Pathological Changes in Pancreatic Carcinogenesis: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathology of Pancreatic Tumors
2.1. Primary Pancreatic Tumors
2.2. Secondary Pancreatic Tumors
3. Pathology of Precancerous Lesions of the Pancreas
3.1. Cancer-Related Lesions of the Pancreas
3.2. Pancreatic Intraepithelial Neoplasia (PanIN)
3.3. Pancreatic Cystic Lesions
4. Animal Models of Pancreatic Carcinogenesis
4.1. Hamster
4.2. Mouse
4.3. Other Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Pandol, S.; Gukovskaya, A.; Edderkaoui, M.; Dawson, D.; Eibl, G.; Lugea, A. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. S2), 127–134. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Ahrendt, S.A.; Pitt, H.A. Surgical management of pancreatic cancer. Oncology 2002, 16, 725–734; discussion 734, 736–738, 740, 743. [Google Scholar]
- Iacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J. Clin. Oncol. 2009, 27, 1806–1813. [Google Scholar] [CrossRef] [Green Version]
- Aichler, M.; Seiler, C.; Tost, M.; Siveke, J.; Mazur, P.K.; Da Silva-Buttkus, P.; Bartsch, D.K.; Langer, P.; Chiblak, S.; Durr, A.; et al. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: A comparative study in transgenic mice and human tissues. J. Pathol. 2012, 226, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Esposito, I.; Seiler, C.; Bergmann, F.; Kleeff, J.; Friess, H.; Schirmacher, P. Hypothetical progression model of pancreatic cancer with origin in the centroacinar-acinar compartment. Pancreas 2007, 35, 212–217. [Google Scholar] [CrossRef]
- LeBlanc, J.K.; Chen, J.H.; Al-Haddad, M.; Luz, L.; McHenry, L.; Sherman, S.; Juan, M.; Dewitt, J. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis?: A retrospective study of pathologic correlation. Pancreas 2014, 43, 849–854. [Google Scholar] [CrossRef]
- Murtaugh, L.C. Pathogenesis of pancreatic cancer: Lessons from animal models. Toxicol. Pathol. 2014, 42, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Ishiwata, T.; Yachida, S.; Suzuki, A.; Hamashima, Y.; Hamayasu, H.; Yoshimura, H.; Honma, N.; Aida, J.; Takubo, K.; et al. Clinicopathological Features of 15 Occult and 178 Clinical Pancreatic Ductal Adenocarcinomas in 8339 Autopsied Elderly Patients. Pancreas 2016, 45, 234–240. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [Green Version]
- Cannon, A.; Thompson, C.; Hall, B.R.; Jain, M.; Kumar, S.; Batra, S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer 2018, 9, 78–86. [Google Scholar] [CrossRef]
- Matsuda, Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol. Int. 2019, 69, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Furukawa, T.; Yachida, S.; Nishimura, M.; Seki, A.; Nonaka, K.; Aida, J.; Takubo, K.; Ishiwata, T.; Kimura, W.; et al. The Prevalence and Clinicopathological Characteristics of High-Grade Pancreatic Intraepithelial Neoplasia: Autopsy Study Evaluating the Entire Pancreatic Parenchyma. Pancreas 2017, 46, 658–664. [Google Scholar] [CrossRef]
- Kimura, W.; Nagai, H.; Kuroda, A.; Muto, T.; Esaki, Y. Analysis of small cystic lesions of the pancreas. Int. J. Pancreatol. 1995, 18, 197–206. [Google Scholar]
- Mukada, T.; Yamada, S. Dysplasia and carcinoma in situ of the exocrine pancreas. Tohoku J. Exp. Med. 1982, 137, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Luttges, J.; Reinecke-Luthge, A.; Mollmann, B.; Menke, M.A.; Clemens, A.; Klimpfinger, M.; Sipos, B.; Kloppel, G. Duct changes and K-ras mutations in the disease-free pancreas: Analysis of type, age relation and spatial distribution. Virchows Arch. 1999, 435, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Andea, A.; Sarkar, F.; Adsay, V.N. Clinicopathological correlates of pancreatic intraepithelial neoplasia: A comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod. Pathol. 2003, 16, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Detlefsen, S.; Sipos, B.; Feyerabend, B.; Kloppel, G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005, 447, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Volkan Adsay, N.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar] [PubMed]
- Neesse, A.; Algul, H.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebours, V.; Gaujoux, S.; d’Assignies, G.; Sauvanet, A.; Ruszniewski, P.; Levy, P.; Paradis, V.; Bedossa, P.; Couvelard, A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin. Cancer Res. 2015, 21, 3522–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishiwata, T.; Izumiyama-Shimomura, N.; Hamayasu, H.; Fujiwara, M.; Tomita, K.; Hiraishi, N.; Nakamura, K.; Ishikawa, N.; Aida, J.; et al. Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS ONE 2015, 10, e0117575. [Google Scholar] [CrossRef]
- Yokode, M.; Akita, M.; Fujikura, K.; Kim, M.J.; Morinaga, Y.; Yoshikawa, S.; Terada, T.; Matsukiyo, H.; Tajiri, T.; Abe-Suzuki, S.; et al. High-grade PanIN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology 2018, 73, 247–258. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Cruz-Monserrate, Z.; Davis, B.M.; Edderkaoui, M.; Eibl, G.; Epouhe, A.Y.; Gedeon, J.Y.; Gorelick, F.S.; Grippo, P.J.; et al. Animal Models: Challenges and Opportunities to Determine Optimal Experimental Models of Pancreatitis and Pancreatic Cancer. Pancreas 2019, 48, 759–779. [Google Scholar] [CrossRef]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kitazawa, S.; Ito, S.; Takashima, Y.; Tsutsumi, M.; Denda, A.; Konishi, Y. Cycles of repeated augmentation pressure in rapid production of pancreatic and cholangiocellular carcinomas in hamsters initiated with N-nitrosobis(2-oxopropyl)amine. Carcinogenesis 1989, 10, 1457–1459. [Google Scholar] [CrossRef]
- Banerjee, J.; Papu John, A.M.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer in a hamster model by cAMP decrease. Oncotarget 2016, 7, 44430–44441. [Google Scholar] [CrossRef] [Green Version]
- Adachi, T.; Tajima, Y.; Kuroki, T.; Mishima, T.; Kitasato, A.; Fukuda, K.; Tsutsumi, R.; Kanematsu, T. Bile-reflux into the pancreatic ducts is associated with the development of intraductal papillary carcinoma in hamsters. J. Surg. Res. 2006, 136, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hori, M.; Mutoh, M.; Wakabayashi, K.; Nakagama, H. Experimental animal models of pancreatic carcinogenesis for prevention studies and their relevance to human disease. Cancers 2011, 3, 582–602. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Bardeesy, N.; Aguirre, A.J.; Chu, G.C.; Cheng, K.H.; Lopez, L.V.; Hezel, A.F.; Feng, B.; Brennan, C.; Weissleder, R.; Mahmood, U.; et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. USA 2006, 103, 5947–5952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, A.J.; Bardeesy, N.; Sinha, M.; Lopez, L.; Tuveson, D.A.; Horner, J.; Redston, M.S.; DePinho, R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003, 17, 3112–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izeradjene, K.; Combs, C.; Best, M.; Gopinathan, A.; Wagner, A.; Grady, W.M.; Deng, C.X.; Hruban, R.H.; Adsay, N.V.; Tuveson, D.A.; et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 2007, 11, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Kojima, K.; Vickers, S.M.; Adsay, N.V.; Jhala, N.C.; Kim, H.G.; Schoeb, T.R.; Grizzle, W.E.; Klug, C.A. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res 2007, 67, 8121–8130. [Google Scholar] [CrossRef] [Green Version]
- Hanlon, L.; Avila, J.L.; Demarest, R.M.; Troutman, S.; Allen, M.; Ratti, F.; Rustgi, A.K.; Stanger, B.Z.; Radtke, F.; Adsay, V.; et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res 2010, 70, 4280–4286. [Google Scholar] [CrossRef] [Green Version]
- Ijichi, H.; Chytil, A.; Gorska, A.E.; Aakre, M.E.; Fujitani, Y.; Fujitani, S.; Wright, C.V.; Moses, H.L. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006, 20, 3147–3160. [Google Scholar] [CrossRef] [Green Version]
- Skoulidis, F.; Cassidy, L.D.; Pisupati, V.; Jonasson, J.G.; Bjarnason, H.; Eyfjord, J.E.; Karreth, F.A.; Lim, M.; Barber, L.M.; Clatworthy, S.A.; et al. Germline Brca2 heterozygosity promotes Kras(G12D)-driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell 2010, 18, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Guerra, C.; Schuhmacher, A.J.; Canamero, M.; Grippo, P.J.; Verdaguer, L.; Perez-Gallego, L.; Dubus, P.; Sandgren, E.P.; Barbacid, M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007, 11, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Ji, B.; Tsou, L.; Wang, H.; Gaiser, S.; Chang, D.Z.; Daniluk, J.; Bi, Y.; Grote, T.; Longnecker, D.S.; Logsdon, C.D. Ras activity levels control the development of pancreatic diseases. Gastroenterology 2009, 137, 1072–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, J.L.; von Figura, G.; Mayes, E.; Liu, F.F.; Dubois, C.L.; Morris, J.P.; Pan, F.C.; Akiyama, H.; Wright, C.V.; Jensen, K.; et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 22, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Strobel, O.; Dor, Y.; Alsina, J.; Stirman, A.; Lauwers, G.; Trainor, A.; Castillo, C.F.; Warshaw, A.L.; Thayer, S.P. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007, 133, 1999–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suklabaidya, S.; Dash, P.; Das, B.; Suresh, V.; Sasmal, P.K.; Senapati, S. Experimental models of pancreatic cancer desmoplasia. Lab. Investig. 2018, 98, 27–40. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, K.; Guo, M.; Liu, Y.; Zheng, J. Progress in Animal Models of Pancreatic Ductal Adenocarcinoma. J. Cancer 2020, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
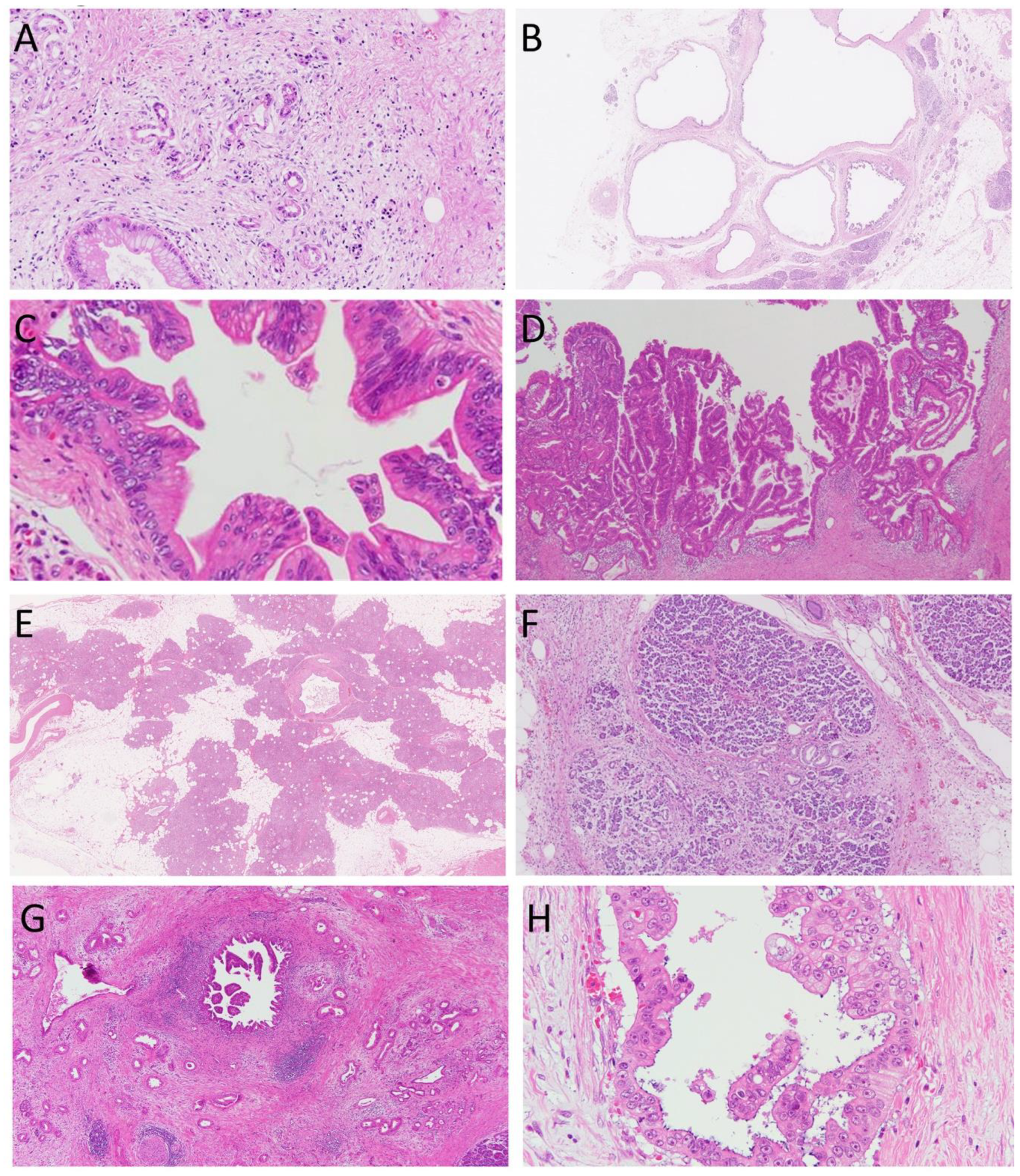
| Precursor lesions of pancreatic cancer |
| Acinar-to-ductal metaplasia (ADM) |
| Cyst |
| Pancreatic intraepithelial neoplasia (PanIN) |
| Intraductal papillary mucinous neoplasm (IPMN) |
| Surrogate markers of pancreatic cancer |
| Focal fatty replacement |
| Lobulocentric atrophy |
| Duct dilatation/Cyst/IPMN |
| Hamster | Precursor Lesions | |
|---|---|---|
| BOP | PanIN | Takahashi, 2018 |
| BOP, ethionine-methionine | PanIN | Mizumoto, 1989 |
| Bile reflux into the pancreatic duct | IPMN | Adachi, 2006 |
| Mouse | ||
| LSL-KrasG12D; Pdx1-Cre | PanIN | Hingorani, 2003 |
| LSL-KrasG12D; Ptf/p48-Cre | PanIN | Hingorani, 2003 |
| KIC model, Pdx-Cre; LSL-KrasG12D; Ink4a/Arflox/lox | PanIN | Aguirre, 2003 |
| Pdx-Cre; LSL-KrasG12D; Trp53lox/lox; Ink4a/Arflox/lox | PanIN | Bardeesy, 2006 |
| KPC model, Pdx1-Cre; LSL-KrasG12D; LSL-Trp53R172H/+ | PanIN | Hingorani, 2005 |
| KD model, Pdx-Cre; LSL-KrasG12D; SMAD4lox/lox | PanIN, IPMN | Kojima, 2007 |
| Ptf1a-Cre; LSL-KrasG12D; SMAD4lox/lox | PanIN, MCN | Izeradjene, 2007 |
| Ptf1a-Cre; LSL-KrasG12D; Notch1lox/lox | PanIN | Hanlon, 2010 |
| Ptf1a-Cre; LSL-KrasG12D; Tgfr2lox/lox | PanIN | Ijichi, 2006 |
| Pdx1-Cre; LSL-KrasG12D; Brca2tr/Δ11 | PanIN | Skoulidis, 2010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamakawa, K.; Ye, J.; Nakano-Narusawa, Y.; Matsuda, Y. Pathological Changes in Pancreatic Carcinogenesis: A Review. Cancers 2021, 13, 686. https://doi.org/10.3390/cancers13040686
Yamakawa K, Ye J, Nakano-Narusawa Y, Matsuda Y. Pathological Changes in Pancreatic Carcinogenesis: A Review. Cancers. 2021; 13(4):686. https://doi.org/10.3390/cancers13040686
Chicago/Turabian StyleYamakawa, Keiko, Juanjuan Ye, Yuko Nakano-Narusawa, and Yoko Matsuda. 2021. "Pathological Changes in Pancreatic Carcinogenesis: A Review" Cancers 13, no. 4: 686. https://doi.org/10.3390/cancers13040686






