Long-Term Outcome and Role of Biology within Risk-Adapted Treatment Strategies: The Austrian Neuroblastoma Trial A-NB94
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
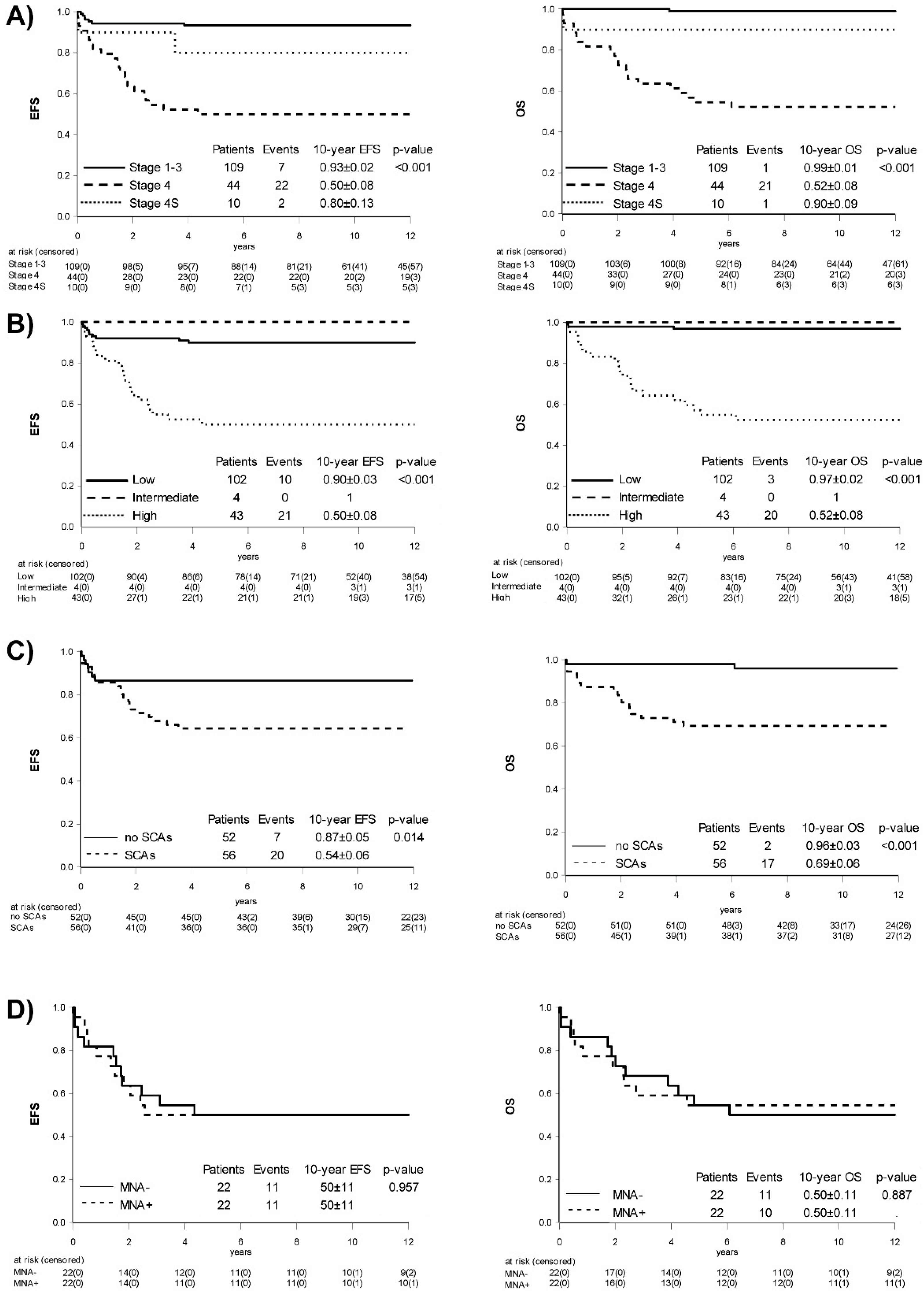
2.1. Trial Population and Overall Outcome
2.2. Influence of Stage
2.3. Role of Age
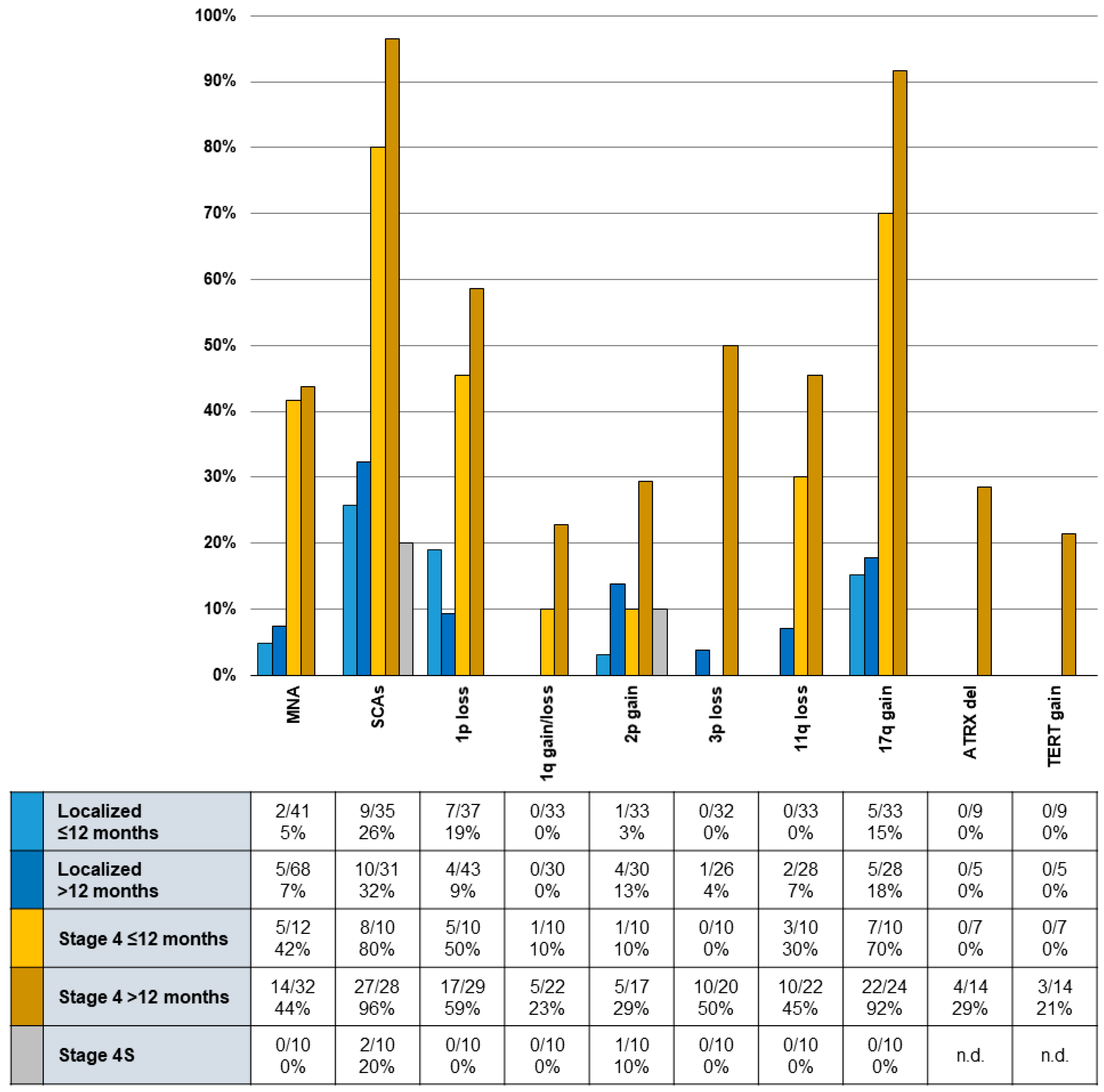
2.4. Occurrence and Influence of Biomarkers
2.5. Role of Treatment Elements to Achieve Remission Induction
2.5.1. Surgery
2.5.2. High-Dose Therapy (HDT)
2.6. Acute Toxicities and Long-Term Morbidity
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Treatment Concepts
4.3. Disease and Response Assessment
4.4. Toxicity Evaluation
4.5. Biologic Studies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| A-NB94 Staging Criteria | Retrospective INRG Risk Classification | ||||||
|---|---|---|---|---|---|---|---|
| MYCN | Stage | Age | Low | Intermediate | High | n.e. | Total |
| amplified | 1 | ≤12 | 1 | 1 | |||
| >12 | 1 | 1 | |||||
| 2 | ≤12 | ||||||
| >12 | |||||||
| 3 | ≤12 | 1 | 1 | ||||
| >12 | 4 | 4 | |||||
| 4 | ≤12 | 5 | 5 | ||||
| >12 | 14 | 14 | |||||
| non-amplified | 1 | ≤12 | 15 | 15 | |||
| >12 | 46 | 46 | |||||
| 2 | ≤12 | 9 | 9 | ||||
| >12 | 10 | 10 | |||||
| 3 | ≤12 | 8 | 4 | 12 | |||
| >12 | 3 | 3 | 1 | 7 | |||
| 4 | ≤12 | 4 | 1 | 1 | 6 | ||
| >12 | 17 | 1 | 18 | ||||
| 4S | ≤12 | 4 | 6 | 10 | |||
| heterogeneously amplified | 1 | ≤12 | 1 | 1 | |||
| >12 | |||||||
| 2 | ≤12 | 1 | 1 | ||||
| >12 | |||||||
| 3 | ≤12 | 1 | 1 | ||||
| >12 | |||||||
| 4 | ≤12 | 1 | 1 | ||||
| >12 | |||||||
| Total | 102 | 4 | 43 | 14 | 163 | ||
| Type of Toxicity | Induction Phase | Surgery | HDT Phase | |
|---|---|---|---|---|
| LD/ID | HD | |||
| infection | 4 | 10 | 4 | 13 |
| gastrointestinal | 3 | 8 | ||
| hepatic injury | 3 | |||
| venous occlusive disease | 1 | |||
| cardiac | 2 | 3 | 4 | |
| renal failure | 2 | |||
| hemorrhagic cystitis | 1 | |||
| respiratory insufficiency | 1 | |||
| oral-intestinal mucositis | 5 | 12 | ||
| tumor lysis syndrome | 1 | |||
| capillary leak syndrome | 1 | 1 | ||
| hemolytic uremic syndrome | 1 | |||
| autoimmune hemolytic anemia | 1 | |||
| non-febrile seizures | 1 | |||
| intraoperative tumor rupture | 2 | |||
| severe bleeding | 4 | |||
| lymphatic fistula | 4 | |||
| Horner’s syndrome | 3 | |||
| pleural effusion | 2 | |||
| pneumothorax | 1 | |||
| cava vein thrombosis | 1 | |||
| Total | 6 | 26 | 21 | 46 |
| Stage | Therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | Surgery | ||||||||
| IIA | Surgery | If macroscopic residual tumor: second surgery, or radiotherapy | |||||||
| IIB | Surgery | CV | Re-surgery | Radiotherapy | |||||
| IIIA | Surgery | 3–4 × alternating DAMO/MVDOC | Re-surgery | Radiotherapy | (Re-surgery) | ||||
| IIIB | Surgery | 3–5 × alternating DAMO/MVDOC/IPE | Re-surgery | Radiotherapy | Re-surgery | ||||
| IV | Surgery | 4 × alternating MVDOC/IPE | Re-surgery | HDT/TBI/ASCR | Radiotherapy | ||||
| Details on Chemotherapy | |||||||||
| Chemotherapy | Abbreviation | Substance | Dosage | Days Given | |||||
| DAMO | D | dacarbazine | 850 mg/m² | 1 | |||||
| A | doxorubicin | 30 mg/m² | 1, 2 | ||||||
| M | mustargen | 6 mg/m² | 1 | ||||||
| O | vincristine | 1.5 mg/m² | 1 + 5 | ||||||
| MVDOC | M | mustargen | 6 mg/m² | 1 | |||||
| V | teniposide | 150 mg/m² | 1 | ||||||
| D | dacarbacine | 850 mg/m² | 1 | ||||||
| O | vincristine | 1.5 mg/m² | 1 | ||||||
| C | cyclophosphamide | 850 mg/m² | 1 | ||||||
| IPE | I | ifosfamide | 3 g/m² | 1, 2 | |||||
| P | cisplatin | 40 mg/m² | 1–5 | ||||||
| E | etoposide | 150 mg/m² | 3–5 | ||||||
References
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Cohn, S.L.; Rademaker, A.W.; Salwen, H.R.; Franklin, W.A.; Gonzales-Crussi, F.; Rosen, S.T.; Bauer, K.D. Analysis of DNA ploidy and proliferative activity in relation to histology and N-myc amplification in neuroblastoma. Am. J. Pathol. 1990, 136, 1043–1052. [Google Scholar] [CrossRef][Green Version]
- Jereb, B.; Bretsky, S.S.; Vogel, R.; Helson, L. Age and prognosis in neuroblastoma. Review of 112 patients younger than 2 years. Am. J. Pediatr. Hematol. Oncol. 1984, 6, 233–243. [Google Scholar] [CrossRef]
- Paul, S.R.; Tarbell, N.J.; Korf, B.; Kretschmar, C.S.; Lavally, B.; Grier, H.E. Stage IV neuroblastoma in infants. Long-term survival. Cancer 1991, 67, 1493–1497. [Google Scholar] [CrossRef]
- Nitschke, R.; Smith, E.I.; Shochat, S.; Altshuler, G.; Travers, H.; Shuster, J.J.; Hayes, F.A.; Patterson, R.; McWilliams, N. Localized neuroblastoma treated by surgery: A Pediatric Oncology Group Study. J. Clin. Oncol. 1988, 6, 1271–1279. [Google Scholar] [CrossRef]
- Matthay, K.K.; Sather, H.N.; Seeger, R.C.; Haase, G.M.; Hammond, G.D. Excellent outcome of stage II neuroblastoma is independent of residual disease and radiation therapy. J. Clin. Oncol. 1989, 7, 236–244. [Google Scholar] [CrossRef]
- Garaventa, A.; De Bernardi, B.; Pianca, C.; Donfrancesco, A.; Cordero di Montezemolo, L.; Di Tullio, M.T.; Bagnulo, S.; Mancini, A.; Carli, M.; Pession, A.; et al. Localized but unresectable neuroblastoma: Treatment and outcome of 145 cases. Italian Cooperative Group for Neuroblastoma. J. Clin. Oncol. 1993, 11, 1770–1779. [Google Scholar] [CrossRef]
- Perez, C.A.; Matthay, K.K.; Atkinson, J.B.; Seeger, R.C.; Shimada, H.; Haase, G.M.; Stram, D.O.; Gerbing, R.B.; Lukens, J.N. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: A children’s cancer group study. J. Clin. Oncol. 2000, 18, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ambros, P.F.; Ambros, I.M.; Strehl, S.; Bauer, S.; Luegmayr, A.; Kovar, H.; Ladenstein, R.; Fink, F.M.; Horcher, E.; Printz, G. Regression and progression in neuroblastoma. Does genetics predict tumour behaviour? Eur. J. Cancer 1995, 31A, 510–515. [Google Scholar] [CrossRef]
- Ambros, P.F.; Ambros, I.M.; Kerbl, R.; Luegmayr, A.; Rumpler, S.; Ladenstein, R.; Amann, G.; Kovar, H.; Horcher, E.; De Bernardi, B.; et al. Intratumoural heterogeneity of 1p deletions and MYCN amplification in neuroblastomas. Med. Pediatr. Oncol. 2001, 36, 1–4. [Google Scholar] [CrossRef]
- Berbegall, A.; Villamón, E.; Piqueras, M.; Tadeo, I.; Djos, A.; Ambros, P.; Martinsson, T.; Ambros, I.; Cañete, A.; Castel, V.; et al. Comparative genetic study of intratumoral heterogenous MYCN amplified neuroblastoma versus aggressive genetic profile neuroblastic tumors. Oncogene 2015. [Google Scholar] [CrossRef] [PubMed]
- Berbegall, A.P.; Bogen, D.; Pötschger, U.; Beiske, K.; Bown, N.; Combaret, V.; Defferrari, R.; Jeison, M.; Mazzocco, K.; Varesio, L.; et al. Heterogeneous MYCN amplification in neuroblastoma: A SIOP Europe Neuroblastoma Study. Br. J. Cancer 2018, 118, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Philip, T.; Ladenstein, R.; Zucker, J.M.; Pinkerton, R.; Bouffet, E.; Louis, D.; Siegert, W.; Bernard, J.L.; Frappaz, D.; Coze, C.; et al. Double megatherapy and autologous bone marrow transplantation for advanced neuroblastoma: The LMCE2 study. Br. J. Cancer 1993, 67, 119–127. [Google Scholar] [CrossRef][Green Version]
- Ambros, I.M.; Zellner, A.; Roald, B.; Amann, G.; Ladenstein, R.; Printz, D.; Gadner, H.; Ambros, P.F. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N. Engl. J. Med. 1996, 334, 1505–1511. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef]
- Kerbl, R.; Urban, C.E.; Ambros, I.M.; Dornbusch, H.J.; Schwinger, W.; Lackner, H.; Ladenstein, R.; Strenger, V.; Gadner, H.; Ambros, P.F. Neuroblastoma mass screening in late infancy: Insights into the biology of neuroblastic tumors. J. Clin. Oncol. 2003, 21, 4228–4234. [Google Scholar] [CrossRef]
- Schilling, F.H.; Spix, C.; Berthold, F.; Erttmann, R.; Fehse, N.; Hero, B.; Klein, G.; Sander, J.; Schwarz, K.; Treuner, J.; et al. Neuroblastoma screening at one year of age. N. Engl. J. Med. 2002, 346, 1047–1053. [Google Scholar] [CrossRef]
- Fritsch, P.; Kerbl, R.; Lackner, H.; Urban, C. “Wait and see” strategy in localized neuroblastoma in infants: An option not only for cases detected by mass screening. Pediatr. Blood Cancer 2004, 43, 679–682. [Google Scholar] [CrossRef]
- Ladenstein, R.; Ambros, P.F.; Urban, C.; Ambros, I.M.; Fink, F.M.; Zoubek, A.; Grienberger, H.; Schmitt, K.; Kerbl, R.; Horcher, E.; et al. Value of prognostic factors in the Austrian A-NB87 Neuroblastoma Study. Klin. Pädiatrie 1996, 208, 210–220. [Google Scholar] [CrossRef]
- Baker, D.L.; Schmidt, M.L.; Cohn, S.L.; Maris, J.M.; London, W.B.; Buxton, A.; Stram, D.; Castleberry, R.P.; Shimada, H.; Sandler, A.; et al. Outcome after Reduced Chemotherapy for Intermediate-Risk Neuroblastoma. N. Engl. J. Med. 2010, 363, 1313–1323. [Google Scholar] [CrossRef]
- Bagatell, R.; Beck-Popovic, M.; London, W.B.; Zhang, Y.; Pearson, A.D.J.J.; Matthay, K.K.; Monclair, T.; Ambros, P.F.; Cohn, S.L.; International Neuroblastoma Risk Group. Significance of MYCN Amplification in International Neuroblastoma Staging System Stage 1 and 2 Neuroblastoma: A Report From the International Neuroblastoma Risk Group Database. J. Clin. Oncol. 2009, 27, 365–370. [Google Scholar] [CrossRef]
- Laprie, A.; Michon, J.; Hartmann, O.; Munzer, C.; Leclair, M.-D.D.; Coze, C.; Valteau-Couanet, D.; Plantaz, D.; Carrie, C.; Habrand, J.-L.L.; et al. High-dose chemotherapy followed by locoregional irradiation improves the outcome of patients with international neuroblastoma staging system Stage II and III neuroblastoma with MYCN amplification. Cancer 2004, 101, 1081–1089. [Google Scholar] [CrossRef]
- Bagatell, R.; Rumcheva, P.; London, W.B.; Cohn, S.L.; Look, A.T.; Brodeur, G.M.; Frantz, C.; Joshi, V.; Thorner, P.; Rao, P.V.; et al. Outcomes of Children with Intermediate-Risk Neuroblastoma After Treatment Stratified by MYCN Status and Tumor Cell Ploidy. J. Clin. Oncol. 2005, 23, 8819–8827. [Google Scholar] [CrossRef]
- Defferrari, R.; Mazzocco, K.; Ambros, I.M.; Ambros, P.F.; Bedwell, C.; Beiske, K.; Bénard, J.; Berbegall, A.P.; Bown, N.; Combaret, V.; et al. Influence of segmental chromosome abnormalities on survival in children over the age of 12 months with unresectable localised peripheral neuroblastic tumours without MYCN amplification. Br. J. Cancer 2015, 112, 290–295. [Google Scholar] [CrossRef]
- Ambros, I.M.; Tonini, G.-P.; Pötschger, U.; Gross, N.; Mosseri, V.; Beiske, K.; Berbegall, A.P.; Bénard, J.; Bown, N.; Caron, H.; et al. Age Dependency of the Prognostic Impact of Tumor Genomics in Localized Resectable MYCN -Nonamplified Neuroblastomas. Report from the SIOPEN Biology Group on the LNESG Trials and a COG Validation Group. J. Clin. Oncol. 2020, 38, 3685–3697. [Google Scholar] [CrossRef]
- Lavarino, C.; Cheung, N.-K.V.; Garcia, I.; Domenech, G.; de Torres, C.; Alaminos, M.; Rios, J.; Gerald, W.L.; Kushner, B.; LaQuaglia, M.; et al. Specific gene expression profiles and chromosomal abnormalities are associated with infant disseminated neuroblastoma. BMC Cancer 2009, 9, 44. [Google Scholar] [CrossRef]
- Iehara, T.; Hiyama, E.; Tajiri, T.; Yoneda, A.; Hamazaki, M.; Fukuzawa, M.; Hosoi, H.; Sugimoto, T.; Sawada, T. Is the prognosis of stage 4s neuroblastoma in patients 12 months of age and older really excellent? Eur. J. Cancer 2012, 48, 1707–1712. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, N.K.; LaQuaglia, M.P.; Ambros, P.F.; Ambros, I.M.; Bonilla, M.A.; Gerald, W.L.; Ladanyi, M.; Gilbert, F.; Rosenfield, N.S.; et al. Survival from locally invasive or widespread neuroblastoma without cytotoxic therapy. J. Clin. Oncol. 1996, 14, 373–381. [Google Scholar] [CrossRef]
- Taggart, D.R.; London, W.B.; Schmidt, M.L.; DuBois, S.G.; Monclair, T.F.; Nakagawara, A.; De Bernardi, B.; Ambros, P.F.; Pearson, A.D.J.; Cohn, S.L.; et al. Prognostic Value of the Stage 4S Metastatic Pattern and Tumor Biology in Patients with Metastatic Neuroblastoma Diagnosed Between Birth and 18 Months of Age. J. Clin. Oncol. 2011, 29, 4358–4364. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Michon, J.; Ribeiro, A.; Pierron, G.; Mosseri, V.; Rubie, H.; Munzer, C.; Bénard, J.; Auger, N.; Combaret, V.; et al. Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study). Br. J. Cancer 2011, 105, 1940–1948. [Google Scholar] [CrossRef]
- Canete, A.; Gerrard, M.; Rubie, H.; Castel, V.; Di Cataldo, A.; Munzer, C.; Ladenstein, R.; Brichard, B.; Bermúdez, J.D.; Couturier, J.; et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European Neuroblastoma Experience. J. Clin. Oncol. 2009, 27, 1014–1019. [Google Scholar] [CrossRef]
- Katzenstein, H.M.; Bowman, L.C.; Brodeur, G.M.; Thorner, P.S.; Joshi, V.V.; Smith, E.I.; Look, A.T.; Rowe, S.T.; Nash, M.B.; Holbrook, T.; et al. Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: The pediatric oncology group experience—A pediatric oncology group study. J. Clin. Oncol. 1998, 16, 2007–2017. [Google Scholar] [CrossRef]
- Kushner, B.H.; LaQuaglia, M.P.; Bonilla, M.A.; Lindsley, K.; Rosenfield, N.; Yeh, S.; Eddy, J.; Gerald, W.L.; Heller, G.; Cheung, N.K. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J. Clin. Oncol. 1994, 12, 2607–2613. [Google Scholar] [CrossRef]
- Cheung, N.V.; Heller, G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J. Clin. Oncol. 1991, 9, 1050–1058. [Google Scholar] [CrossRef]
- Philip, T.; Ladenstein, R.; Lasset, C.; Hartmann, O.; Zucker, J.M.; Pinkerton, R.; Pearson, A.D.; Klingebiel, T.; Garaventa, A.; European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party; et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. Eur. J. Cancer 1997, 33, 2130–2135. [Google Scholar] [CrossRef]
- George, R.E.; Li, S.; Medeiros-Nancarrow, C.; Neuberg, D.; Marcus, K.; Shamberger, R.C.; Pulsipher, M.; Grupp, S.A.; Diller, L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: Long-term survival update. J. Clin. Oncol. 2006, 24, 2891–2896. [Google Scholar] [CrossRef]
- Granger, M.; Grupp, S.A.; Kletzel, M.; Kretschmar, C.; Naranjo, A.; London, W.B.; Diller, L. Feasibility of a tandem autologous peripheral blood stem cell transplant regimen for high risk neuroblastoma in a cooperative group setting: A Pediatric Oncology Group study: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2012, 59, 902–907. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Bogen, D.; Brunner, C.; Walder, D.; Ziegler, A.; Abbasi, R.; Ladenstein, R.L.; Noguera, R.; Martinsson, T.; Amann, G.; Schilling, F.H.; et al. The genetic tumor background is an important determinant for heterogeneous MYCN-amplified neuroblastoma. Int. J. Cancer 2016. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Janoueix-Lerosey, I.; Ribeiro, A.; Klijanienko, J.; Couturier, J.; Pierron, G.; Mosseri, V.; Valent, A.; Auger, N.; Plantaz, D.; et al. Accumulation of segmental alterations determines progression in neuroblastoma. J. Clin. Oncol. 2010, 28, 3122–3130. [Google Scholar] [CrossRef]
- Janoueix-Lerosey, I.; Schleiermacher, G.; Michels, E.; Mosseri, V.; Ribeiro, A.; Lequin, D.; Vermeulen, J.; Couturier, J.; Peuchmaur, M.; Valent, A.; et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J. Clin. Oncol. 2009, 27, 1026–1033. [Google Scholar] [CrossRef]
- Attiyeh, E.F.; London, W.B.; Mossé, Y.P.; Wang, Q.; Winter, C.; Khazi, D.; McGrady, P.W.; Seeger, R.C.; Look, A.T.; Shimada, H.; et al. Chromosome 1p and 11q Deletions and Outcome in Neuroblastoma. N. Engl. J. Med. 2005, 353, 2243–2253. [Google Scholar] [CrossRef]
- Ambros, I.M.; Brunner, C.; Abbasi, R.; Frech, C.; Ambros, P.F. Ultra-High Density SNParray in Neuroblastoma Molecular Diagnostics. Front. Oncol. 2014, 4, 202. [Google Scholar] [CrossRef]
- Valteau-Couanet, D.; Le Deley, M.-C.; Bergeron, C.; Ducassou, S.; Michon, J.; Rubie, H.; Le Teuff, G.; Coze, C.; Plantaz, D.; Sirvent, N.; et al. Long-term results of the combination of the N7 induction chemotherapy and the busulfan-melphalan high dose chemotherapy. Pediatr. Blood Cancer 2014, 61, 977–981. [Google Scholar] [CrossRef]
- Rubino, C.; Adjadj, E.; Guérin, S.; Guibout, C.; Shamsaldin, A.; Dondon, M.-G.; Valteau-Couanet, D.; Hartmann, O.; Hawkins, M.; de Vathaire, F. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: Role of treatment. Int. J. Cancer 2003, 107, 791–796. [Google Scholar] [CrossRef]
- Flandin, I.; Hartmann, O.; Michon, J.; Pinkerton, R.; Coze, C.; Stephan, J.L.; Fourquet, B.; Valteau-Couanet, D.; Bergeron, C.; Philip, T.; et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1424–1431. [Google Scholar] [CrossRef]
- Irtan, S.; Brisse, H.J.; Minard-Colin, V.; Schleiermacher, G.; Canale, S.; Sarnacki, S. Minimally invasive surgery of neuroblastic tumors in children: Indications depend on anatomical location and image-defined risk factors. Pediatr. Blood Cancer 2015, 62, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, P.; Lassalle, M.; Mercier, G.; Raquin, M.A.; Izzi, G.; Corradini, N.; Hartmann, O. Platinum Compound-Related Ototoxicity in Children: Long-Term Follow-Up Reveals Continuous Worsening of Hearing Loss. J. Pediatr. Hematol. Oncol. 2004, 26, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Méhes, G.; Luegmayr, A.; Ambros, I.M.; Ladenstein, R.; Ambros, P.F. Combined automatic immunological and molecular cytogenetic analysis allows exact identification and quantification of tumor cells in the bone marrow. Clin. Cancer Res. 2001, 7, 1969–1975. [Google Scholar] [PubMed]
- Ambros, P.F.; Méhes, G.; Hattinger, C.; Ambros, I.M.; Luegmayr, A.; Ladenstein, R.; Gadner, H. Unequivocal identification of disseminated tumor cells in the bone marrow by combining immunological and genetic approaches-functional and prognostic information. Leukemia 2001, 15, 275–277. [Google Scholar] [CrossRef][Green Version]
- Ladenstein, R.; Philip, T.; Lasset, C.; Hartmann, O.; Garaventa, A.; Pinkerton, R.; Michon, J.; Pritchard, J.; Klingebiel, T.; Kremens, B.; et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: A report from the European Bone Marrow Transplantation Solid Tumor Registry. J. Clin. Oncol. 1998, 16, 953–965. [Google Scholar] [CrossRef]
- Ambros, P.F.; Ambros, I.M.; Brodeur, G.M.; Haber, M.; Khan, J.; Nakagawara, A.; Schleiermacher, G.; Speleman, F.; Spitz, R.; London, W.B.; et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Cancer 2009, 100, 1471–1482. [Google Scholar] [CrossRef]
| Risk-Adapted Strategy of Treatments in the A-NB94 Trial | ||||||||
| Stage | Age | MYCNNon-Amplified | MYCNAmplified | |||||
| 1, 2 | ≤12 | surgery | surgery if microscopic incomplete resection: 6 × CAV, radiotherapy | |||||
| >12 | surgery if microscopic incomplete resection: 3 × alternating CAV + CBDCA/VP16, radiotherapy | |||||||
| 3 | ≤12 | 4–6 × CV surgery | 3 × alternating CAV + CBDCA/VP16 surgery radiotherapy | |||||
| >12 | 3 × alternating CAV + CBDCA/VP16 surgery | 3 × alternating HD-CAV + CDDP/VP16 surgery radiotherapy | ||||||
| 4 | ≤12 | 3 × alternating HD-CAV + CDDP/VP16 surgery HDT/ASCR (single or multiple) radiotherapy | ||||||
| >12 | 3 × alternating HD-CAV + CDDP/VP16 surgery HDT/ASCR (single or multiple) radiotherapy | |||||||
| 4S | ≤12 | observation (up to 3 months) if progression: surgery if life-threatening symptoms: CV option to escalation: CBDCA/VP16 | - | |||||
| Details on Chemotherapy | ||||||||
| Chemotherapy | Abbreviation | Substance | Dosage | Days Given | ||||
| CV | CYC | cyclophosphamide | 5 mg/kg | 1–5 | ||||
| VCR | vincristine | 0.05 mg/kg | 1 | |||||
| CAV | CYC | cyclophosphamide | 300 mg/m2 | 1–5 | ||||
| ADR | doxorubicin | 60 mg/m2 | 5 | |||||
| VCR | vincristine | 1.5 mg/m2 | 1, 5 | |||||
| CBDCA/VP16 | CBDCA | carboplatin | 200 mg/m2 | 1–3 | ||||
| VP16 | etoposide | 150 mg/m2 | 1–3 | |||||
| HD-CAV | CCY | cyclophosphamide | 70 mg/kg | 1, 2 | ||||
| ADR | doxorubicin | 25 mg/m2 | 1–3 | |||||
| VCR | vincristine | 1 (1.5) mg/m2 | 1–3 (9) | |||||
| CDDP/VP16 | CDDP | cisplatin | 40 mg/m2 | 1–5 | ||||
| VP16 | etoposide | 150 mg/m2 | 3–5 | |||||
| single HDT | VP16 | etoposide | 60 mg/kg | −4 | ||||
| CBDCA | carboplatin | 500 mg/m2 | −4–2 | |||||
| MEL | melphalan | 180 mg/m2 | −2 | |||||
| multiple HDT | 1st course | THIO | thiotepa | 200 mg/m2 | −5–3 | |||
| CBDCA | carboplatin | 500 mg/m2 | −5–3 | |||||
| 2nd course | THIO | thiotepa | 200 mg/m2 | −5–3 | ||||
| CYC | cyclophosphamide | 1500 mg/m2 | −4–2 | |||||
| 3rd course | VP16 | etoposide | 40 mg/kg | −3 | ||||
| MEL | melphalan | 140 mg/m2 | −2 | |||||
| Total | Non-MNA | MNA | hetMNA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | <12 | ≥12 | Total | % | <12 | ≥12 | Total | <12 | ≥12 | Total | <12 | ≥12 | Total | |
| INSS Stage | GNB | 2 | 22 | 24 | 15% | 2 | 22 | 24 | ||||||
| 1 | 16 | 30 | 46 | 28% | 14 | 29 | 43 | 1 | 1 | 2 | 1 | 1 | ||
| 2 | 9 | 7 | 16 | 10% | 8 | 7 | 15 | 1 | 1 | |||||
| 3 | 14 | 9 | 23 | 14% | 12 | 5 | 17 | 1 | 4 | 5 | 1 | 1 | ||
| 4 | 12 | 32 | 44 | 27% | 6 | 18 | 24 | 5 | 14 | 19 | 1 | 1 | ||
| 4S | 10 | 10 | 6% | 10 | 10 | |||||||||
| Total | 63 | 100 | 163 | 100% | 52 | 81 | 133 | 7 | 19 | 26 | 4 | 4 | ||
| % | 39% | 61% | 100% | 32% | 50% | 82% | 4% | 12% | 16% | 2% | 2% | |||
| Biologic Criterion | EFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Marker | n | Events | 10y | p | Deaths | 10y | p | |
| MYCN | normal | 133 | 21 | 84 ± 3 | 0.035 | 14 | 89 ± 3 | 0.008 |
| MNA | 26 | 10 | 60 ± 10 | 9 | 64 ± 10 | |||
| hetMNA | 4 | 0 | 100 | 0 | 100 | |||
| SCAs | absent | 52 | 7 | 87 ± 5 | 0.014 | 2 | 96 ± 3 | <0.001 |
| present | 56 | 20 | 64 ± 6 | 17 | 69 ± 6 | |||
| 1p | normal | 89 | 14 | 84 ± 4 | 0.015 | 10 | 89 ± 3 | 0.004 |
| loss | 33 | 12 | 64 ± 8 | 10 | 69 ± 8 | |||
| 1q | normal | 91 | 18 | 81 ± 4 | <0.001 | 10 | 89 ± 3 | <0.001 |
| gain/loss | 6 | 6 | 0 | 6 | 0 | |||
| 2p | normal | 80 | 18 | 78 ± 5 | 0.702 | 11 | 86 ± 5 | 0.562 |
| gain | 12 | 2 | 83 ± 11 | 2 | 83 ± 11 | |||
| 3p | normal | 83 | 18 | 78 ± 5 | 0.007 | 11 | 87 ± 4 | 0.002 |
| loss | 10 | 6 | 40 ± 15 | 5 | 50 ± 16 | |||
| 4p | normal | 87 | 21 | 76 ± 5 | 0.512 | 14 | 87 ± 5 | 0.119 |
| loss | 7 | 1 | 86 ± 13 | 0 | 100 | |||
| 11q | normal | 80 | 15 | 81 ± 4 | 0.002 | 8 | 90 ± 3 | <0.001 |
| loss | 17 | 9 | 47 ± 12 | 9 | 47 ± 12 | |||
| 14q | normal | 70 | 17 | 75 ± 5 | 0.971 | 12 | 83 ± 5 | 0.982 |
| loss | 12 | 3 | 75 ± 13 | 2 | 83 ± 15 | |||
| 17q | normal | 58 | 9 | 84 ± 5 | 0.006 | 3 | 95 ± 3 | <0.001 |
| gain | 38 | 16 | 58 ± 8 | 15 | 61 ± 8 | |||
| Model | Marker | EFS | OS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CL | p | HR | 95% CL | p | ||
| A | SCAs | 0.67 | 0.15−2.91 | 0.59 | 1.06 | 0.16−6.93 | 0.95 |
| B | 1 ploss | 0.83 | 0.19−3.63 | 0.80 | 0.38 | 0.07−1.97 | 0.25 |
| 1 qgain/loss | 1.68 | 0.43−6.53 | 0.46 | 2.45 | 0.56−10.7 | 0.23 | |
| 3 ploss | 0.95 | 0.26−3.52 | 0.94 | 0.62 | 0.15−2.66 | 0.52 | |
| 11 qloss | 1.69 | 0.46−6.23 | 0.43 | 2.08 | 0.50−8.74 | 0.32 | |
| 17 qgain | 0.30 | 0.03−3.52 | 0.34 | 2.59 | 0.26−26.2 | 0.42 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, S.; Ambros, I.M.; Glogova, E.; Benesch, M.; Urban, C.; Mayer, M.; Ebetsberger-Dachs, G.; Bardi, E.; Jones, N.; Gamper, A.; et al. Long-Term Outcome and Role of Biology within Risk-Adapted Treatment Strategies: The Austrian Neuroblastoma Trial A-NB94. Cancers 2021, 13, 572. https://doi.org/10.3390/cancers13030572
Fiedler S, Ambros IM, Glogova E, Benesch M, Urban C, Mayer M, Ebetsberger-Dachs G, Bardi E, Jones N, Gamper A, et al. Long-Term Outcome and Role of Biology within Risk-Adapted Treatment Strategies: The Austrian Neuroblastoma Trial A-NB94. Cancers. 2021; 13(3):572. https://doi.org/10.3390/cancers13030572
Chicago/Turabian StyleFiedler, Stefan, Inge M. Ambros, Evgenia Glogova, Martin Benesch, Christian Urban, Marlene Mayer, Georg Ebetsberger-Dachs, Edit Bardi, Neil Jones, Agnes Gamper, and et al. 2021. "Long-Term Outcome and Role of Biology within Risk-Adapted Treatment Strategies: The Austrian Neuroblastoma Trial A-NB94" Cancers 13, no. 3: 572. https://doi.org/10.3390/cancers13030572
APA StyleFiedler, S., Ambros, I. M., Glogova, E., Benesch, M., Urban, C., Mayer, M., Ebetsberger-Dachs, G., Bardi, E., Jones, N., Gamper, A., Meister, B., Crazzolara, R., Amann, G., Dieckmann, K., Horcher, E., Kerbl, R., Brunner-Herglotz, B., Ziegler, A., Ambros, P. F., & Ladenstein, R. (2021). Long-Term Outcome and Role of Biology within Risk-Adapted Treatment Strategies: The Austrian Neuroblastoma Trial A-NB94. Cancers, 13(3), 572. https://doi.org/10.3390/cancers13030572







