Predictive Factors for Pancreatic Cancer and Its Early Detection Using Special Pancreatic Ultrasonography in High-Risk Individuals
Abstract
Simple Summary
Abstract
1. Introduction
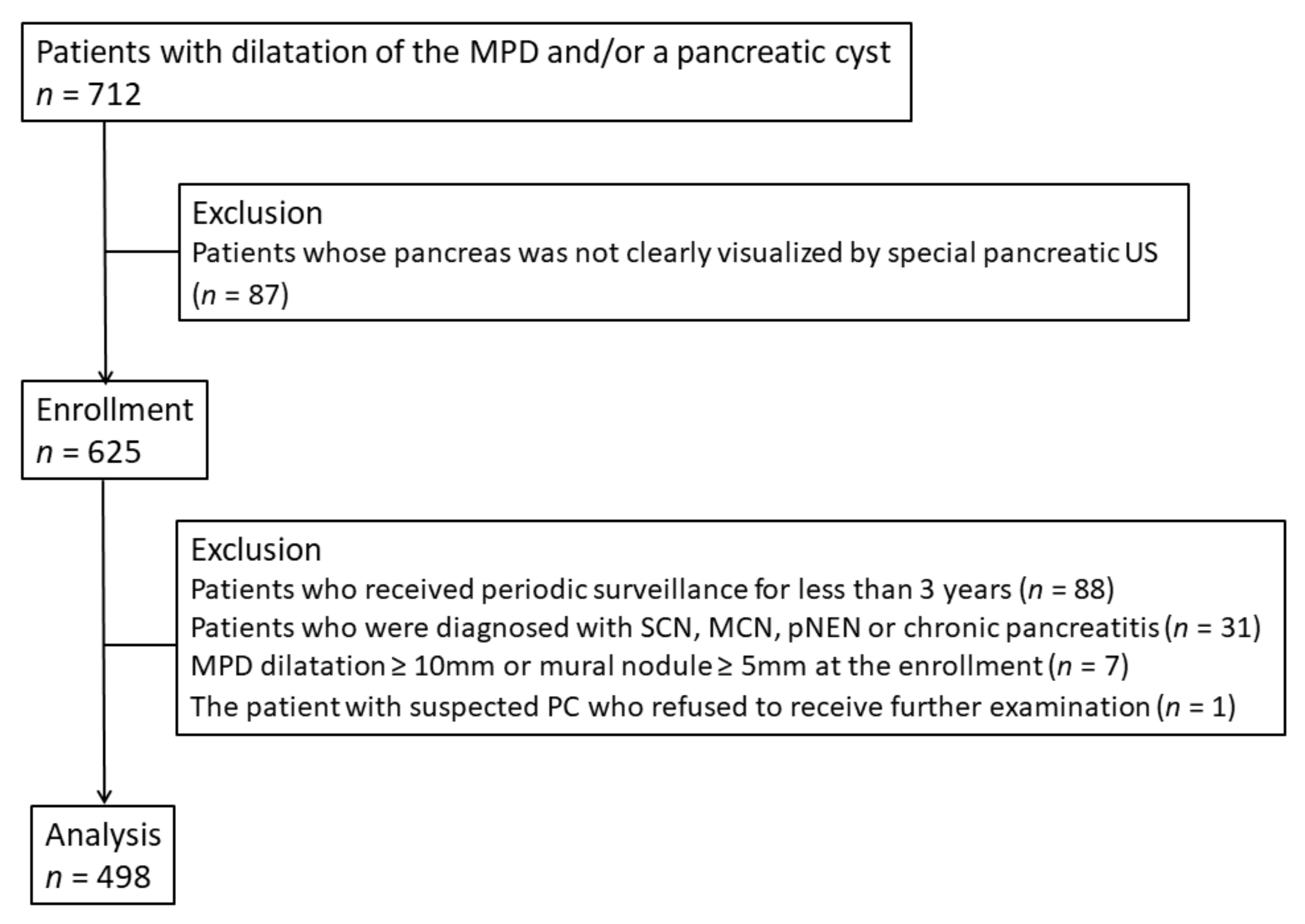
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
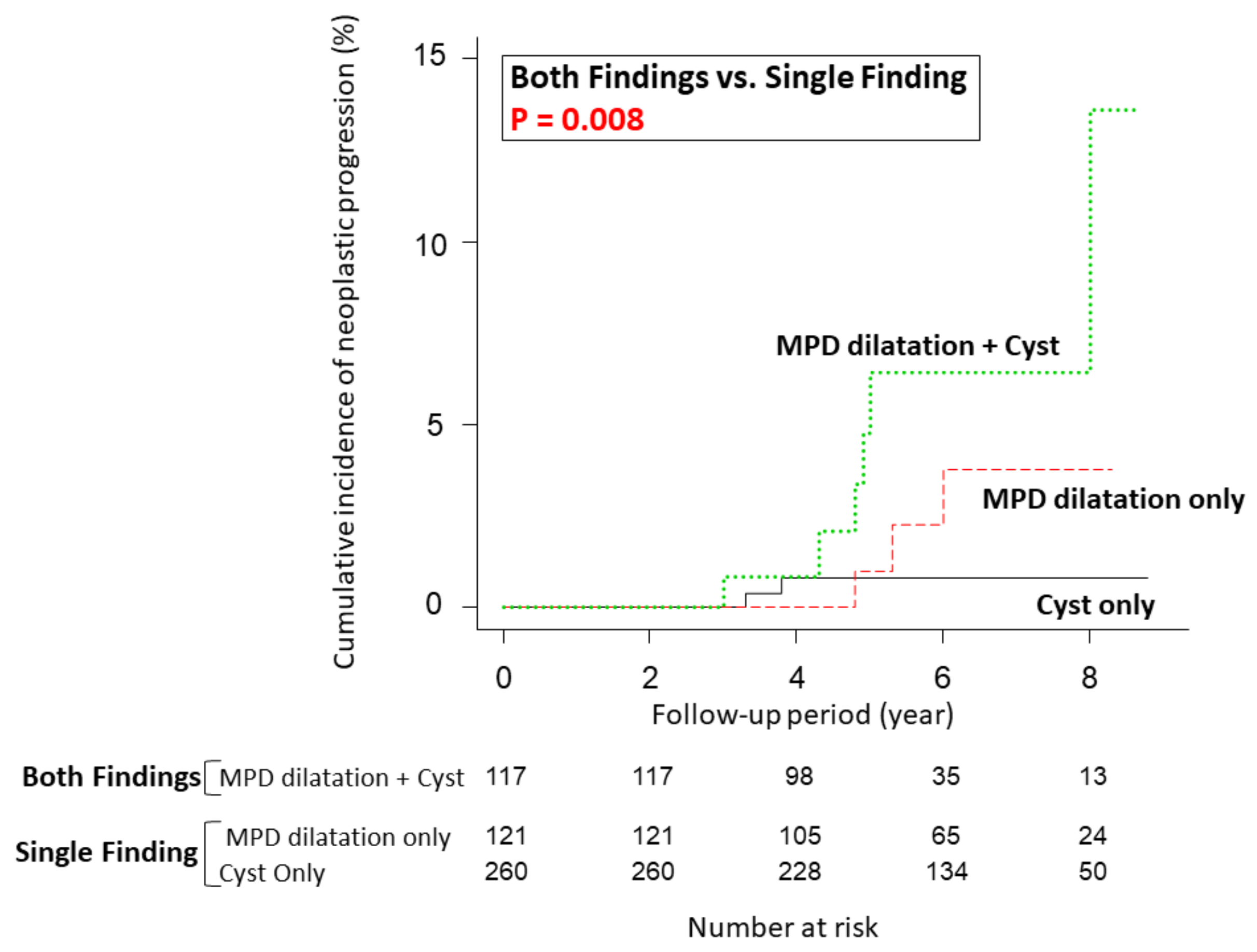
3.2. Cumulative Incidence of Neoplastic Progression
3.3. Factors Associated with Neoplastic Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACG | American College of Gastroenterology |
| ALP | alkaline phosphatase |
| Br | branch-duct |
| CA19-9 | carbohydrate antigen 19-9 |
| CI | confidence interval |
| CT | computed tomography |
| ERCP | endoscopic retrograde cholangiopancreatography |
| EUS | endoscopic ultrasound |
| FBS | fasting blood sugar |
| FNA | fine-needle aspiration |
| HGD | high-grade dysplasia |
| HR | hazard ratio |
| IAP | International Association of Pancreatology |
| IPMN | intraductal papillary mucinous neoplasm |
| IQR | interquartile range |
| MPD | main pancreatic duct |
| MRI | magnetic resonance imaging |
| PC | pancreatic cancer |
| PDAC | pancreatic ductal adenocarcinoma |
| US | ultrasonography |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Takeda, K.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Matsuno, S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004, 28, 235–240. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Kato, S.; Honda, K. Use of biomarkers and imaging for early detection of pancreatic cancer. Cancers 2020, 12, 1965. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Nakao, M.; Katayama, K.; Fukuda, J.; Okagaki, S.; Misu, K.; Miyazaki, S.; Matsuno, N.; Ashida, R.; Ioka, T.; Ito, Y.; et al. Evaluating the ability to detect pancreatic lesions using a special ultrasonography examination focusing on the pancreas. Eur. J. Radiol. 2017, 91, 10–14. [Google Scholar] [CrossRef]
- Ashida, R.; Tanaka, S.; Yamanaka, H.; Okagaki, S.; Nakao, K.; Fukuda, J.; Nakao, M.; Ioka, T.; Katayama, K. The role of transabdominal ultrasound in the diagnosis of early stage pancreatic cancer: Review and single-center experience. Diagnostics 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakaizumi, A.; Ioka, T.; Takakura, R.; Uehara, H.; Nakao, M.; Suzuki, R.; Fukuda, J.; Ishikawa, O.; Ohigashi, H. Periodic Ultrasonography Checkup for the Early Detection of Pancreatic Cancer: Preliminary Report. Pancreas 2004, 28, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Tanaka, S.; Ishida, N.; Ioka, T.; Ikezawa, K.; Takakura, R.; Nakao, M.; Ohkawa, K.; Katayama, K.; Nagata, S. A case of stage IA pancreatic ductal adenocarcinoma accompanied with focal pancreatitis demonstrated by contrast-enhanced ultrasonography. J. Med. Ultrason. 2018, 45, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Takakura, R.; Ioka, T.; Nakao, M.; Fukuda, J.; Suzuki, R.; Ueda, E.; Yoshioka, F.; Ashida, R.; Arimoto, N. Detectability of high-risk signs of pancreatic cancer (pancreatic cysts and main pancreatic duct dilatation): Ultrasonography versus low-dose plain X-ray CT (in Japanese). Jpn J. Med. Ultrason. 2012, 39, 3–7. [Google Scholar] [CrossRef]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Liao, W.C.; Tu, Y.K.; Wu, M.S.; Lin, J.T.; Wang, H.P.; Chien, K.L. Blood glucose concentration and risk of pancreatic cancer: Systematic review and dose-response meta-analysis. BMJ 2015, 349, g7371. [Google Scholar] [CrossRef]
- Yoshioka, T.; Shigekawa, M.; Ikezawa, K.; Tamura, T.; Sato, K.; Urabe, M.; Sueyoshi, H.; Yamai, T.; Suda, T.; Sakamori, R.; et al. Risk Factors for Pancreatic Cancer and the Necessity of Long-Term Surveillance in Patients With Pancreatic Cystic Lesions. Pancreas 2020, 49, 552–560. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Besselink, M.G.; Scholten, L.; Bruno, M.J.; Cahen, D.L.; Gress, T.M.; van Hooft, J.E.; Lerch, M.M.; Mayerle, J.; Hackert, T.; et al. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Lanke, G.; Lee, J.H. Similarities and differences in guidelines for the management of pancreatic cysts. World J. Gastroenterol. 2020, 26, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jang, J.Y.; Kim, S.J.; Lee, K.B.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B.; Kim, S.W. Cyst Growth Rate Predicts Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasms. Clin. Gastroenterol. Hepatol. 2011, 9, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.T.; Lawson, R.D.; Hunt, G.; Fehmi, S.M.; Proudfoot, J.A.; Xu, R.; Giap, A.; Tang, R.S.; Gonzalez, I.; Krinsky, M.L.; et al. Rapid Growth Rates of Suspected Pancreatic Cyst Branch Duct Intraductal Papillary Mucinous Neoplasms Predict Malignancy. Dig. Dis. Sci. 2015, 60, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Pezzilli, R.; Bissolati, M.; Capurso, G.; Romano, L.; Brunori, M.P.; Calculli, L.; Tamburrino, D.; Piccioli, A.; Ruffo, G.; et al. Active Surveillance beyond 5 Years Is Required for Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms Undergoing Non-Operative Management. Am. J. Gastroenterol. 2017, 112, 1153–1161. [Google Scholar] [CrossRef]
- Hasan, A.; Visrodia, K.; Farrell, J.J.; Gonda, T.A. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J. Gastroenterol. 2019, 25, 4405–4413. [Google Scholar] [CrossRef]
- Malleo, G.; Marchegiani, G.; Borin, A.; Capelli, P.; Accordini, F.; Butturini, G.; Pederzoli, P.; Bassi, C.; Salvia, R. Observational Study of the Incidence of Pancreatic and Extrapancreatic Malignancies during Surveillance of Patients with Branch-duct Intraductal Papillary Mucinous Neoplasm. Ann. Surg. 2015, 261, 984–990. [Google Scholar] [CrossRef]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237.e5. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014, 46, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, C.; Alvarez-Sánchez, M.V.; Napoléon, B.; Faiss, S. Diagnostic endoscopic ultrasonography: Assessment of safety and prevention of complications. World J. Gastroenterol. 2012, 18, 4659–4676. [Google Scholar] [CrossRef] [PubMed]

| Total | Progressor | Non-Progressor | p-Value | ||
|---|---|---|---|---|---|
| (n = 498) | (n = 11) | (n = 487) | |||
| Age, median (IQR), years | 65 (59–70) | 65 (59–70) | 65 (59–70) | 0.784 | |
| Sex | Male, n (%) | 205 (41.2) | 4 (36.4) | 201 (41.3) | >0.999 |
| Female, n (%) | 293 (58.8) | 7 (63.6) | 286 (58.7) | ||
| MPD, median (IQR), mm | 2.35 (1.7–3.2) | 3.8 (2.7–9.2) | 2.3 (1.7–6.3) | 0.005 | |
| Cyst | Median number | 1 (1–2) | 2 (0.5–2) | 1 (1–2) | 0.243 |
| Max cyst size median (IQR), mm | 14.0 (9.3–20.0) | 17.5 (15.0–22.5) | 13.0 (9.2–20.0) | 0.087 | |
| CA19-9, median (IQR), IU/mL | 12 (8–22) | 18 (12.5–23.75) | 12 (8–21) | 0.060 | |
| P-amylase, median (IQR), IU/L | 31 (25–38.75) | 22.5 (6–24) | 31 (25–39) | 0.107 | |
| Elastase, median (IQR), ng/dL | 112 (84–156) | 108 (72–169.5) | 113 (84–156) | 0.900 | |
| ALP, median (IQR), IU/L | 213 (177.25–262) | 217 (167–248.5) | 213 (178–252.5) | 0.441 | |
| FBS, median (IQR), mg/dL | 95 (88–101) | 100 (95–116) | 95 (88–101) | 0.030 | |
| Patient Number | Age, Years/Sex | Initial US | Last US | Findings Leading to a Detailed Examination | Diagnostic Method | Final Diagnosis | Management | c/pTNM Classification | Stage (UICC 8th) | OS, Year | Cause of Death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyst | MPD | Cyst | MPD | Imaging Modality | Findings/Location | Time to Detection, Year | |||||||||||
| Number | Size | Number | Size | ||||||||||||||
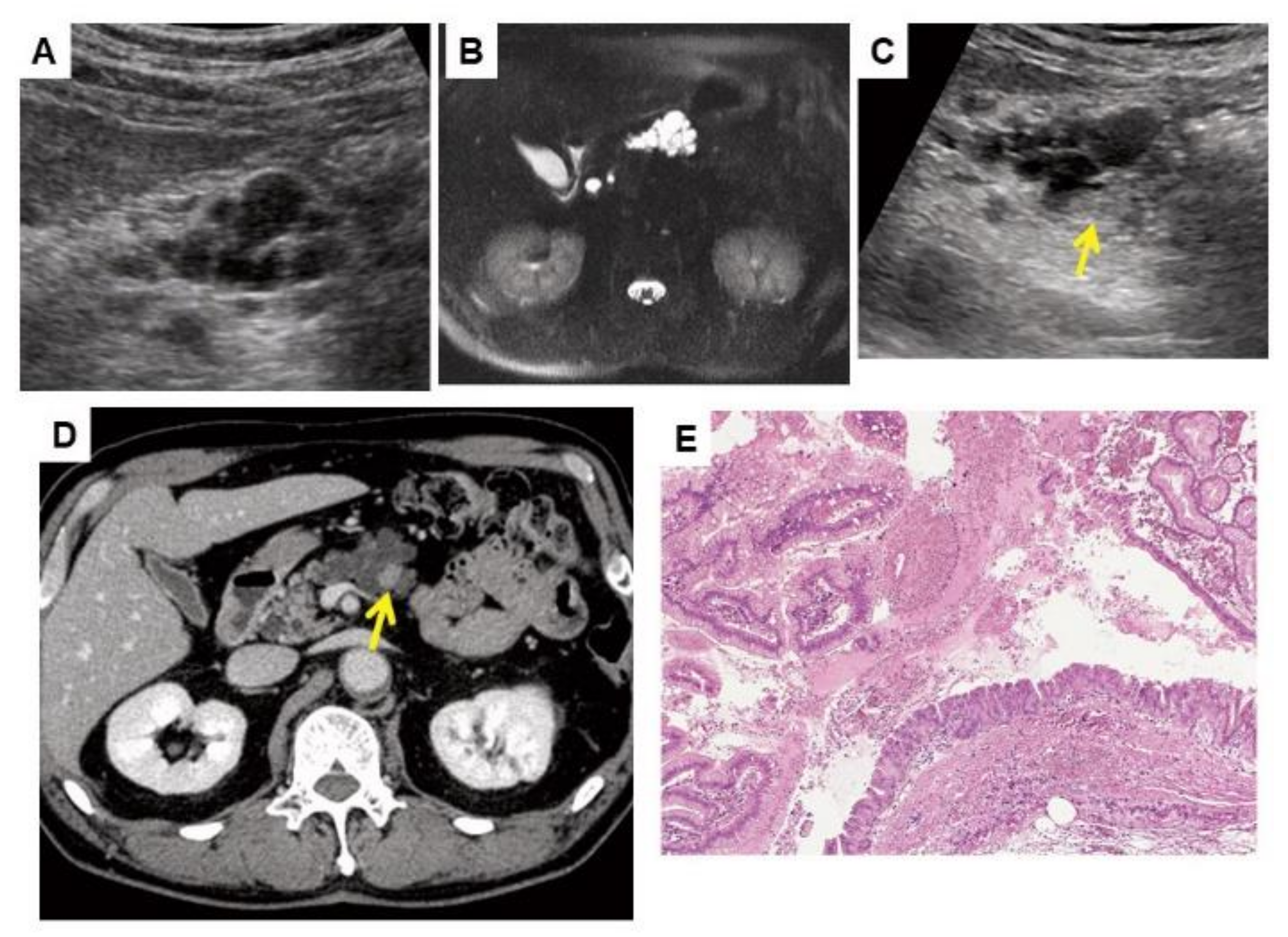
| 1 | 79/M | 2 | 18.0 | 5.1 | 5 | 20.0 | 9.8 | CECT | Hypovascular tumor/head | 4.9 | ERCP/PJC | IPMN with associated invasive carcinoma | No surgery (Chemotherapy) | cT4N0M0 | cStage III | Died, 2.1 | Cancer progression |
| 2 | 80/M | 4 | 33.0 | 9.2 | 4 | 54.0 | 18.4 | US | Hypoechoic mass/head | 8.0 | EUS-FNA | IPMN with associated invasive carcinoma | PD | pT3N2M0 | pStage III | Died, 1.7 | Cancer progression |
| 3 | 75/F | 0 | - | 4.3 | 0 | - | 7.5 | US | Rapid MPD dilatation/body | 6.0 | ERCP/PJC | PDAC (in situ) | PD | pTisN0M0 | pStage 0 | Alive, 8.7 | NA |
| 4 | 74/F | 2 | 16.0 | 3.4 | 4 | 35.0 | 3.5 | US | Cyst growth with appearance of a mural nodule/head | 3.0 | ERCP/PJC | IPMN-HGD | PD | pTisN0M0 | pStage 0 | Died, 8.8 | Other disease |
| 5 | 71/F | 2 | 12.0 | 2.2 | 5 | 19.0 | 3.6 | US | Hypoechoic mass/head | 3.8 | EUS-FNA | IPMN with associated invasive carcinoma | PD | pT2N1M0 | pStage IIB | Died, 5.3 | Cancer progression |
| 6 | 68/F | 1 | 17.0 | 1.2 | 2 | 19.0 | 1.6 | US | Hypoechoic mass/body | 3.3 | ERCP/PJC | IPMN-HGD | DP | pTisN0M0 | pStage 0 | Alive, 11.0 | NA |
| 7 | 70/F | 3 | 19.0 | 4.4 | 4 | 27.0 | 2.7 | Symptoms * | - | 5.0 | ERCP/PJC | PDAC concomitant with IPMN | No surgery (Chemotherapy) | cT2N0M0 | cStage IB | Died, 3.4 | Cancer progression |
| 8 | 64/F | 0 | - | 2.8 | 1 | 17.0 | 4.0 | US | Hypoechoic mass/body-tail | 5.3 | EUS-FNA | PDAC | PD after NACRT | ycT1bN0M0 (ypT0N0M0 <no residual tumor>) | ycStage IA | Alive, 7.5 | NA |
| 9 | 64/F | 0 | - | 2.6 | 0 | - | 5.1 | US | Hypoechoic mass/body | 4.8 | ERCP/PJC | PDAC | PD | pT1bN0M0 | pStage IA | Died, 5.0 | Cancer progression |
| 10 | 62/M | 1 | 40.0 | 6.5 | 2 | 50.0 | 13.0 | US | Cyst growth with appearance of a mural nodule/body | 4.8 | ERCP/PJC | IPMN-HGD | TP | pTisN0M0 | pStage 0 | Alive, 9.3 | NA |
| 11 | 61/M | 2 | 11.0 | 3.8 | 3 | 27.0 | 5.7 | MRI | Solid mass/tail | 4.3 | EUS-FNA | PDAC concomitant with IPMN | DP after NACRT | ycT2N0M0 (ypT1bN0M0) | ycStage IB | Alive, 3.4 | NA |
| Factor | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | <65 years | 1 | |||
| ≥65 years | 1.725 (0.502–5.935) | 0.39 | |||
| Sex | Female | 1 | |||
| Male | 0.850 (0.250–2.885) | 0.79 | |||
| MPD dilatation (≥2.5 mm) and/or pancreatic cysts (≥5 mm) | Single finding (either MPD dilatation or cysts) | 1 | 1 | ||
| Both findings | 5.082 (1.537–16.810) | 0.008 | 5.095 (1.519–17.090) | 0.008 | |
| Max cyst size | <15 mm | 1 | |||
| ≥15 mm | 3.742 (0.745–18.810) | 0.11 | |||
| CA19-9 | <15 IU/mL | 1 | |||
| ≥15 IU/mL | 2.162 (0.610–7.661) | 0.23 | |||
| P-amylase | <30 IU/L | 1 | |||
| ≥30 IU/L | 0.469 (0.148–1.490) | 0.2 | |||
| Elastase | <110 IU/mL | 1 | |||
| ≥110 IU/mL | 0.726 (0.226–2.337) | 0.59 | |||
| ALP | <215 IU/mL | 1 | |||
| ≥215 IU/mL | 1.204 (0.363–3.988) | 0.76 | |||
| FBS | <95 mg/dL | 1 | 1 | ||
| ≥95 mg/dL | 4.397 (0.998–19.37) | 0.05 | 4.412 (1.027–18.950) | 0.046 | |
| Factor | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| MPD growth rate/year | <0.2 mm | 1 | 1 | ||
| ≥0.2 mm | 30.150 (7.055–128.800) | <0.001 | 17.600 (3.547–87.310) | <0.001 | |
| Increase in cyst number | No | 1 | 1 | ||
| Yes | 4.793 (1.251–18.360) | 0.022 | 3.339 (0.986–11.310) | 0.053 | |
| Cyst size growth rate/year | <2 mm | 1 | 1 | ||
| ≥2 mm | 11.740 (3.342–41.260) | <0.001 | 4.417 (1.240–15.740) | 0.022 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, J.; Ikezawa, K.; Nakao, M.; Okagaki, S.; Ashida, R.; Ioka, T.; Takada, R.; Yamai, T.; Fukutake, N.; Uehara, H.; et al. Predictive Factors for Pancreatic Cancer and Its Early Detection Using Special Pancreatic Ultrasonography in High-Risk Individuals. Cancers 2021, 13, 502. https://doi.org/10.3390/cancers13030502
Fukuda J, Ikezawa K, Nakao M, Okagaki S, Ashida R, Ioka T, Takada R, Yamai T, Fukutake N, Uehara H, et al. Predictive Factors for Pancreatic Cancer and Its Early Detection Using Special Pancreatic Ultrasonography in High-Risk Individuals. Cancers. 2021; 13(3):502. https://doi.org/10.3390/cancers13030502
Chicago/Turabian StyleFukuda, Junko, Kenji Ikezawa, Miho Nakao, Suetsumi Okagaki, Reiko Ashida, Tatsuya Ioka, Ryoji Takada, Takuo Yamai, Nobuyasu Fukutake, Hiroyuki Uehara, and et al. 2021. "Predictive Factors for Pancreatic Cancer and Its Early Detection Using Special Pancreatic Ultrasonography in High-Risk Individuals" Cancers 13, no. 3: 502. https://doi.org/10.3390/cancers13030502
APA StyleFukuda, J., Ikezawa, K., Nakao, M., Okagaki, S., Ashida, R., Ioka, T., Takada, R., Yamai, T., Fukutake, N., Uehara, H., Nagata, S., Takahashi, H., Tabuchi, T., Tanaka, S., Ohkawa, K., & Katayama, K. (2021). Predictive Factors for Pancreatic Cancer and Its Early Detection Using Special Pancreatic Ultrasonography in High-Risk Individuals. Cancers, 13(3), 502. https://doi.org/10.3390/cancers13030502






