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 2019, 19, 215–227. [Google Scholar] [CrossRef]

| Characteristics | N (%) |
|---|---|
| Age (years) | |
| Median (range) | 62 (29–81) |
| Primary site | |
| Tubo-ovarian | 216/245 (88.2) |
| Peritoneal | 29/245 (11.8) |
| FIGO stage | |
| III | 105/245 (42.9) |
| IV | 125/245 (51.0) |
| Advanced, not otherwise specified | 15/245 (6.1) |
| BRCA status (n = 127) | |
| BRCA positive | 28/127 (22.0) |
| BRCA negative | 99/127 (78.0) |
| Cycles of NACT | |
| 3 or 4 | 168/245 (68.6) |
| >4 | 75/245 (30.6) |
| Unknown | 2/245 (0.8) |
| Total of ≥6 cycles of chemotherapy | |
| Neoadjuvant+adjuvant | 194/245 (79.2) |
| Regimen of NACT | |
| Carboplatin/paclitaxel only | 217/245 (88.6) |
| Carboplatin/paclitaxel and additional agent(s) | 17/245 (6.9) |
| Platinum-based therapy (no paclitaxel) | 11/245 (4.5) |
| Scored site | |
| Omentum | 101/245 (41.2) |
| Adnexa | 144/245 (58.8) |
| Follow-up (months) | |
| Median (range) | 27 (5–160) |
| Site | CRS | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Events | Median | p-Value * | N | Events | Median | p-Value * | ||
| Omentum | 3 tier | 0.055 | 0.022 | ||||||
| 1 | 35 | 25 | 40.5 | 35 | 33 | 11.7 | |||
| 2 | 50 | 28 | 34.4 | 49 | 43 | 12.7 | |||
| 3 | 16 | 6 | 59.9 | 15 | 10 | 20.3 | |||
| 2 tier | 0.018 | 0.021 | |||||||
| 1/2 | 85 | 53 | 36.0 | 84 | 76 | 12.2 | |||
| 3 | 16 | 6 | 59.9 | 15 | 10 | 20.3 | |||
| Adnexa | 3 tier | 0.008 | 0.004 | ||||||
| 1 | 60 | 36 | 26.9 | 60 | 54 | 11.5 | |||
| 2 | 71 | 31 | 45.2 | 71 | 57 | 14.0 | |||
| 3 | 13 | 4 | NE | 12 | 7 | 22.3 | |||
| 2 tier | 0.042 | 0.035 | |||||||
| 1/2 | 131 | 67 | 39.5 | 131 | 111 | 12.6 | |||
| 3 | 13 | 4 | NE | 12 | 7 | 22.3 | |||
| Entire cohort | 3 tier | 0.002 | <0.001 | ||||||
| 1 | 95 | 61 | 35.9 | 95 | 87 | 11.6 | |||
| 2 | 121 | 59 | 39.1 | 120 | 100 | 14.0 | |||
| 3 | 29 | 10 | 81.8 | 27 | 17 | 20.3 | |||
| 2 tier | 0.002 | 0.001 | |||||||
| 1/2 | 216 | 120 | 38.0 | 215 | 187 | 12.4 | |||
| 3 | 29 | 10 | 81.8 | 27 | 17 | 20.3 | |||
| Survival | Histopathology | HR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| LB | UB | ||||
| OS | 2 tier CRS (1/2 versus 3) | 0.49 | 0.25 | 0.95 | 0.034 |
| Oncocytic change (0/1 versus 2/3) | 0.63 | 0.42 | 0.93 | 0.020 | |
| Inflammation (0/1 versus 2/3) | 0.60 | 0.41 | 0.87 | 0.007 | |
| Desmoplasia (0/1 versus 2/3) | 1.67 | 1.13 | 2.47 | 0.010 | |
| PFS | 2 tier CRS (1/2 versus 3) | 0.52 | 0.31 | 0.87 | 0.012 |
| Inflammation (0/1 versus 2/3) | 0.63 | 0.46 | 0.85 | 0.003 | |
| Desmoplasia (0/1 versus 2/3) | 1.48 | 1.09 | 2.01 | 0.011 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Liu, J.; Li, X.; Westin, S.N.; Malpica, A.; Lawson, B.C.; Lee, S.; Fellman, B.M.; Coleman, R.L.; Sood, A.K.; et al. A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy. Cancers 2021, 13, 704. https://doi.org/10.3390/cancers13040704
Zhong Y, Liu J, Li X, Westin SN, Malpica A, Lawson BC, Lee S, Fellman BM, Coleman RL, Sood AK, et al. A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy. Cancers. 2021; 13(4):704. https://doi.org/10.3390/cancers13040704
Chicago/Turabian StyleZhong, Yanping, Jinsong Liu, Xiaoran Li, Shannon N. Westin, Anais Malpica, Barrett C. Lawson, Sanghoon Lee, Bryan M. Fellman, Robert L. Coleman, Anil K. Sood, and et al. 2021. "A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy" Cancers 13, no. 4: 704. https://doi.org/10.3390/cancers13040704
APA StyleZhong, Y., Liu, J., Li, X., Westin, S. N., Malpica, A., Lawson, B. C., Lee, S., Fellman, B. M., Coleman, R. L., Sood, A. K., & Fleming, N. D. (2021). A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy. Cancers, 13(4), 704. https://doi.org/10.3390/cancers13040704






