Lipid Level, Lipid Variability, and Risk of Multiple Myeloma: A Nationwide Population-Based Study of 3,527,776 Subjects
Abstract
Simple Summary
Abstract
1. Background
2. Methods
2.1. Data Source
2.2. Study Design and Ethics Statement
2.3. Study Population
2.4. Data Collection and Measurements
2.5. Definition of Lipid Variability
2.6. Definition of Covariates
2.7. Study Outcomes and Follow-up
2.8. Data Analyses
3. Results
3.1. Baseline Characteristics of Study Population
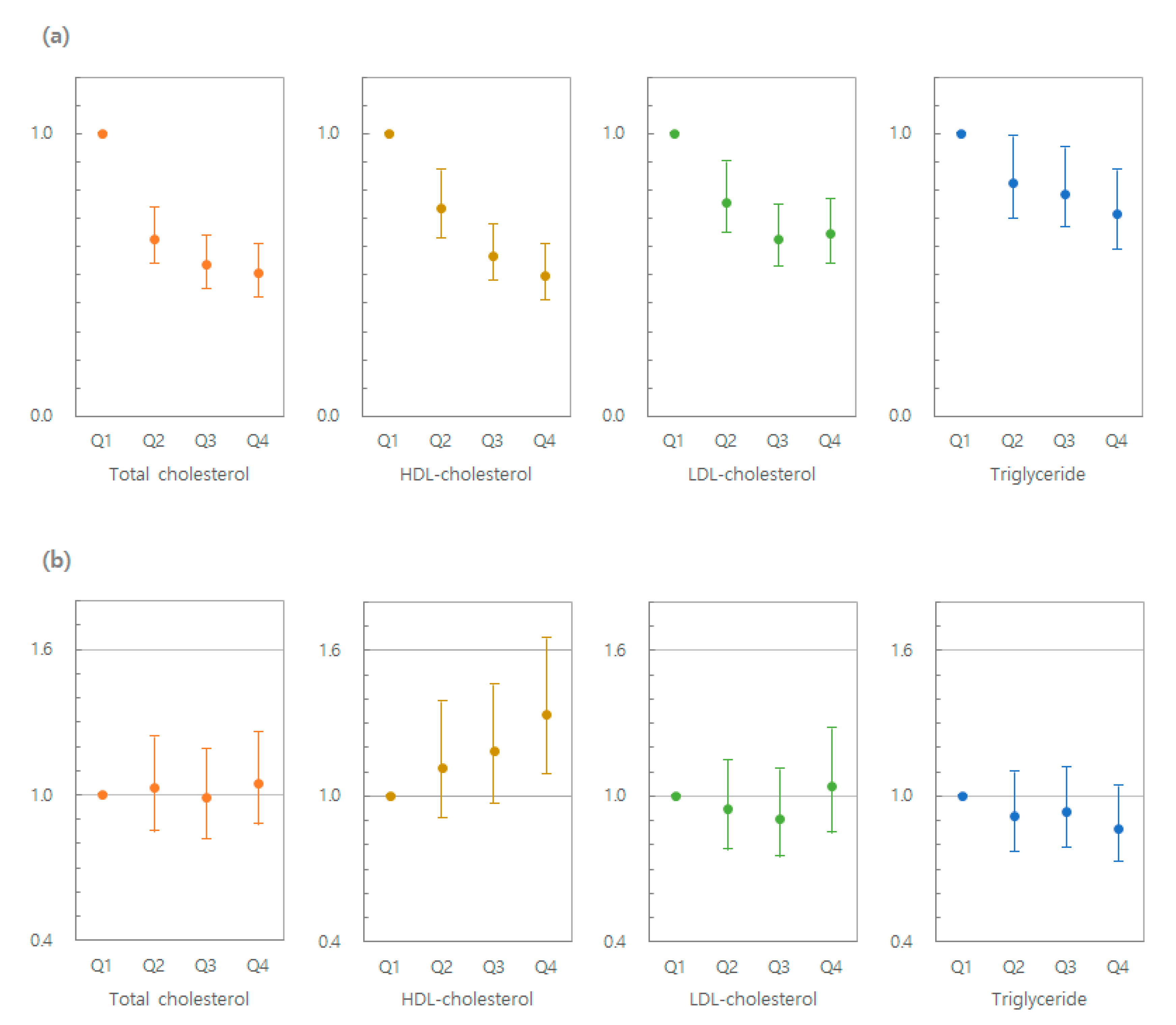
3.2. Lipid Levels and Risk of MM
3.3. Lipid Variability and Risk of MM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ARV | Average Real Variability |
| BMI | Body mass index |
| CV | Coefficient of Variation |
| HR | Hazard ratio |
| HDL-C | High-density lipoprotein cholesterol |
| ICD | International Classification of Diseases |
| LDL-C | Low-density lipoprotein cholesterol |
| MM | Multiple myeloma |
| NHIS | National Health Insurance System |
| Q | Quartile |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglyceride |
| VIM | Variability independent of the mean |
References
- Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1975–2016). 2019. Available online: www.seer.cancer.gov (accessed on 1 April 2019).
- Usmani, S.Z.; Hoering, A.; Cavo, M.; Miguel, J.S.; Goldschimdt, H.; Hajek, R.; Turesson, I.; Lahuerta, J.J.; Attal, M.; Barlogie, B.; et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—an IMWG Research Project. J. Blood Cancer 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Jung, S.-H.; Richardson, P.G.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: A randomised, double-blind, phase 3 trial. Lancet Haematol. 2017, 4, e431–e442. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and car-cinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar] [PubMed]
- Lu, C.-W.; Lo, Y.-H.; Chen, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Chen, P.-J.; Yang, Y.-F.; Wang, C.-H.; Tan, C.-H.; Hou, M.-F.; et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017, 388, 130–138. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; López-Vilaró, L.; Nasarre, L.; Perez-Olabarria, M.; Del Olmo, T.V.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortes, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Byon, C.H.; Hardy, R.W.; Ren, C.; Ponnazhagan, S.; Welch, D.; McDonald, J.M.; Chen, Y. Free fatty acids enhance breast cancer cell migration through plasminogen activator inhibitor-1 and SMAD4. Lab. Investig. 2009, 89, 1221–1228. [Google Scholar] [CrossRef]
- Kitahara, C.M.; De González, A.B.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef]
- Furberg, A.-S.; Veierød, M.B.; Wilsgaard, T.; Bernstein, L.; Thune, I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J. Natl. Cancer Inst. 2004, 96, 1152–1160. [Google Scholar] [CrossRef]
- Furberg, A.-S.; Espetvedt, S.; Emaus, A.; Khan, N.; Thune, I. Low high-density lipoprotein cholesterol may signal breast cancer risk: Recent findings and new hypotheses. Biomarkers Med. 2007, 1, 121–131. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.K.; Han, W.; Kim, D.-H.; Hong, Y.C.; Ha, E.-H.; Ahn, S.-H.; Noh, D.-Y.; Kang, D.; Yoo, K.Y. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol. Biomark. Prev. 2009, 18, 508–515. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Cheng, J.-X.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; et al. Cholesteryl ester accumulation induced by pten loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Broadbent, H.; Law, P.J.; Sud, A.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Mendelian randomisation implicates hyperlipidaemia as a risk factor for colorectal cancer. Int. J. Cancer 2017, 140, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; VHM & PP Study Group; Borena, W.; Rapp, K.; Klenk, J.; Strasak, A.; Diem, G.; Concin, H.; Nagel, G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br. J. Cancer 2009, 101, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, P.; Wang, L.; Zhang, C.; Wang, M.; Ouyang, J.; Chen, B. Cholesterol levels provide prognostic information in patients with multiple myeloma. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Hungria, V.T.; Latrilha, M.C.; Rodrigues, D.G.; Bydlowski, S.P.; Chiattone, C.S.; Maranhao, R.C. Me-tabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and a pre-liminary clinical study of LDE as a drug vehicle for the treatment of the disease. Cancer Chemother. Pharmacol. 2004, 53, 51–60. [Google Scholar] [CrossRef]
- Yavaşoğlu, I.; Tombuloglu, M.; Kadikoylu, G.; Donmez, A.; Cagirgan, S.; Bolaman, Z.; Cagırgan, S. Cholesterol levels in patients with multiple myeloma. Ann. Hematol. 2007, 87, 223–228. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Chlebowski, R.T.; Vitolins, M.Z.; Wassertheil-Smoller, S.; Rohan, T.E. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control. 2017, 29, 13–24. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Çolak, Y.; Bojesen, S.E.; Nordestgaard, B.G. Low high-density lipoprotein and in-creased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J. Hematol. Oncol. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Jeong, S.C.T.; Kim, D.; Han, K.; Kim, S.J.; Rhee, S.Y.; Giovannucci, E.L.; Shin, D.W. Association between high-density lipoprotein cholesterol level and risk of hematologic malignancy. Leukemia 2020. [Google Scholar] [CrossRef]
- Nazir, D.J.; Roberts, R.S.; Hill, S.A.; McQueen, M.J. Monthly intra-individual variation in lipids over a 1-year period in 22 normal subjects. Clin. Biochem. 1999, 32, 381–389. [Google Scholar] [CrossRef]
- Smith, S.J.; Cooper, G.R.; Myers, G.L.; Sampson, E.J. Biological variability in concentrations of serum lipids: Sources of variation among results from published studies and composite predicted values. Clin. Chem. 1993, 39, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Smit, R.A.; Jukema, J.W.; Postmus, I.; Ford, I.; Slagboom, P.E.; Heijmans, B.T.; Le Cessie, S.; Trompet, S. Visit-to-visit lipid variability: Clinical significance, effects of lipid-lowering treatment, and (pharmaco) genetics. J. Clin. Lipidol. 2018, 12, 266–276.e3. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Breazna, A.; Demicco, D.A.; Wun, C.-C.; Messerli, F.H. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes. J. Am. Coll. Cardiol. 2015, 65, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Fayyad, R.; Messerli, F.H.; Laskey, R.; Demicco, D.A.; Kastelein, J.J.; Waters, D.D. Relation of variability of low-density lipoprotein cholesterol and blood pressure to events in patients with previous myocardial infarction from the ideal trial. Am. J. Cardiol. 2017, 119, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kreger, B.E.; Odell, P.M.; D’Agostino, R.B.; Wilson, P.F. Long-term intraindividual cholesterol varia-bility: Natural course and adverse impact on morbidity and mortality--the Framingham Study. Am. Heart J. 1994, 127, 1607–1614. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Kim, H.-S.; Park, Y.-M.; Kwon, H.-S.; Yoon, K.-H.; Lee, S.-H. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: A nationwide population-based study. Eur. Hear. J. 2017, 38, 3560–3566. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Koh, E.S.; Kim, H.-S.; Kwon, H.-S.; Park, Y.; Yoon, K.-H.; Lee, S. Variability in total cholesterol is associated with the risk of end-stage renal disease. Arter. Thromb. Vasc. Biol. 2017, 37, 1963–1970. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.-Y.; Lee, E.-J.; Shin, S.A.; Khang, Y.-H.; Park, J.H.; Kang, H.-J.; Lee, H.; Do, C.-H.; Song, J.-S.; et al. Data resource profile: The national health information database of the national health insurance service in south korea. Int. J. Epidemiol. 2016, 46, 799–800. [Google Scholar] [CrossRef]
- Jeong, S.; Jang, W.; Shin, D.W. Association of statin use with Parkinson’s disease: Dose–response relationship. Mov. Disord. 2019, 34, 1014–1021. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Shin, D.W.; Lee, J.E.; Hyeon, J.H.; Lee, J.; Kim, S. Anemia is associated with incidence of dementia: A national health screening study in Korea involving 37,900 persons. Alzheimer’s Res. Ther. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Lee, K.R.; Hwang, I.C.; Han, K.D.; Jung, J.; Seo, M.H. Waist circumference and risk of breast cancer in Korean women: A nationwide cohort study. Int. J. Cancer 2017, 142, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Jochem, C.; Leitzmann, M.F.; Keimling, M.; Schmid, D.; Behrens, G. Physical activity in relation to risk of hematologic cancers: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.A. Obesity and the Risk for a Hematological Malignancy: Leukemia, Lymphoma, or Myeloma. Oncol. 2010, 15, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Salvayre, A.N.; Dousset, N.; Ferretti, G.; Bacchetti, T.; Curatola, G.; Salvayre, R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic. Biol. Med. 2006, 41, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Gaman, M.A.; Epingeac, M.E.; Gaman, A.M. The evaluation of oxidative stress and high-density lipoprotein cholesterol levels in diffuse large b-cell lymphoma. Revista de Chimie 2019, 70, 977–980. [Google Scholar] [CrossRef]
- Giles, F.J.; Krawczyk, J.; O’Dwyer, M.; Swords, R.; Freeman, C. The role of inflammation in leukaemia. Adv. Exp. Med. Biol. 2014, 816, 335–360. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Pagler, T.; Tall, A.R.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010, 328, 1689–1693. [Google Scholar] [CrossRef]
- Zhu, X.; Parks, J.S. New roles of HDL in inflammation and hematopoiesis. Annu. Rev. Nutr. 2012, 32, 161–182. [Google Scholar] [CrossRef]
- Yui, Y.; Aoyama, T.; Morishita, H.; Takahashi, M.; Takatsu, Y.; Kawai, C. Serum prostacyclin stabilizing factor is identical to apolipoprotein A-I (Apo A-I). A novel function of Apo A-I. J. Clin. Investig. 1988, 82, 803–807. [Google Scholar] [CrossRef]
- Halton, J.M.; Nazir, D.J.; McQueen, M.J.; Barr, R.D. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 83, 379–384. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.R.; Tugnoli, V. Cholesteryl esters in malignancy. Clin. Chim. Acta 2005, 359, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Lamanuzzi, A.; Saltarella, I.; DeSantis, V.; Frassanito, M.A.; Leone, P.; Racanelli, V.; Nico, B.; Ribatti, D.; Ditonno, P.; Prete, M.; et al. Inhibition of mTOR complex 2 restrains tumor angiogenesis in multiple myeloma. Oncotarget 2018, 9, 20563–20577. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Kumar, S.K. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk. Lymphoma 2018, 59, 2524–2534. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; D’Souza, A. Signaling pathways and emerging therapies in multiple myeloma. Curr. Hematol. Malign-Rep. 2016, 11, 156–164. [Google Scholar] [CrossRef]
- Fruhwürth, S.; Krieger, S.; Winter, K.; Rosner, M.; Mikula, M.; Weichhart, T.; Bittman, R.; Hengstschläger, M.; Stangl, H. Inhibition of mTOR down-regulates scavenger receptor, class B, type I (SR-BI) expression, reduces endothelial cell migration and impairs nitric oxide production. Biochim. Biophys. Acta (BBA) Bioenergy 2014, 1841, 944–953. [Google Scholar] [CrossRef]
- Pugliese, L.; Bernardini, I.; Pacifico, N.; Peverini, M.; Damaskopoulou, E.; Cataldi, S.; Albi, E. Severe hypocholesterolaemia is often neglected in haematological malignancies. Eur. J. Cancer 2010, 46, 1735–1743. [Google Scholar] [CrossRef]
- Codini, M.; Cataldi, S.; Lazzarini, A.; Tasegian, A.; Ceccarini, M.R.; Floridi, A.; Lazzarini, R.; Ambesi-Impiombato, F.S.; Curcio, F.; Beccari, T.; et al. Why high cholesterol levels help hematological malignancies: Role of nuclear lipid microdomains. Lipids Heal. Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Yavaşoğlu, I.; Sargin, G.; Donmez, A.; Yegin, Z.A.; Bilgir, O.; Tiftik, N.; Ertop, S.; Ayyildiz, O.; Sonmez, M.; Pektas, G.; et al. Cholesterol levels in patients with chronic lymphocytic leukemia. J. Natl. Med. Assoc. 2017, 109, 23–27. [Google Scholar] [CrossRef]
- Scribano, D.; Baroni, S.; Pagano, L.; Zuppi, C.; Leone, G.; Giardina, B. Return to normal values of li-pid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica 1996, 81, 343–345. [Google Scholar]
- Spiegel, R.J.; Schaefer, E.J.; Magrath, I.T.; Edwards, B.K. Plasma lipid alterations in leukemia and lymphoma. Am. J. Med. 1982, 72, 775–782. [Google Scholar] [CrossRef]
- Chiu, B.C.-H.; Chen, J.-H.; Yen, Y.-C.; Calip, G.S.; Chien, C.-R.; Ahsan, H.; Shih, Y.-C.T.; Cheng, K.-F. Long term statin use and risk of multiple myeloma among 15.5 million taiwanese adults: A retrospective cohort study. Blood 2015, 126, 4198. [Google Scholar] [CrossRef]
- Epstein, M.M.; Divine, G.; Chao, C.R.; Wells, K.E.; Feigelson, H.S.; Scholes, D.; Roblin, U.; Yood, M.U.; Engel, L.S.; Taylor, A.; et al. Statin use and risk of multiple myeloma: An analysis from the cancer research network. Int. J. Cancer 2017, 141, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Keller, J.; Gage, B.F.; Luo, S.; Wang, T.-F.; Moskowitz, G.; Gumbel, J.; Blue, B.; O’Brian, K.; Carson, K.R. Statins are associated with reduced mortality in multiple myeloma. J. Clin. Oncol. 2016, 34, 4008–4014. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC); Gutierrez, L. Repositioning of the global epicentre of non-optimal cholesterol. Nat. Cell Biol. 2020, 582, 73–77. [Google Scholar] [CrossRef]
- Lin, C.-F.; Chang, Y.-H.; Chien, S.-C.; Lin, Y.-H.; Yeh, H.-Y. Epidemiology of dyslipidemia in the Asia Pacific region. Int. J. Gerontol. 2018, 12, 2–6. [Google Scholar] [CrossRef]
| Variability Independent of Mean (VIM) of HDL | |||||
|---|---|---|---|---|---|
| Characteristics | Q1 | Q2 | Q3 | Q4 | p |
| N | 881,919 | 881,952 | 881,989 | 881,916 | |
| Age, years | 51.2 ± 8.6 | 51.5 ± 8.7 | 52.0 ± 8.9 | 53.1 ± 9.5 | <0.0001 |
| Sex (Male) | 483,552 (54.8) | 544,238 (61.7) | 587,485 (66.6) | 635,738 (72.1) | <0.0001 |
| Lipid profiles (mg/dL) | |||||
| TC | 201.3 ± 34.6 | 199.2 ± 35.2 | 197.2 ± 35.8 | 193.0 ± 37.0 | <0.0001 |
| HDL-C | 62.0 ± 13.2 | 56.8 ± 12.5 | 52.6 ± 14.0 | 47.0 ± 16.0 | <0.0001 |
| LDL-C | 116.8 ± 33.2 | 117.5 ± 34.2 | 117.22 ± 35.08 | 114.47 ± 36.95 | <0.0001 |
| TG * | 99.8 (99.7–99.9) | 110.4 (110.2–110.5) | 121.5 (121.4–121.7) | 141.1 (140.9–141.2) | <0.0001 |
| Living place (Urban) | 407,633 (46.2) | 400,125 (45.4) | 393,781 (44.7) | 378,010 (42.9) | <0.0001 |
| Household income (Low) | 195,558 (22.2) | 190,524 (21.6) | 192,546 (21.8) | 201,809 (22.9) | <0.0001 |
| BMI, kg/m2 | 23.5 ± 3.0 | 23.8 ± 3.0 | 24.1 ± 2.9 | 24.5 ± 2.9 | <0.0001 |
| WC, cm | 79.3 ± 8.6 | 80.7 ± 8.5 | 81.8 ± 8.3 | 83.4 ± 8.2 | <0.0001 |
| Smoking habits | <0.0001 | ||||
| No | 527,768 (59.8) | 483,106 (54.8) | 451,638 (51.2) | 415,426 (47.1) | |
| Ex | 168,446 (19.1) | 185,731 (21.1) | 194,658 (22.1) | 198,829 (22.6) | |
| Current | 185,705 (21.1) | 213,115 (24.2) | 235,693 (26.7) | 267,661 (30.4) | |
| Alcohol consumption | <0.0001 | ||||
| No | 416,162 (47.2) | 408,334 (46.3) | 412,138 (46.7) | 435,252 (49.4) | |
| Mild (<30 mg/d) | 401,658 (45.5) | 406,167 (46.1) | 402,049 (45.6) | 381,322 (43.2) | |
| Heavy (≥30 mg/d) | 64,099 (7.3) | 67,451 (7.7) | 67,802 (7.7) | 65,342 (7.4) | |
| Regular exercise, Yes | 205,121 (23.3) | 205,180 (23.3) | 203,589 (23.1) | 197,258 (22.4) | <0.0001 |
| Hypertension, Yes | 226,922 (25.7) | 245,354 (27.8) | 266,667 (30.2) | 303,871 (34.5) | <0.0001 |
| Diabetes, Yes | 69,405 (7.9) | 82,132 (9.3) | 97,362 (11.0) | 126,127 (14.3) | <0.0001 |
| Dyslipidemia, Yes | 210642 (23.9) | 207,056 (23.5) | 203,745 (23.1) | 196,157 (22.2) | <0.0001 |
| Blood glucose, mg/dL | 97.7 ± 20.7 | 98.8 ± 22.1 | 99.9 ± 23.6 | 101.9 ± 26.3 | <0.0001 |
| SBP, mmHg | 121.9 ± 14.3 | 122.7 ± 14.2 | 123.3 ± 14.1 | 124.2 ± 14.1 | <0.0001 |
| DBP, mmHg | 76.4 ± 9.8 | 76.9 ± 9.7 | 77.4 ± 9.7 | 77.8 ± 9.7 | <0.0001 |
| Lipid Levels | N | Case | Duration | IR (100,000 PY) | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| TC | |||||||
| Q1 | 867,617 | 377 | 4,449,425.7 | 8.5 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 905,931 | 234 | 4,654,278.6 | 5.0 | 0.63 (0.54, 0.74) | 0.63 (0.54, 0.74) | 0.63 (0.54, 0.74) |
| Q3 | 885,382 | 190 | 4,550,159.6 | 4.2 | 0.54 (0.45, 0.64) | 0.54 (0.45, 0.64) | 0.54 (0.45, 0.64) |
| Q4 | 868,846 | 168 | 4,455,274.4 | 3.8 | 0.51 (0.42, 0.61) | 0.51 (0.42, 0.61) | 0.51 (0.42, 0.61) |
| HDL-C | |||||||
| Q1 | 846,909 | 362 | 4,350,341.4 | 8.3 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 873,920 | 255 | 4,493,565.9 | 5.7 | 0.73 (0.63, 0.86) | 0.74 (0.63, 0.87) | 0.74 (0.63, 0.87) |
| Q3 | 921,806 | 197 | 4,737,000.3 | 4.2 | 0.56 (0.47, 0.67) | 0.57 (0.48, 0.68) | 0.57 (0.48, 0.68) |
| Q4 | 885,141 | 155 | 4,528,230.7 | 3.4 | 0.49 (0.41, 0.59) | 0.51 (0.41, 0.62) | 0.50 (0.41, 0.61) |
| LDL-C | |||||||
| Q1 | 890,525 | 326 | 4,561,655.5 | 7.1 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 861,343 | 240 | 4,424,092.2 | 5.4 | 0.77 (0.65, 0.91) | 0.76 (0.65, 0.90) | 0.76 (0.65, 0.90) |
| Q3 | 888,661 | 202 | 4,567,970.3 | 4.4 | 0.64 (0.54, 0.76) | 0.63 (0.53, 0.75) | 0.63 (0.53, 0.75) |
| Q4 | 887,247 | 201 | 4,555,420.3 | 4.4 | 0.66 (0.55, 0.79) | 0.65 (0.54, 0.77) | 0.65 (0.54, 0.77) |
| TG | |||||||
| Q1 | 889,167 | 268 | 4,558,628.0 | 5.9 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 880,387 | 248 | 4,517,069.2 | 5.5 | 0.85 (0.71, 1.01) | 0.83 (0.70, 0.99) | 0.83 (0.70, 0.99) |
| Q3 | 877,719 | 245 | 4,508,015.7 | 5.4 | 0.82 (0.69, 0.98) | 0.80 (0.67, 0.95) | 0.79 (0.67, 0.95) |
| Q4 | 880,503 | 208 | 4,525,425.4 | 4.6 | 0.75 (0.63, 0.90) | 0.72 (0.60, 0.87) | 0.72 (0.59, 0.87) |
| VIM | N | Case | Duration | IR (100,000 PY) | HR (95% C.I.) | |||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| TC | ||||||||
| Q1 | 881,943 | 223 | 4,511,433.7 | 4.9 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 881,943 | 230 | 4,546,886.3 | 5.1 | 1.04 (0.86, 1.25) | 1.04 (0.86, 1.25) | 1.04 (0.86, 1.25) | 1.03 (0.85, 1.24) |
| Q3 | 881,945 | 231 | 4,543,898.0 | 5.1 | 1.02 (0.85, 1.23) | 1.02 (0.85, 1.23) | 1.02 (0.85, 1.23) | 0.99 (0.82, 1.19) |
| Q4 | 881,945 | 285 | 4,506,920.3 | 6.3 | 1.16 (0.98, 1.38) | 1.16 (0.97, 1.38) | 1.16 (0.97, 1.38) | 1.05 (0.88, 1.26) |
| HDL-C | ||||||||
| Q1 | 881,919 | 158 | 4,508,215.7 | 3.5 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 881,952 | 203 | 4,538,730.6 | 4.5 | 1.22 (0.99, 1.51) | 1.21 (0.99, 1.49) | 1.21 (0.99, 1.49) | 1.12 (0.91, 1.39) |
| Q3 | 881,989 | 249 | 4,540,299.4 | 5.5 | 1.40 (1.15, 1.71) | 1.38 (1.13, 1.68) | 1.38 (1.13, 1.68) | 1.19 (0.97, 1.46) |
| Q4 | 881,916 | 359 | 4,521,892.8 | 7.9 | 1.77 (1.47, 2.14) | 1.72 (1.43, 2.09) | 1.73 (1.43, 2.09) | 1.34 (1.09, 1.65) |
| LDL-C | ||||||||
| Q1 | 881,936 | 212 | 4,516,264.2 | 4.7 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 881,952 | 218 | 4,541,908.7 | 4.8 | 1.02 (0.84, 1.23) | 1.02 (0.84, 1.23) | 1.02 (0.84, 1.23) | 0.95 (0.78, 1.15) |
| Q3 | 881,942 | 230 | 4,535,323.7 | 5.1 | 1.05 (0.87, 1.27) | 1.05 (0.87, 1.27) | 1.05 (0.87, 1.27) | 0.91 (0.75, 1.11) |
| Q4 | 881,946 | 309 | 4,515,641.8 | 6.8 | 1.34 (1.12, 1.59) | 1.35 (1.13, 1.61) | 1.35 (1.13, 1.61) | 1.04 (0.85, 1.28) |
| TG | ||||||||
| Q1 | 881,944 | 265 | 4,499,237.3 | 5.9 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 881,944 | 239 | 4,540,799.6 | 5.3 | 0.92 (0.78, 1.10) | 0.93 (0.78, 1.10) | 0.93 (0.78, 1.10) | 0.92 (0.77, 1.10) |
| Q3 | 881,944 | 242 | 4,545,779.6 | 5.3 | 0.94 (0.79, 1.12) | 0.95 (0.79, 1.13) | 0.95 (0.79, 1.13) | 0.94 (0.79, 1.12) |
| Q4 | 881,944 | 223 | 4,523,321.9 | 4.9 | 0.88 (0.73, 1.05) | 0.89 (0.74, 1.06) | 0.89 (0.74, 1.06) | 0.87 (0.73, 1.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, T.; Choi, I.Y.; Han, K.; Jeong, S.-M.; Yoo, J.E.; Rhee, S.Y.; Park, Y.-G.; Shin, D.W. Lipid Level, Lipid Variability, and Risk of Multiple Myeloma: A Nationwide Population-Based Study of 3,527,776 Subjects. Cancers 2021, 13, 540. https://doi.org/10.3390/cancers13030540
Choi T, Choi IY, Han K, Jeong S-M, Yoo JE, Rhee SY, Park Y-G, Shin DW. Lipid Level, Lipid Variability, and Risk of Multiple Myeloma: A Nationwide Population-Based Study of 3,527,776 Subjects. Cancers. 2021; 13(3):540. https://doi.org/10.3390/cancers13030540
Chicago/Turabian StyleChoi, Taewoong, In Young Choi, Kyungdo Han, Su-Min Jeong, Jung Eun Yoo, Sang Youl Rhee, Yong-Gyu Park, and Dong Wook Shin. 2021. "Lipid Level, Lipid Variability, and Risk of Multiple Myeloma: A Nationwide Population-Based Study of 3,527,776 Subjects" Cancers 13, no. 3: 540. https://doi.org/10.3390/cancers13030540
APA StyleChoi, T., Choi, I. Y., Han, K., Jeong, S.-M., Yoo, J. E., Rhee, S. Y., Park, Y.-G., & Shin, D. W. (2021). Lipid Level, Lipid Variability, and Risk of Multiple Myeloma: A Nationwide Population-Based Study of 3,527,776 Subjects. Cancers, 13(3), 540. https://doi.org/10.3390/cancers13030540





