Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.2.1. Evaluation Items
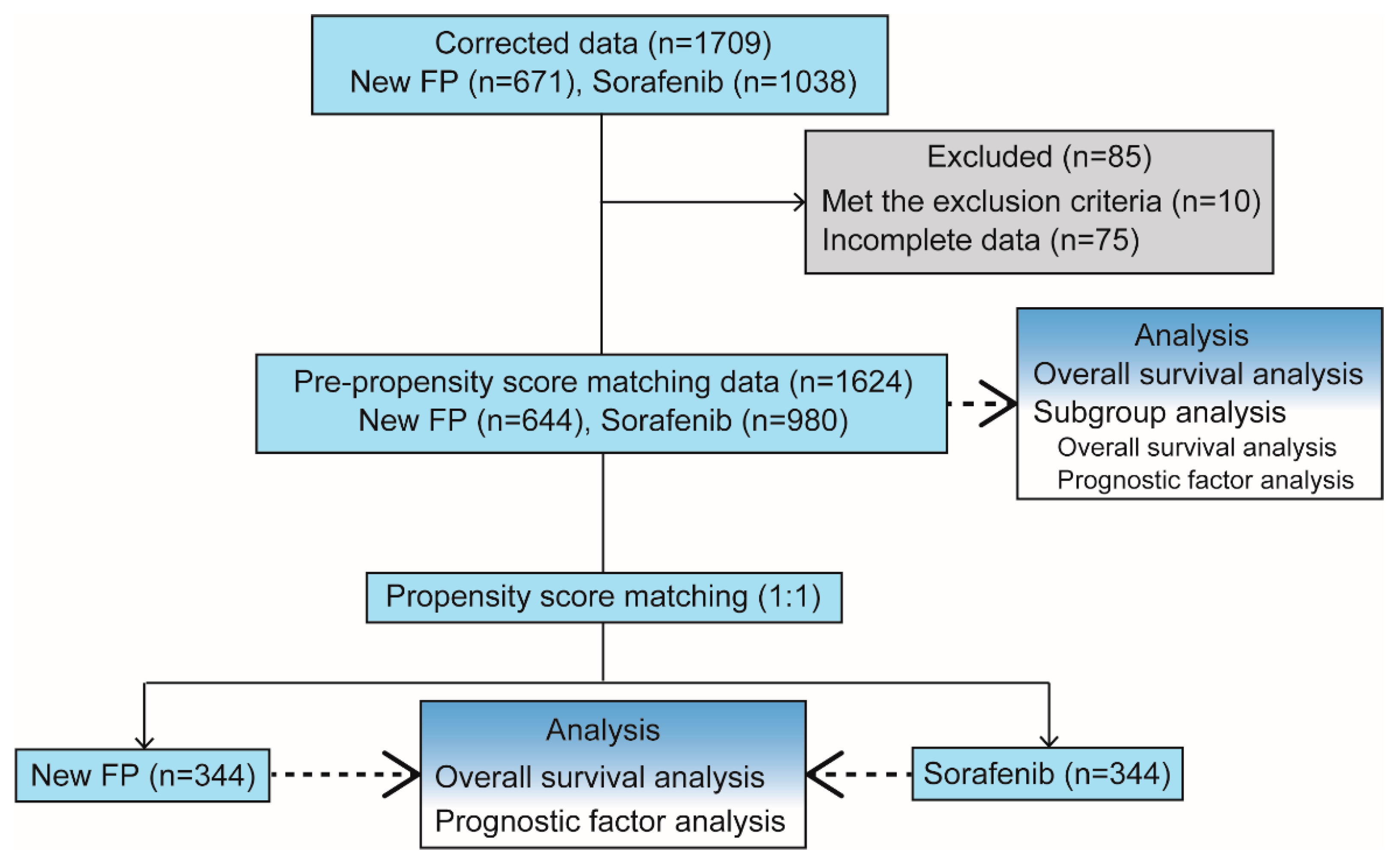
2.2.2. Refinement to Avoid Bias
2.2.3. PSM
2.3. Treatment Protocol
2.3.1. Sorafenib
2.3.2. New-FP
2.3.3. Catheter Implantation Procedure for HAIC
2.3.4. HAIC Regimen: New-FP
2.4. Statistical Analyses
3. Conclusions
3.1. Patient and Tumor Characteristics
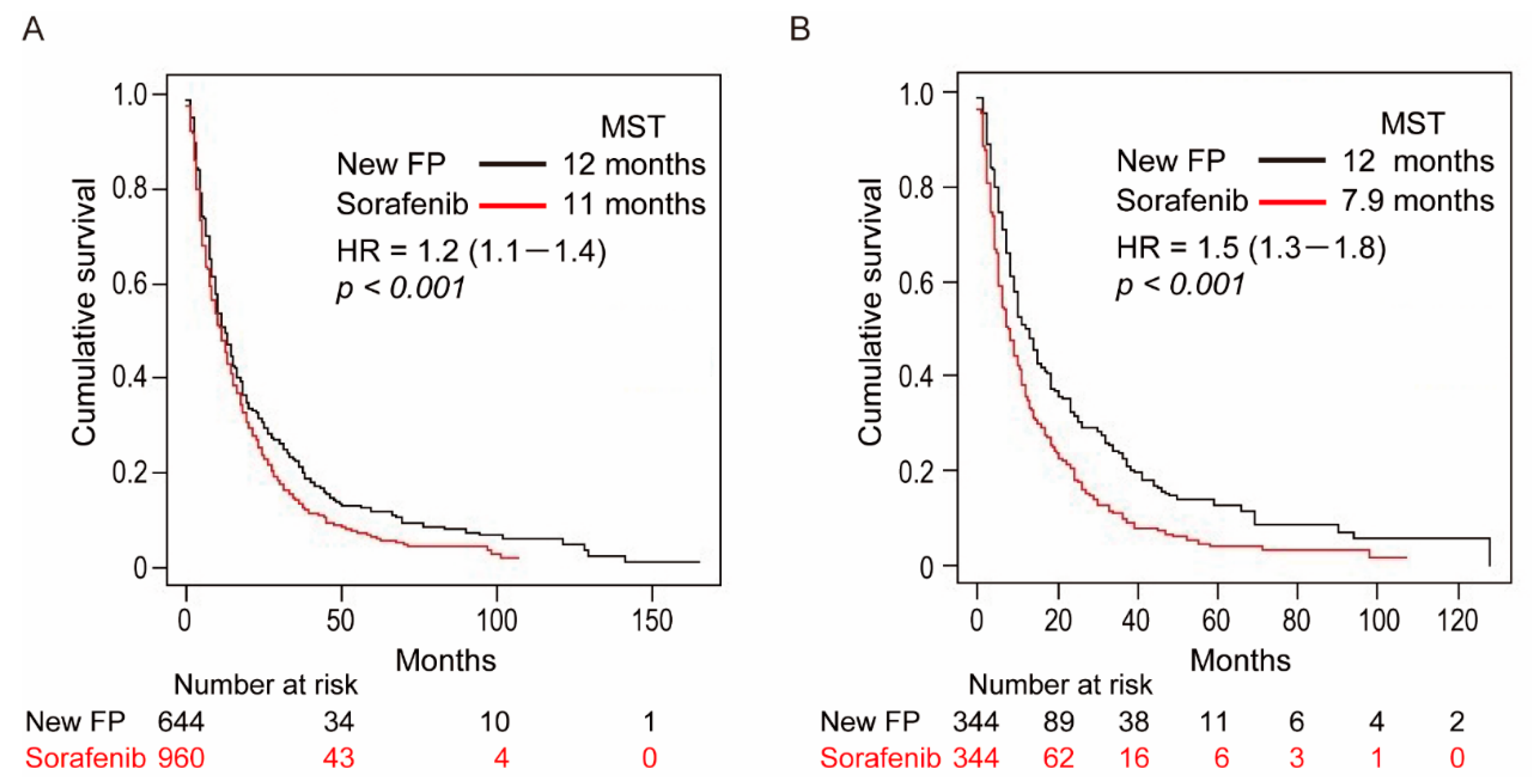
3.2. OS Curves before PSM
3.3. OS Curves after PSM
3.4. Prognostic Analysis for Survival before and after PSM
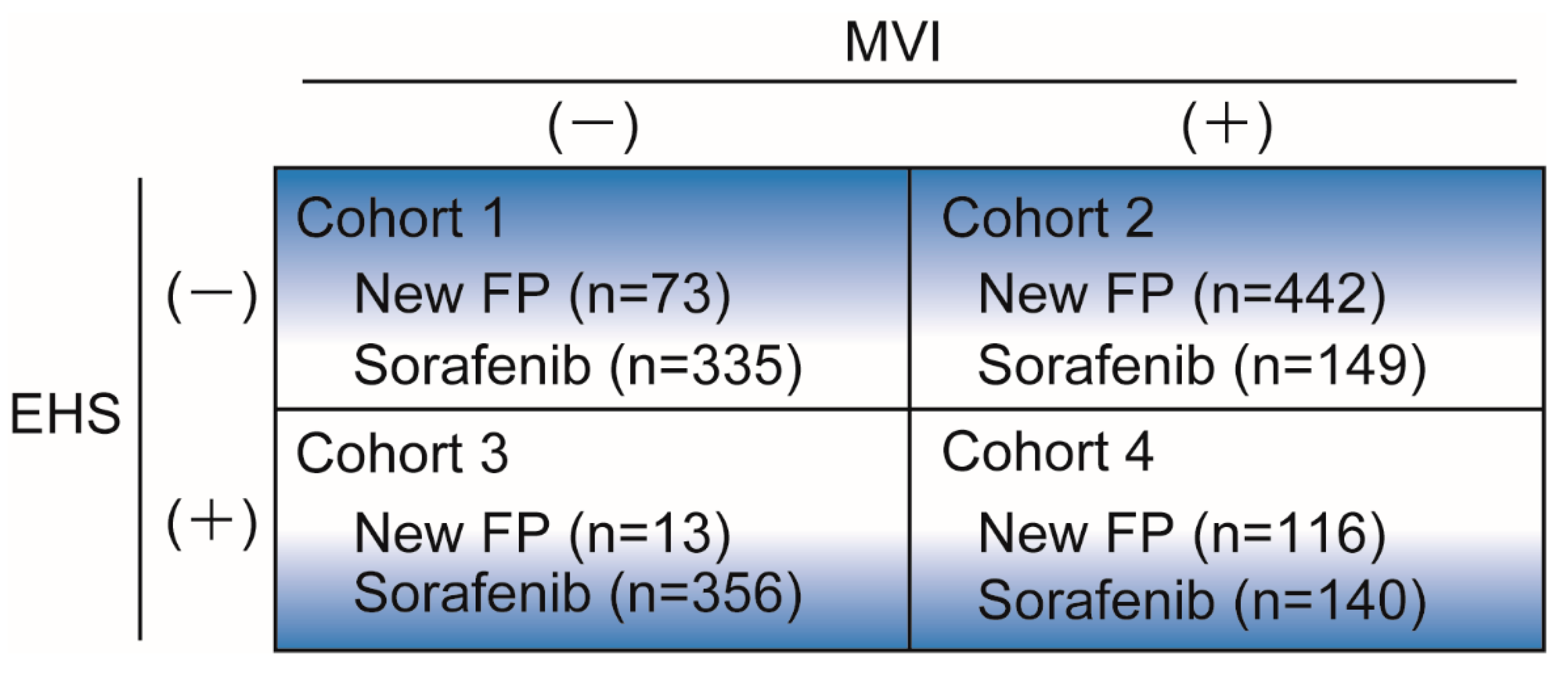
3.5. Subgroup Analysis in the Four Cohort Groups
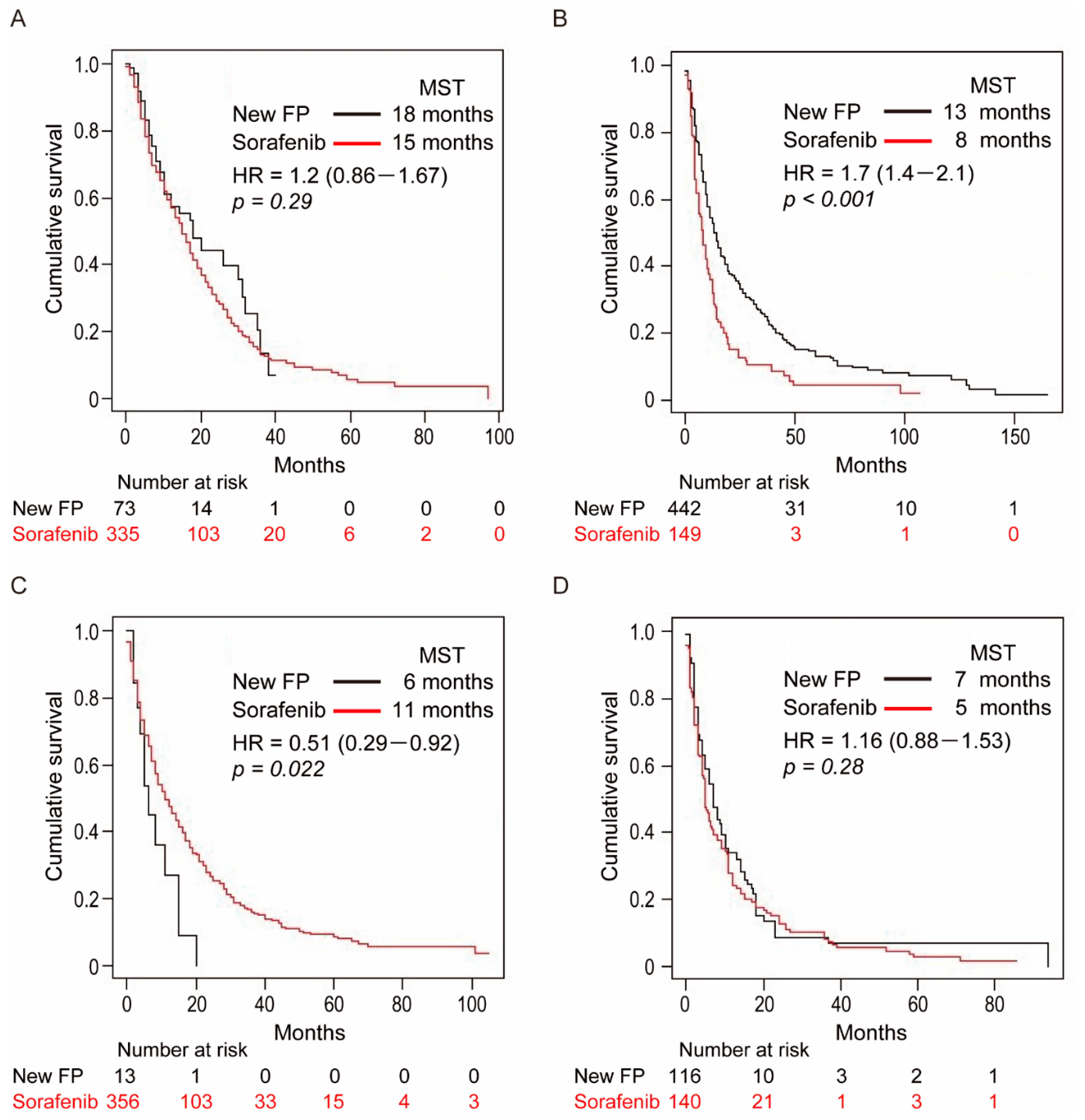
3.6. Cohort-1: Without MVI and EHS
3.7. Cohort-2: With MVI and without EHS
3.8. Cohort-3: Without MVI and with EHS
3.9. Cohort-4: With MVI and EHS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M. Management of Hepatocellular Carcinoma in Japan: Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Aino, H.; Sumie, S.; Niizeki, T.; Kuromatsu, R.; Tajiri, N.; Nakano, M.; Satani, M.; Yamada, S.; Okamura, S.; Shimose, S.; et al. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol. Clin. Oncol. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ando, E.; Tanaka, M.; Yamashita., F.; Kuromatsu, R.; Yutani, S.; Fukumori, K.; Sumie, S.; Yano, Y.; Okuda, K.; Sata, M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer 2002, 95, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Okusaka, T.; Furuse, J.; Mitsunaga, S.; Ueno, H.; Yamaura, H.; Inaba, Y.; Takeuchi, Y.; Satake, M.; Arai, Y. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2013, 72, 463–470. [Google Scholar] [CrossRef]
- Niizeki, T.; Sumie, S.; Torimura, T.; Kurogi, J.; Kuromatsu, R.; Iwamoto, H.; Aino, H.; Nakano, M.; Kawaguchi, A.; Kakuma, T.; et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J. Gastroenterol. 2012, 47, 686–695. [Google Scholar] [CrossRef]
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Hepatic Arterial Infusion Chemotherapy Using Low-Dose 5-Fluorouracil and Cisplatin for Advanced Hepatocellular Carcinoma. Oncology 2010, 78 (Suppl. S1), 148–153. [Google Scholar] [CrossRef] [PubMed]
- Murakami, E.; Aikata, H.; Miyaki, D.; Nagaoki, Y.; Katamura, Y.; Kawaoka, T.; Takaki, S.; Hiramatsu, A.; Waki, K.; Takahashi, S.; et al. Hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic interferon-α for advanced hepatocellular carcinoma in combination with or without three-dimensional conformal radiotherapy to venous tumor thrombosis in hepatic vein or inferior vena. Hepatol. Res. 2011, 42, 442–453. [Google Scholar] [CrossRef]
- Obi, S.; Sato, S.; Kawai, T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer 2015, 4, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kimura, T.; Kurokawa, F.; Aoyama, K.; Ishikawa, T.; Tajima, K.; Yokoyama, Y.; Takami, T.; Omori, K.; Kawaguchi, K.; et al. Prognostic factors in patients with advanced hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. J. Gastroenterol. 2005, 40, 70–78. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A randomized clinical trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimizu, S.; Sato, T.; Morimoto, M.; Kojima, Y.; Inaba, Y.; Hagihara, A.; Kudo, M.; Nakamori, S.; Kaneko, S.; et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann. Oncol. 2016, 27, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Nagamatsu, H.; Hiraki, M.; Mizukami, N.; Yoshida, H.; Iwamoto, H.; Sumie, S.; Torimura, T.; Sata, M. Intra-arterial therapy with cisplatin suspension in lipiodol and 5-fluorouracil for hepatocellular carcinoma with portal vein tumour thrombosis. Aliment. Pharmacol. Ther. 2010, 32, 543–550. [Google Scholar] [CrossRef]
- Nagamatsu, H.; Sumie, S.; Niizeki, T.; Tajiri, N.; Iwamoto, H.; Aino, H.; Nakano, M.; Shimose, S.; Satani, M.; Okamura, S.; et al. Hepatic arterial infusion chemoembolization therapy for advanced hepatocellular carcinoma: Multicenter phase II study. Cancer Chemother. Pharmacol. 2016, 77, 243–250. [Google Scholar] [CrossRef]
- Nakano, M.; Niizeki, T.; Nagamatsu, H.; Tanaka, M.; Kuromatsu, R.; Satani, M.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; et al. Clinical effects and safety of intra-arterial infusion therapy of cisplatin suspension in lipiodol combined with 5-fluorouracil versus sorafenib, for advanced hepatocellular carcinoma with macroscopic vascular invasion without extra-hepatic spread: A prospective cohort study. Mol. Clin. Oncol. 2017, 7, 1013–1020. [Google Scholar] [CrossRef][Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumor-itropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Shimose, S.; Tanaka, M.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Aino, H.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol. Res. 2019, 49, 919–928. [Google Scholar] [CrossRef]
- Sastre, J.; Díaz-Beveridge, R.; García-Foncillas, J.; Guardeño, R.; Lopez, C.; Pazo, R.; Rodríguez-Salas, N.; Salgado, M.; Salvia, A.S.; Feliú, J. Clinical guideline SEOM: Hepatocellular carcinoma. Clin. Transl. Oncol. 2015, 17, 988–995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, D.-Y.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: Updates and insights. HepatoBiliary Surg. Nutr. 2020, 9, 452–463. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, S.H.; McCarthy, C.J.; Lavelle, L.P.; Moran, D.E.; Cantwell, C.P.; Skehan, S.J.; Gibney, R.G.; Malone, D.E. Hepatocellular Carcinoma: Illustrated Guide to Systematic Radiologic Diagnosis and Staging According to Guidelines of the American Association for the Study of Liver Diseases. Radiographics 2013, 33, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL–EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Chong, C.C.N.; Chan, A.W.H.; Poon, D.M.C.; Chok, K.S.H. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J. Gastroenterol. 2016, 22, 7289–7300. [Google Scholar] [CrossRef]
- Jeong, S.W.; Jang, J.Y.; Shim, K.Y.; Lee, S.H.; Kim, S.G.; Cha, S.-W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; Kim, B.S.; et al. Practical Effect of Sorafenib Monotherapy on Advanced Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis. Gut Liver 2013, 7, 696–703. [Google Scholar] [CrossRef]
- Okuda, K.; Tanaka, M.; Shibata, J.; Ando, E.; Ogata, T.; Kinoshita, H.; Eriguchi, N.; Aoyagi, S.; Tanikawa, K. Hepatic arterial infusion chemotherapy with continuous low dose administration of cisplatin and 5-fluorouracil for multiple recurrence of hepatocellular carcinoma after surgical treatment. Oncol. Rep. 1999, 6, 587–678. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Bajwa, R.; Madoff, D.C. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers 2020, 12, 791. [Google Scholar] [CrossRef]
- Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Terashima, T.; Yamashita, T.; Mizukoshi, E.; Sakai, A.; Nakamoto, Y.; Honda, M.; et al. Randomized, Phase II Study Comparing Interferon Combined with Hepatic Arterial Infusion of Fluorouracil plus Cisplatin and Fluorouracil Alone in Patients with Advanced Hepatocellular Carcinoma. Oncology 2011, 81, 281–290. [Google Scholar] [CrossRef]
- Nouso, K.; Miyahara, K.; Uchida, D.; Kuwaki, K.; Izumi, N.; Omata, M.; Ichida, T.; Kudo, M.; Ku, Y.; Kokudo, N.; et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br. J. Cancer 2013, 109, 1904–1907. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.-L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chiba, Y.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Objective Response by mRECIST Is an Independent Prognostic Factor for Overall Survival in Hepatocellular Carcinoma Treated with Sorafenib in the SILIUS Trial. Liver Cancer 2019, 8, 505–519. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Merle, P. The New Immuno-Oncology-Based Therapies and Their Perspectives in Hepatocellular Carcinoma. Cancers 2021, 13, 238. [Google Scholar] [CrossRef]
- Li, S.; Ji, J.; Zhang, Z.; Peng, Q.; Hao, L.; Guo, Y.; Zhou, W.; Cui, Q.; Shi, X. Cisplatin promotes the expression level of PD-L1 in the microenvironment of hepatocellular carcinoma through YAP1. Mol. Cell. Biochem. 2020, 475, 79–91. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Y.; Che, X.; Hou, K.; Zhang, C.; Li, C.; Wen, T.; Wang, S.; Cheng, Y.; Liu, Y.; et al. 5-FU-Induced Upregulation of Exosomal PD-L1 Causes Immunosuppression in Advanced Gastric Cancer Patients. Front. Oncol. 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; He, B.; Mu, W.; Liu, K.; Liu, L.; Zeng, H.; Liu, Y.; Jiang, L.; Zhou, P.; Huang, Z.; et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics 2021, 11, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
| Before Matching n = 1624 | After Matching n = 688 | |||||
|---|---|---|---|---|---|---|
| New-FP n = 644 | Sorafenib n = 980 | p-Value | New-FP n = 344 | Sorafenib n = 344 | p-Value | |
| Patient characteristics | ||||||
| Age (years) | 67.94±11.02 | 70.11±9.52 | <0.001 | 68.50 ± 11.02 | 68.35 ± 10.33 | 0.856 |
| Sex Male/Female | 505/139 | 771/209 | 0.951 | 267/77 | 271/73 | 0.782 |
| HCV | 300/344 | 546/414 | <0.001 | 168/176 | 152/192 | 0.252 |
| HBV | 123/521 | 179/801 | 0.721 | 71/273 | 76/268 | 0.710 |
| Child-Pugh class A/B/C | 405/218/21 | 809/169/2 | <0.001 | 244 /98/2 | 252/90/2 | 0.791 |
| Tumor characteristics | ||||||
| BCLC stage B/C/D | 71/571/2 | 350/630/0 | <0.001 | 70/274/0 | 89/255/0 | 0.0857 |
| Tumor size (mm) | 110.95 ± 52.83 | 86.84 ± 60.43 | <0.001 | 109.74 ± 53.42 | 107.49 ± 54.58 | 0.585 |
| MVI | 558/86 | 289/691 | <0.001 | 259/85 | 260/84 | 1.000 |
| Severe MVI | 331/313 | 168/812 | <0.001 | 129/215 | 152/192 | 0.088 |
| EHS | 129/515 | 496/484 | <0.001 | 114/230 | 122/222 | 0.574 |
| AFP (ng/mL) | 624.20 ± 360.28 | 609.99 ± 350.87 | 0.430 | 598.04 ± 358.41 | 619.26 ± 342.57 | 0.428 |
| DCP (mAU/mL) | 591.42 ± 346.46 | 599.33 ± 349.91 | 0.655 | 589.06 ± 342.95 | 612.01 ± 365.21 | 0.396 |
| Factors | Unit | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Sorafenib treatment | N/A | 1.41 | 1.19–1.68 | <0.001 |
| Presence of severe MVI | N/A | 1.53 | 1.28–1.82 | <0.001 |
| Presence of EHS | N/A | 1.51 | 1.26–1.81 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Nomura, T.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers 2021, 13, 646. https://doi.org/10.3390/cancers13040646
Iwamoto H, Niizeki T, Nagamatsu H, Ueshima K, Nomura T, Kuzuya T, Kasai K, Kooka Y, Hiraoka A, Sugimoto R, et al. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers. 2021; 13(4):646. https://doi.org/10.3390/cancers13040646
Chicago/Turabian StyleIwamoto, Hideki, Takashi Niizeki, Hiroaki Nagamatsu, Kazuomi Ueshima, Takako Nomura, Teiji Kuzuya, Kazuhiro Kasai, Yohei Kooka, Atsushi Hiraoka, Rie Sugimoto, and et al. 2021. "Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma" Cancers 13, no. 4: 646. https://doi.org/10.3390/cancers13040646
APA StyleIwamoto, H., Niizeki, T., Nagamatsu, H., Ueshima, K., Nomura, T., Kuzuya, T., Kasai, K., Kooka, Y., Hiraoka, A., Sugimoto, R., Yonezawa, T., Ishihara, A., Deguchi, A., Arai, H., Shimose, S., Shirono, T., Nakano, M., Okamura, S., Noda, Y., ... Kurume Liver Cancer Study Group of Japan. (2021). Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers, 13(4), 646. https://doi.org/10.3390/cancers13040646








