Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
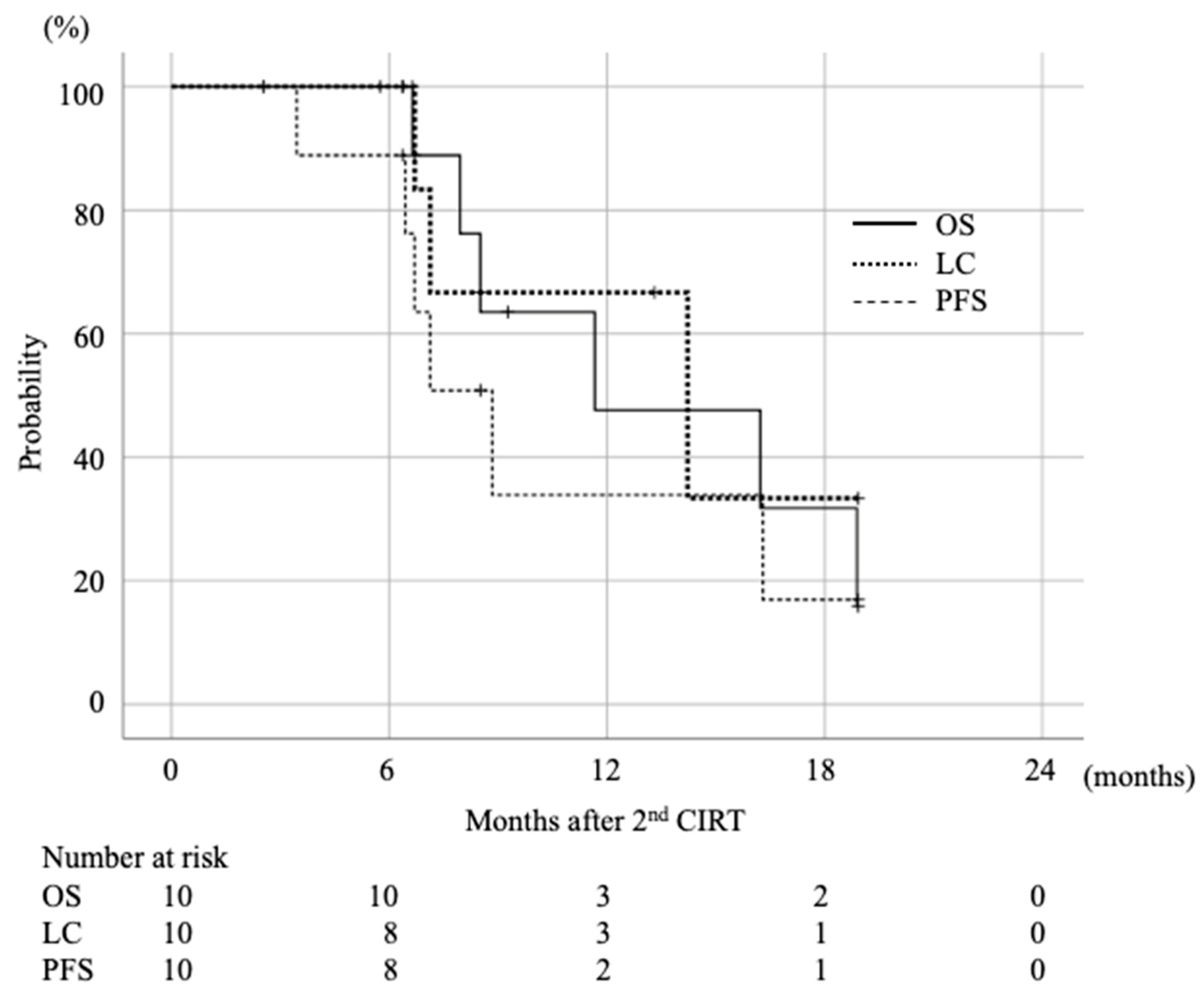
2.2. Survival and Local Control Rate
2.3. Toxicities
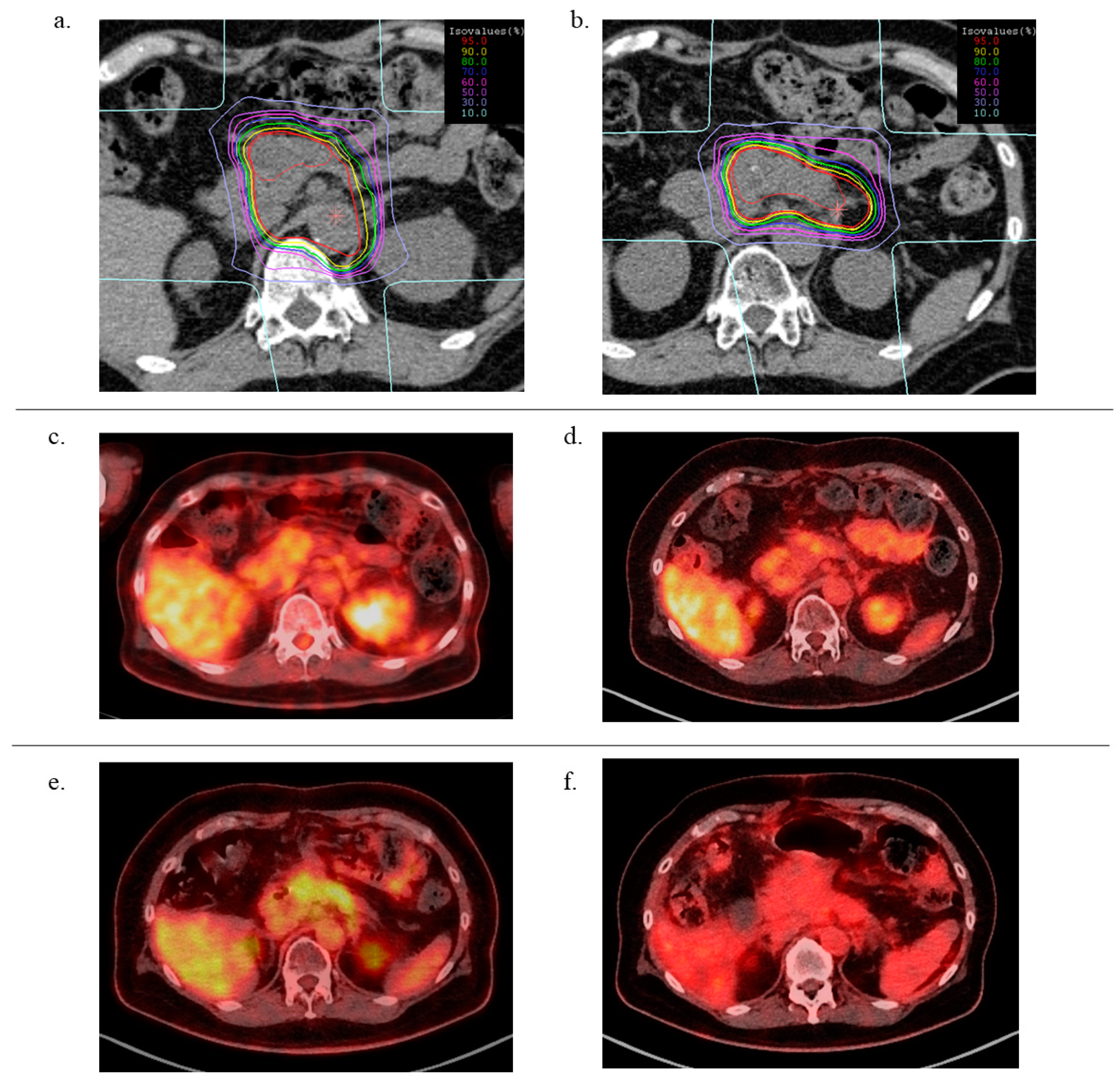
2.4. DVH Comparison
3. Discussion
4. Materials and Methods
4.1. Patient Eligibility
4.2. Carbon Ion Radiotherapy
4.3. Treatment Planning
4.4. Evaluation and Follow-Up
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Sun, X.-J.; Jiang, T.-H.; Mao, A.-W. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Kim, T.H.; Woo, S.M.; Lee, W.J.; Lee, J.H.; Youn, S.H.; Han, S.S.; Park, S.J.; Kim, D.Y. Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost-intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer. Radiat. Oncol. J. 2018, 36, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Nakamura, A.; Ashida, R.; Sakanaka, K.; Itasaka, S.; Shibuya, K.; Matsumoto, S.; Kanai, M.; Isoda, H.; Masui, T.; et al. Clinical evaluation of intensity-modulated radiotherapy for locally advanced pancreatic cancer. Radiat. Oncol. 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-Ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, Y.; Ohno, T.; Kato, S.; Suzuki, M.; Morita, S.; Sato, S.; Oka, K.; Tsujii, H. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin. Cancer Res. 2006, 12, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Oonishi, K.; Tsujii, H.; Yasuda, T.; Matsumoto, Y.; Furusawa, Y.; Akashi, M.; Kamada, T.; Okayasu, R. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011, 71, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Asano, T.; et al. Carbon Ion Radiation Therapy with Concurrent Gemcitabine for Patients with Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kawashiro, S.; Yamada, S.; Okamoto, M.; Ohno, T.; Nakano, T.; Shinoto, M.; Shioyama, Y.; Nemoto, K.; Isozaki, Y.; Tsuji, H.; et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Azuma, R.; Imai, T.; Shimokawa, T. Enhancement of mTOR signaling contributes to acquired X-ray and C-ion resistance in mouse squamous carcinoma cell line. Cancer Sci. 2017, 108, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nitta, N.; Aoki, I.; Imai, T.; Shimokawa, T. Repeated photon and C-ion irradiations in vivo have different impact on alteration of tumor characteristics. Sci. Rep. 2018, 8, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Yamada, S.; Isozaki, Y.; Takiyama, H.; Shinoto, M.; Kawashiro, S.; Bhattacharyya, T.; Nemoto, K.; Tsuji, H. Efficacy and feasibility of re-irradiation using carbon ions for pancreatic cancer that recurs after carbon-ion radiotherapy. Clin. Transl. Radiat. Oncol. 2021, 26, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lominska, C.E.; Unger, K.; Nasr, N.M.; Haddad, N.; Gagnon, G. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat. Oncol. 2012, 7, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dagoglu, N.; Callery, M.; Moser, J.; Tseng, J.; Kent, T.; Bullock, A.; Miksad, R.; Mancias, J.D.; Mahadevan, A. Stereotactic body radiotherapy (SBRT) reirradiation for recurrent pancreas cancer. J. Cancer 2016, 7, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Sutera, P.; Bernard, M.E.; Wang, H.; Bahary, N.; Burton, S.; Zeh, H.; Heron, D.E. Stereotactic body radiation therapy for locally progressive and recurrent pancreatic cancer after prior radiation. Front. Oncol. 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.T.; Hiniker, S.M.; Chang, D.T.; Tran, P.T.; Khashab, M.A.; Limaye, M.R.; Laheru, D.A.; Le, D.T.; Kumar, R.; Pai, J.S.; et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J. Gastrointest. Oncol. 2013, 4, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Boimel, P.J.; Berman, A.T.; Li, J.; Apisarnthanarax, S.; Both, S.; Lelionis, K.; Larson, G.L.; Teitelbaum, U.; Lukens, J.N.; Ben-Josef, E.; et al. Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2017, 8, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Koto, M.; Ikawa, H.; Hagiwara, Y.; Tsuji, H.; Ogawa, K.; Kamada, T. Feasibility of Re-irradiation using carbon ions for recurrent head and neck malignancies after carbon-ion radiotherapy. Radiother. Oncol. 2019, 136, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, N.; Karube, M.; Nakajima, M.; Tsuji, H.; Ogawa, K.; Kamada, T. Feasibility of carbon-ion radiotherapy for re-irradiation of locoregionally recurrent, metastatic, or secondary lung tumors. Cancer Sci. 2018, 109, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Karube, M.; Yamamoto, N.; Tsuji, H.; Kanematsu, N.; Nakajima, M.; Yamashita, H.; Nakagawa, K.; Kamada, T. Carbon-ion re-irradiation for recurrences after initial treatment of stage I non-small cell lung cancer with carbon-ion radiotherapy. Radiother. Oncol. 2017, 125, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Kanematsu, N.; Matsufuji, N.; Kanai, T.; Shirai, T.; Noda, K.; Tsuji, H.; Kamada, T.; Tsujii, H. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys. Med. Biol. 2015, 60, 3271–3286. [Google Scholar] [CrossRef] [PubMed]
| pt.# | Age | Sex | 1st CIRT | 2nd CIRT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNM (UICC 7th) | RT Dose (Gy(RBE)) | Tumor Location | Concurrent CT | Adjuvant CT | CIRT Interval (M) | Tumor Location | RT Dose (Gy(RBE)) | Concurrent CT | Adjuvant CT | |||
| 1 | 68 | F | T4N0M0 | 55.2 | Pb | GEM | GEM | 25.3 | in-field | 52.8 | GEM | GEM |
| 2 | 63 | F | T4N1M0 | 55.2 | Pt | S-1 | S-1 | 24.6 | in-field | 55.2 | GEM | GEM |
| 3 | 78 | M | T4N0M0 | 55.2 | Ph | S-1 | GnP | 12.7 | out-field | 55.2 | S-1 | S-1 |
| 4 | 49 | M | T4N0M0 | 55.2 | Pb | GEM | GEM | 50.1 | in-field | 55.2 | GEM | GEM |
| 5 | 45 | M | T4N0M0 | 55.2 | Pb | S-1 | S-1 | 8.0 | in-field | 55.2 | S-1 | S-1 |
| 6 | 51 | M | T4N0M0 | 55.2 | Pb | GEM | GEM | 9.3 | out-field | 55.2 | GEM | GnP |
| 7 | 70 | F | T4N0M0 | 55.2 | Pb | S-1 | S-1 | 11.0 | in-field | 55.2 | S-1 | S-1 |
| 8 | 55 | F | T4N0M0 | 55.2 | Pb | S-1 | S-1 | 13.8 | in-field | 55.2 | S-1 | none |
| 9 | 67 | M | T4N0M0 | 55.2 | Ph | GEM | GnP | 26.0 | in-field | 55.2 | none | S-1 |
| 10 | 78 | M | T4N0M0 | 55.2 | Pb | GEM | GEM | 17.8 | in-field | 55.2 | none | GEM |
| Factor | Category | Number | 95% CI | p-Value |
|---|---|---|---|---|
| age | ≥65 | 5 | 6.9–8.8 | 0.695 |
| <65 | 5 | 9.3–18.9 | ||
| duration for recurrence | ≥1 year | 7 | 11.0–21.0 | 0.224 |
| <1 year | 3 | 3.1–17.8 | ||
| recurrence site | in field | 8 | 9.5–18.9 | 0.450 |
| out of field | 2 | 6.4–8.9 | ||
| GTV V95 (%) | ≥90 | 7 | 13.3–19.8 | 0.018 |
| <90 | 3 | 6.5–7.3 | ||
| GTV V90 (%) | ≥90 | 8 | 10.3–18.9 | 0.247 |
| <90 | 2 | 7.1–7.1 |
| Adverse Event | Grade 0–1 | Grade 2 | Grade 3 | Grade 4–5 |
|---|---|---|---|---|
| upper GI | 8 | 2 | 0 | 0 |
| lower GI | 7 | 2 | 1 | 0 |
| biliary tract | 4 | 6 | 0 | 0 |
| portal vein | 7 | 3 | 0 | 0 |
| dermatitis | 10 | 0 | 0 | 0 |
| peripheral nerve | 10 | 0 | 0 | 0 |
| pain | 10 | 0 | 0 | 0 |
| DVH Parameters | Mean (SD) | p-Value | |
|---|---|---|---|
| GTV volume (cc) | 1st CIRT | 21.9 (16.8) | 0.47 |
| 2nd CIRT | 16.6 (15.7) | ||
| CTV volume (cc) | 1st CIRT | 140.1 (37.6) | <0.001 |
| 2nd CIRT | 38.6 (22.9) | ||
| GTV V90%dose (%) | 1st CIRT | 96.8 (3.5) | 0.97 |
| 2nd CIRT | 96.7 (3.6) | ||
| GTV V95%dose (%) | 1st CIRT | 93.4 (6.3) | 0.79 |
| 2nd CIRT | 92.6 (7.1) | ||
| Stomach—D2cc (Gy(RBE)) | 1st CIRT | 32.1 (6.5) | 0.004 |
| 2nd CIRT | 17.5 (11.5) | ||
| Stomach—V30Gy (cc) | 1st CIRT | 4.3 (3.0) | 0.004 |
| 2nd CIRT | 0.7 (0.9) | ||
| Duodenum—D2cc (Gy(RBE)) | 1st CIRT | 29.5 (7.0) | 0.21 |
| 2nd CIRT | 24.9 (8.4) | ||
| Duodenum—V30Gy (cc) | 1st CIRT | 3.4 (2.8) | 0.47 |
| 2nd CIRT | 2.4 (3.0) | ||
| Intestine—D2cc (Gy(RBE)) | 1st CIRT | 32.1 (7.2) | 0.02 |
| 2nd CIRT | 22.1 (10.2) | ||
| Intestine—V30Gy (cc) | 1st CIRT | 6.4 (3.7) | 0.004 |
| 2nd CIRT | 1.7 (2.7) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, M.; Shiba, S.; Okazaki, S.; Miyasaka, Y.; Shibuya, K.; Kiyohara, H.; Ohno, T. Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer. Cancers 2021, 13, 665. https://doi.org/10.3390/cancers13040665
Okamoto M, Shiba S, Okazaki S, Miyasaka Y, Shibuya K, Kiyohara H, Ohno T. Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer. Cancers. 2021; 13(4):665. https://doi.org/10.3390/cancers13040665
Chicago/Turabian StyleOkamoto, Masahiko, Shintaro Shiba, Shohei Okazaki, Yuhei Miyasaka, Kei Shibuya, Hiroki Kiyohara, and Tatsuya Ohno. 2021. "Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer" Cancers 13, no. 4: 665. https://doi.org/10.3390/cancers13040665
APA StyleOkamoto, M., Shiba, S., Okazaki, S., Miyasaka, Y., Shibuya, K., Kiyohara, H., & Ohno, T. (2021). Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer. Cancers, 13(4), 665. https://doi.org/10.3390/cancers13040665






