1. Introduction
Affibody molecules are small (molecular weight 7 kDa), engineered scaffold proteins that can be selected to bind with high affinity to a broad spectrum of biomolecules [
1]; they belong to a class of engineered scaffold proteins with potential for cancer diagnostics and therapy [
2]. Affibody molecules are well suited as radionuclide imaging probes due to their small size, high affinity, and selectivity to cancer-associated targets [
1]. Another advantage of affibody molecules is the high-yield recombinant production in prokaryotes. Affibody-based imaging probes for epidermal growth factor receptor (EGFR or HER1), human epidermal growth factor receptor type 2 (HER2), human epidermal growth factor receptor type 3 (HER3), platelet-derived growth factor receptor β (PDGFRβ), insulin-like growth factor-1 receptor (IGF-1R), vascular endothelial growth factor receptor 2 (VEGFR2), and programmed death ligand 1 (PD-L1) have demonstrated very promising features in preclinical experiments [
3]. Furthermore, excellent imaging of HER2 has been demonstrated in clinics [
4,
5].
The targeting of HER2 using monoclonal antibodies and antibody–drug conjugates extends the survival of breast and gastroesophageal cancer patients. However, an onset of resistance to such therapies is inevitable despite preserved HER2 expression [
6,
7]. A HER2-targeted radionuclide therapy might be a solution in this case. However, the mainstream approach to targeted radionuclide therapy, i.e., the use of radiolabeled monoclonal antibodies, is inefficient in solid tumors due to their long residence in circulation, causing excessive irradiation of bone marrow [
8]. Direct application of affibody molecules for radionuclide therapy is complicated due to high renal reabsorption and long retention of activity in the case of radiometal labels [
9]. In this case, the renal uptake exceeded tumor uptake by 10–20 fold [
3]. Common methods applied for the reduction of renal uptake of radiolabeled proteins and peptides have turned out to be inefficient for affibody molecules [
10,
11].
Our solution to the problem of high renal reabsorption of radiolabeled affibody molecules is applying pretargeting, a methodology that separates the acts of molecular recognition of cancer-associated abnormalities and radionuclide delivery [
12,
13]. In pretargeting, a target-specific primary agent, coupled to a recognition tag, is injected to localize in the tumor. After clearance of the primary agent from blood, a radiolabeled secondary probe with a high affinity to the recognition tag is injected. Low uptake of the secondary probe in kidneys is critical for successful affibody-based pretargeted therapy. Affibody molecules are attractive candidates as primary probes because they clear rapidly from blood and are slowly internalized by cancer cells [
14]. Importantly, affibody molecules are rapidly internalized by proximal tubule epithelial cells after renal reabsorption [
3]. Thus, affibody-based primary probes disappear from the lumen of the proximal tubule and cannot react with secondary probes in urine. After an evaluation of different approaches [
15,
16], the hybridization of complementary peptide nucleic acid (PNA) probes was selected for affibody-based pretargeting as it provided the best retention of activity in tumors. PNA is a class of synthetic DNA analogs capable of Watson–Crick base-pairing [
17,
18]. The PNA backbone is built up of repeating N-(2-aminoethyl)-glycine units connected by amide bonds, and purine and pyrimidine nucleobases are connected to this scaffold via carboxymethyl linkers. PNAs are resistant to degradation by nucleases and proteases and have shown excellent stability in human blood serum [
19]. They are nonimmunogenic and have low general toxicity. The molecular design of the first-generation of the primary agent Z
HER2:342-SR-
HP1 and the secondary probe
HP2 was successful as it provided high affinity and specificity of PNA hybridization, specific accumulation of the primary probes in tumors, and efficient specific delivery of radiometals [
16,
20]. Labeling of
HP2 with
177Lu [
13,
21],
111In [
16,
20], and
68Ga [
22] resulted in appreciably higher uptake in tumors than in kidneys, although the renal uptake was the highest among normal tissues. Experimental therapy using the Z
HER2:342-SR-
HP1/[
177Lu]Lu-
HP2 pretargeting system significantly increased the median survival of mice bearing HER2-expressing xenografts (66 days for the treated group compared to 32 days for [
177Lu]Lu-
HP2 only) without observable bone marrow and renal toxicity [
21]. However, a further increase in the ratio of absorbed dose to tumor, compared to doses to normal tissues, first and foremost to the kidneys, is necessary to obtain a curative effect of such treatment.
A possible optimization parameter is the length of the secondary probe. Intuitively, a reduction of the length would reduce the hydrodynamic radius of the probe, which might facilitate both its extravasation and diffusion in the tumor interstitium, improving both the localization in tumors and the uniformity of distribution inside the tumor. There are, however, apparent risks associated with a reduction of the secondary probe’s size. First, a reduction of the number of nucleobases might decrease the strength of hybridization with the primary probe. Second, modification of the base composition might affect off-target interactions, resulting in elevated uptake in normal tissues. For example, minor structural changes associated with the substitution of
177Lu by
111In or
68Ga resulted in significant differences in renal uptake [
22] or uptake in blood, liver, and bone [
23]. The biodistribution is further dependent not only on the number and nature of nucleobases but also on their order in a PNA sequence. The scrambling of nucleobases in
99mTc-labeled antisense PNA binding to mRNA encoding MYC protein resulted in more than a two-fold decrease of uptake in normal tissues [
24,
25]. Thus, experimental in vivo studies were required to evaluate if the second-generation secondary probes would provide a better biodistribution and dosimetry profile.
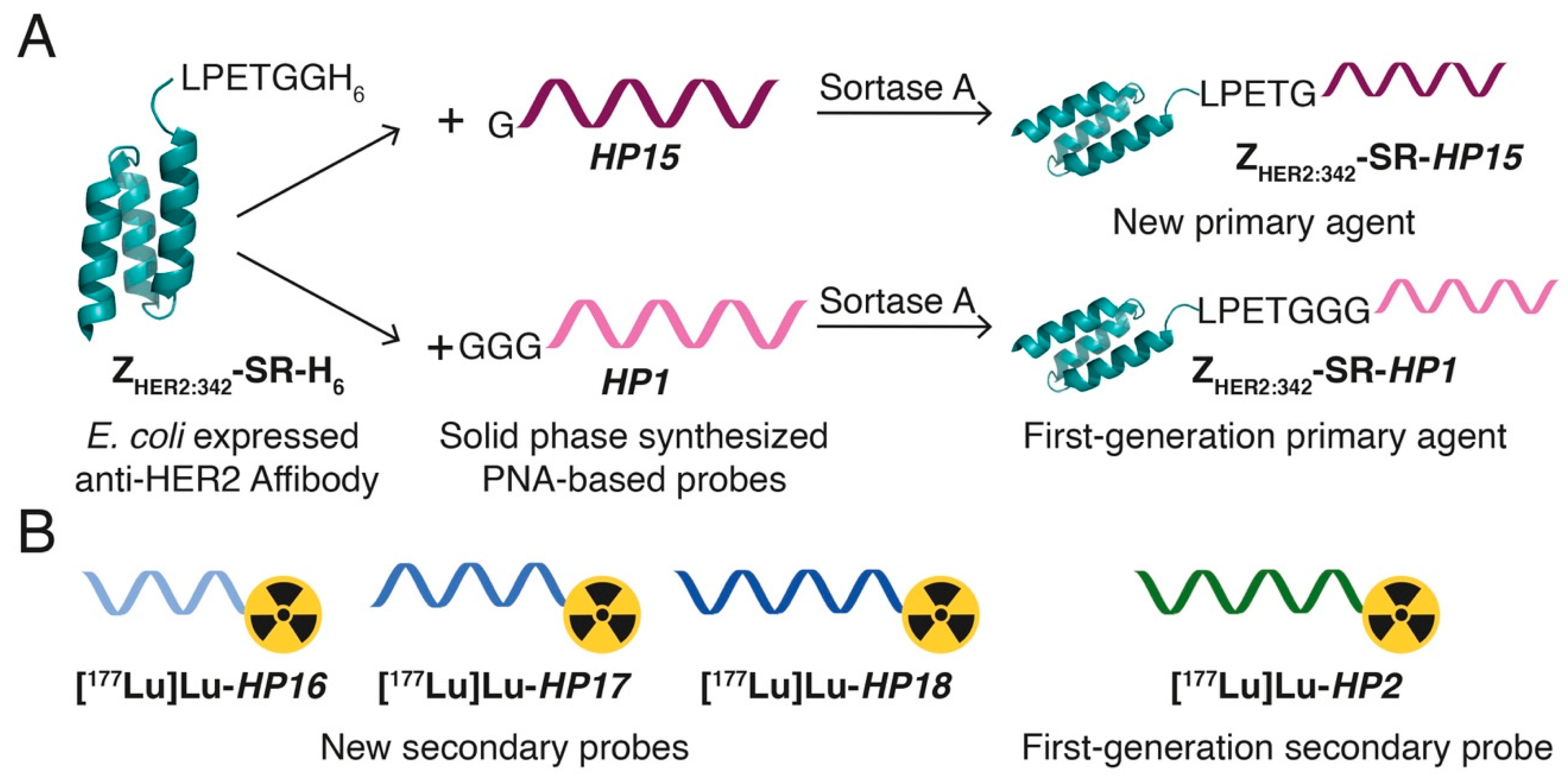
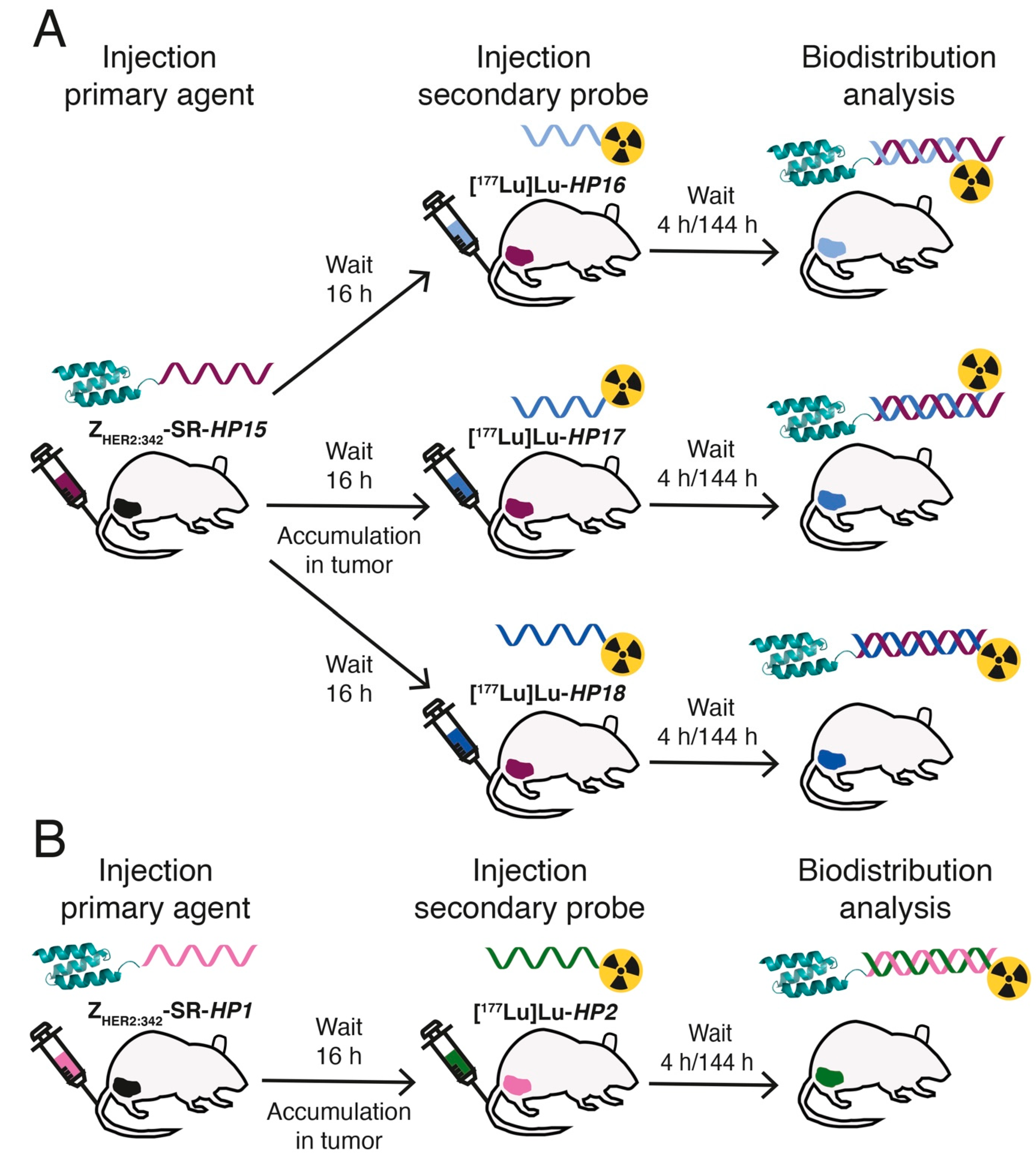
In this study, we have designed second-generation hybridization probes: the primary agent
HP15 and a set of secondary probes—
HP16 (9-mer PNA),
HP17 (12-mer PNA), and
HP18 (15-mer PNA) (
Table 1,
Figure 1). The versatile chelator DOTA was incorporated into the secondary probes to enable labeling with a variety of therapeutic nuclides (e.g.,
90Y,
177Lu,
225Ac). The same chelator was also incorporated in
HP15 to permit site-specific radiometal labeling of the primary targeting agent, facilitating preclinical cellular processing and biodistribution studies and clinical pharmacokinetics evaluation. The specificity and strength of pretargeting, as well as the cellular processing of the primary agent and secondary probes, were evaluated in vitro. Biodistribution of the new
177Lu-labeled secondary probes and pretargeting specificity were evaluated in vivo and compared with the biodistribution of the first-generation secondary probe [
177Lu]Lu-
HP2 (
Figure 2). For estimation of absorbed doses to tumors and kidneys, a clinically validated two-time point approach was used [
26].
3. Discussion
Our previously published PNA pretargeting studies were based on PNA probes having 15 complementary bases, resulting in a very strong affinity between the hybridized probes. Measurement of the binding interaction between these probes by SPR-based biosensor analysis showed that the dissociation rate was extremely slow and that less than 5% of hybridized PNA were dissociated during a 17-h long experiment [
20]. It is likely that such a high affinity is not necessary and that shorter complementary probes would have a sufficiently high binding affinity for successful in vivo pretargeting. In this study, we evaluated how the length of the PNA sequences in the secondary probes would influence pretargeting efficacy. Instead of simply shortening the previously described secondary probe
HP2, we decided to redesign a new set of primary and secondary PNA probes and studied their efficacy in affibody-mediated pretargeting.
Three secondary PNA probes with varying lengths (9-mer, 12-mer and 15-mer) were designed, aiming for an improved biodistribution profile, facilitated production, and improved aqueous solubility while retaining high specificity and affinity between the complementary PNA hybridization probes. The probes were designed to avoid self-complementary sequences as well as extended stretches of purines (A and G), which are known to promote aggregation and be difficult to synthesize and purify after synthesis [
27].
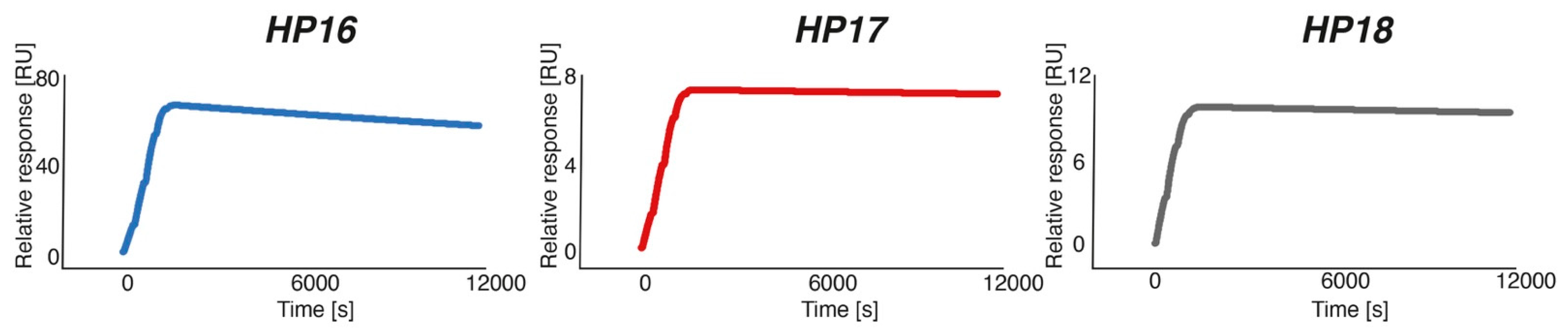
Characterization by SPR showed that the new secondary probes associated rapidly with Z
HER2:342-SR-
HP15 (k
a was in the range of 4.3–5.7 × 10
4 M
−1s
−1), although the association rate was slightly lower compared to the rate for Z
HER2:342-SR-
HP1-
HP2 hybridization (k
a = 1.7 × 10
5 M
−1 s
−1). The
HP17 (12-mer) and
HP18 (15-mer) probes both had such slow dissociation rates after binding to Z
HER2:342-SR-
HP15 that the rate constants could not reliably be estimated from the SPR analysis. A K
D of 280 pM could be determined for the (weaker) interaction between Z
HER2:342-SR-
HP15 and
HP16 (9-mer), which was expected to be of sufficiently high affinity for the intended pretargeting application. Bispecific monoclonal antibodies binding to the haptens used in pretargeting have been reported to be in the nanomolar affinity range [
28]. One example is the earlier demonstrated pretargeting using a bispecific antibody construct binding the tumor-associated antigen CEA and a radiolabeled hapten, with an estimated K
D of only 10
−9–10
−10 M for binding to the
111In-labeled benzyl EDTA derivative [
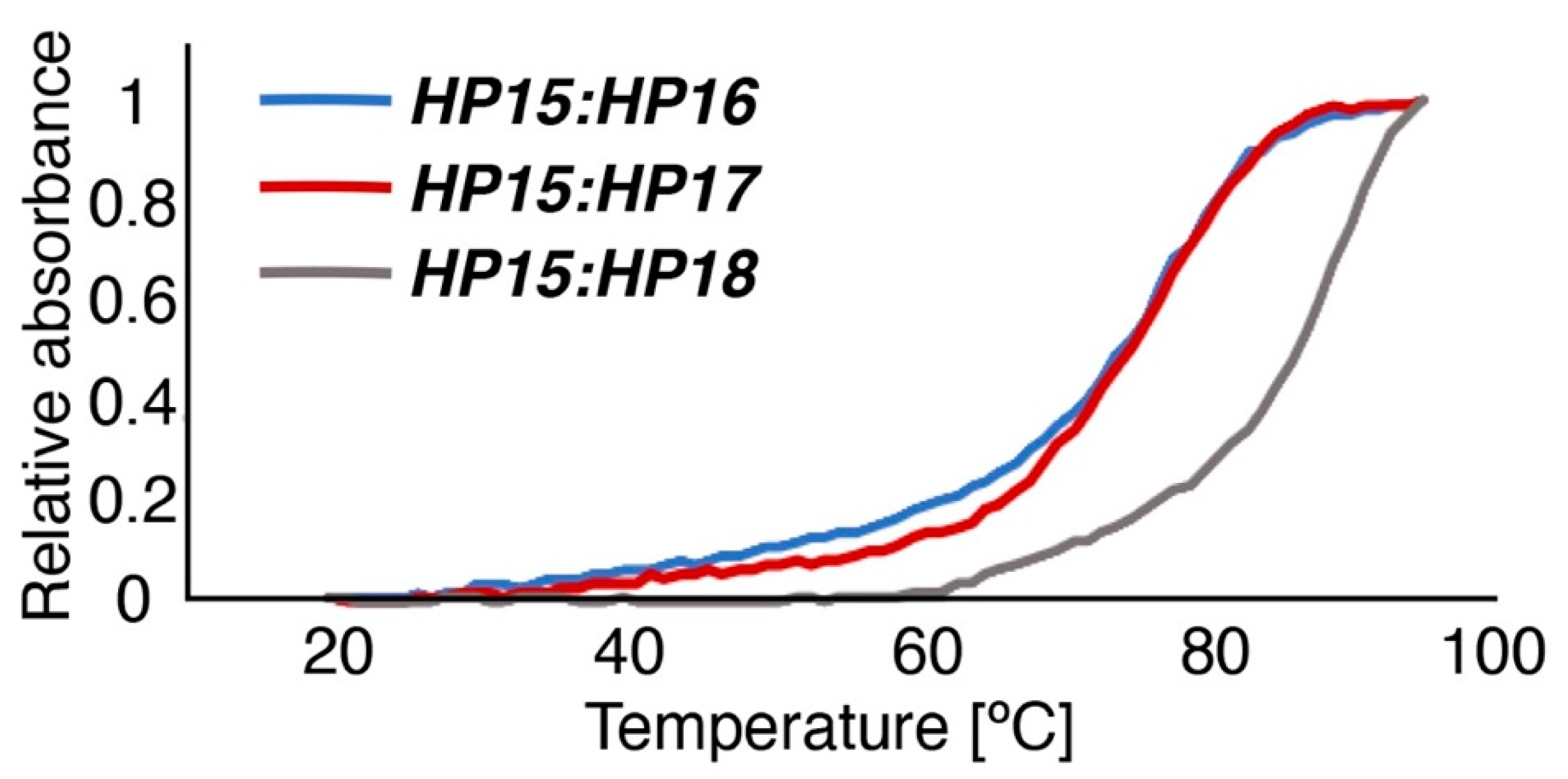
29]. The melting temperatures for duplexes of primary and secondary probes, measured by UV spectroscopy, were nearly equal for
HP15:
HP18 (87 °C) and
HP1:
HP2 (86 °C), i.e., the hybridization strength was approximately the same for the 15-mer probes of first and second generations. Compared to
HP15:
HP18, the melting temperatures for
HP15:
HP16 and
HP15:
HP17 showed a drop in Tm to 73 and 75 °C, respectively, confirming that the stability of the duplexes correlates with the length of the PNA sequences.
The radiolabeling of the new secondary probes was efficient and stable (
Table 3). The binding of [
177Lu]Lu-Z
HER2:342-SR-
HP15 to HER2-expressing cell lines in vitro was HER2-specific (
Figure 5A). The slow internalization of [
177Lu]Lu-Z
HER2:342-SR-
HP15 was favorable for pretargeting application as this enables the long persistence of the primary hybridization probe on the targeted cells’ surface (
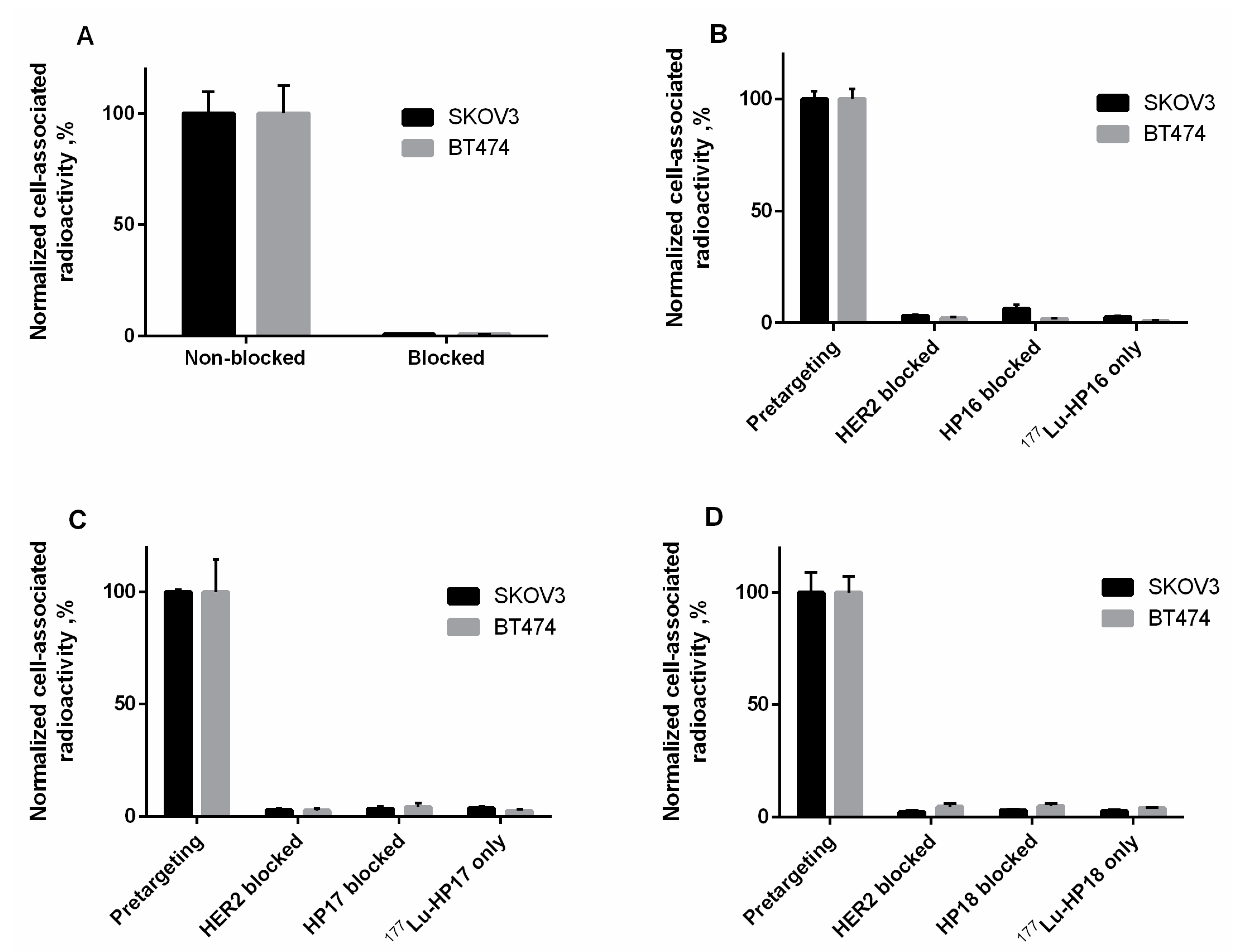
Figure S14). A set of in vitro tests (
Figure 5B–D) demonstrated that pretargeting of new secondary probes in vitro was dependent on the specific binding of primary probes to cells (dramatic reduction of binding in the case of blocking HER2 or in the absence of primary probes) and that interaction between primary and secondary probes was PNA-mediated (dramatic reduction in the case of presaturation with unlabeled PNA). LigandTracer measurements of the kinetics of binding to living SKOV3 cells, which were pretreated with Z
HER2:342-SR-
HP15, demonstrated that the binding of all secondary probes was extremely strong. There was no difference in apparent dissociation constants between secondary probes (
Table S1). Thus, reduction of length of PNA from 15 to 9 nitrogenous bases was not associated with any observable reduction of their binding to primary probes in the cell assay.
The biodistribution of the new secondary probes without the preinjection of primary probes demonstrated rapid clearance from normal tissues (
Table 4). The shorter probes, [
177Lu]Lu-
HP16 and [
177Lu]Lu-
HP17, had lower uptake in blood, liver, and kidneys than [
177Lu]Lu-
HP18. A comparison with previously reported radiolabeled PNA–peptide chimeras indicates that our design of secondary probes was quite fortunate. For example,
111In-labeled DOTA-anti-bcl-2-PNA-Ala [
3,
4,
5,
6], a short peptide-18-mer-PNA chimera [
30], had, at 4 h after injection, at least one order of magnitude higher blood uptake and two- to three-fold higher liver uptake compared to our conjugates. The difference was even more striking when compared with
64Cu-labeled chimeras [
64Cu]Cu-DOTA-Y-PNA50-K4 (18-mer) and [
64Cu]Cu-DOTA-Y-PNA50S-K4 (15-mer), having renal uptake at 4 h after injection of 36.1 ± 3.6% and 60.5 ± 3.6% ID/kidney, respectively [
31]. It can be noted that [
64Cu]Cu-DOTA-Y-PNA50S-K4 had the same length of PNA as [
177Lu]Lu-
HP18 but a much higher renal uptake. This suggests that the length of PNA is not the only factor determining uptake in normal tissue; the composition of nucleobases and flanking amino acids also play an important role, which has not been sufficiently investigated and will require regular structure–property relationship studies in the future.
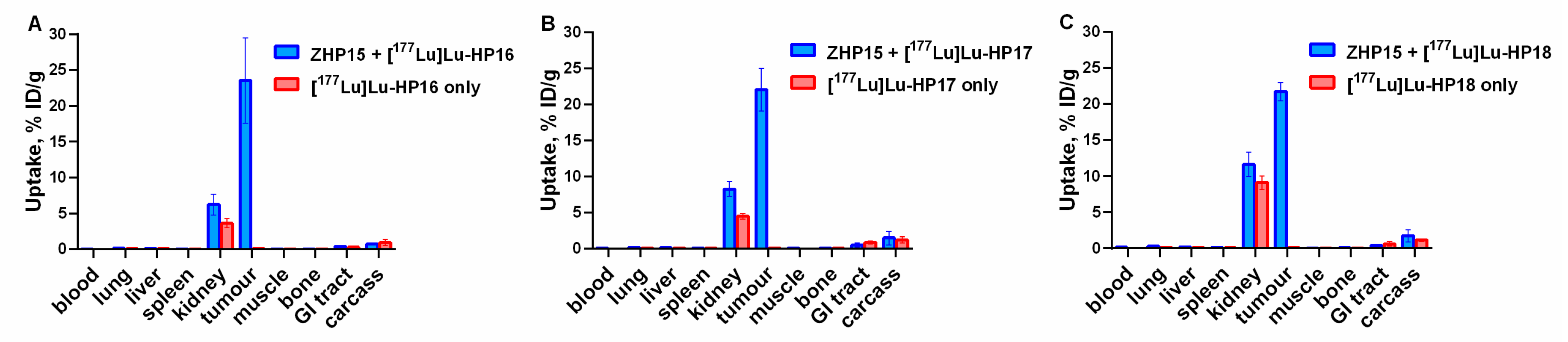
The dramatic increase of uptake of the secondary probes in tumors (
Figure 6) after preinjection of primary agents convincingly shows the specificity of pretargeting. Interestingly, an increased uptake of secondary probes after injection of Z
HER2:342-SR-
HP15 was also observed in blood, kidneys, spleen, and muscles. This might be explained by the association of the secondary probes with the primary agent, which had not completely cleared from circulation or had re-entered the blood flow after dissociation from receptors in the tumor. This effect was less pronounced for [
177Lu]Lu-
HP16, which was unexpected as it can be anticipated that the level of the primary agent is the same in all preinjected mice.
Another interesting observation was the relatively uniform distribution of activity in xenografts for all variants after pretargeting (
Figures S15 and S16). The observed heterogeneity was rather associated with the distribution of malignant and connective tissues within a tumor than with the length of PNA in the secondary targeting probe. Thus, macroscopic uniformity of distribution of our [
177Lu]-labeled probes is not dependent on the length of PNA. It has to be noted that the existing nonuniformity would be compensated by the cross-fire effect.
Tumor uptake exceeded, by several hundred-fold, the uptake in the majority of tissues. It is apparent that only the kidneys would be a dose-limiting organ in a therapeutic context. Thus, the best choice would be a variant that provides the highest ratio between absorbed doses to tumor and to kidneys. A simplified assessment of this was performed by comparing areas under time-activity plots. This estimation suggests that the original [
177Lu]Lu-
HP2 and the newly designed [
177Lu]Lu-
HP18 15-mer-based probes provide similar ratios of doses to tumor and kidneys. The tumor-to-kidney dose ratio for [
177Lu]Lu-
HP17 (12-mer) was 1.5-fold higher than for [
177Lu]Lu-
HP2 and [
177Lu]Lu-
HP18. [
177Lu]Lu-
HP16 provided the highest tumor-to-kidney dose ratio. Thus, in the case of a successful overall design of the secondary PNA-based probe, a shorter PNA sequence provides the best dose ratio between tumor and kidneys. Currently, the renal dose limit for
177Lu-based radionuclide therapy is considered 28–40 Gy, depending on risks associated with the status of the patient’s renal function [
32]. If the preclinical data was translated to humans, the use of [
177Lu]Lu-
HP16 would result in a tumor-absorbed dose of 120-160 Gy. Such level of tumor dose is associated with a pronounced tumor response during
177Lu-based radionuclide therapy in the clinic [
33]. An important aspect of the use of our system for radionuclide therapy is a washout of activity from tumors. The tumor-associated activity of [
177Lu]Lu-
HP16 was reduced from 24 ± 6% ID/g at 4 h to 3 ± 1% ID/g at 144 h after injection. Such washout effect can be observed for other pretargeting systems [
34,
35] and also for short peptides having short residence in circulation (see, e.g., [
36] for somatostatin and [
37] for bombesin analogs). This might be explained partially by dissociation of the targeting agent from the cell surface target (in the case of a pretargeting system, this might be the dissociation of the secondary probe from the primary probe or the dissociation of the whole primary–secondary probe complex from the target). The release of internalizing [
177Lu]-DOTA-TATE [
34] from the tumor suggests that a release of intracellular metabolites might play a certain role even in the case of residualizing radiometal labels. In principle, this might be a challenge for the delivery of a sufficient absorbed dose to a tumor. However, the use of Z
HER2:342-SR-
HP1 in combination with [
177Lu]Lu-
HP2 provided a significant extension of survival of treated tumor-bearing mice [
21]. The profile of activity retention in tumor for that system (17 ± 3% ID/g at 4 h and 3.4 ± 0.6% ID/g at 144 h after injection) was similar to the profile for the Z
HER2:342-SR-
HP15:[
177Lu]Lu-
HP16 system. This suggests that radionuclide therapy using the Z
HER2:342-SR-
HP15:[
177Lu]Lu-
HP16 system is feasible. One or a few additional pretargeting cycles might be necessary to obtain the full effect. Apparently, these additional cycles will not cause an unacceptably high dose to kidneys.
4. Materials and Methods
4.1. Synthesis and Purification of PNA Pretargeting Probes
Peptide nucleic acid monomers, Fmoc-PNA-A(Bhoc)-OH, Fmoc-PNA-G(Bhoc)-OH, Fmoc-PNA-C(Bhoc)-OH and Fmoc-PNA-T-OH, were purchased from PolyOrg Inc. (Leominster, MA, USA). Rink Amide resin (ChemMatrix, 0.50 mmol/g) was purchased from Biotage (Uppsala, Sweden). 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was purchased from CheMatech (Dijon, France). Fmoc-NH-(PEG)2-CH2COOH (AEEA) was purchased from Merck KGaA (Darmstadt, Germany). Solvents and reagents for solid phase synthesis were obtained from commercial suppliers and used without further purification.
HP15 was synthesized on a Biotage Initiator+ Alstra microwave peptide synthesizer using Rink Amide resin (ChemMatrix, 0.50 mmol/g) on a 50-μmol scale in a 10-mL reactor vial. Fmoc deprotection was performed at RT in two stages by treating the resin with piperidine–DMF (1:4) for 3 min, followed by piperidine–DMF (1:4) for 10 min. Couplings were performed using 5 eq of PNA or amino acid monomer, 5 eq Oxyma, and 5 eq DIC in DMF. A coupling time of 10 min at 75 °C was used throughout the sequence, followed by a capping step using NMP–lutidine–acetic anhydride (89:6:5) for 2 min.
Orthogonally protected Lys(Mtt) was introduced for the possibility of site-specific introduction of DOTA. Automated synthesis was interrupted after four coupling steps (Fmoc-E-K(Mtt)-[AEEA]-E-resin) for selective side-chain deprotection of Lys(Mtt) with 5–10 additions of fresh TFA:TIS:DCM (1:2:97) followed by 1 min vortexing. Coupling of DOTA was performed using 5 eq of DOTA, 5 eq Oxyma, and 5 eq DIC in DMF at RT for 1 h. After resuming the automated synthesis and completion of all cycles, the resin was washed with DMF, DCM, and, finally, with MeOH and then dried overnight. The PNA–peptide hybrid was cleaved from the solid support using a mixture of TFA:H2O:TIS (95:2.5:2.5) for 4 h at RT. The PNA product was finally extracted between diethyl ether and water and lyophilized from the aqueous phase.
The synthesis of the complementary PNA probes
HP16,
HP17, and
HP18 was performed as described previously [
20]. In brief, coupling was performed with an excess of PNA monomers, benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP; Sigma Aldrich), and the presence of DIEA in NMP and DMF. After coupling, a capping step, followed by the removal of Fmoc-protecting groups, was conducted. After completed synthesis, the PNA probes were cleaved from the resin and ether extracted analogously to
HP15.
RP-HPLC purification was performed using a semipreparative Zorbax 300 SB-C18 column (9.4 × 250 mm, 5 µm particle size; Agilent, Santa Clara, CA, USA) with a linear gradient of 5–50% B, where A = 0.1% TFA-H2O and B = 0.1% TFA-CH3CN, for over 25 min, using a flow rate of 3 mL/min, a column temperature of 70 °C, and UV detection at 220 and 260 nm. Collected fractions were analyzed by MALDI-TOF (4800 MALDI-TOF/TOF, AB SCIEX) using an α-cyano-4-hydroxycinnamic acid matrix. Fractions containing the correct products were pooled and lyophilized.
The purity of
HP16,
HP17, and
HP18 was confirmed using analytical RP-HPLC on a Zorbax 300SB-C18 column (4.6 × 150 mm, 3.5 µm particle size; Agilent;
Figure S1), followed by MALDI-TOF analysis (
Figures S2–S4). Extinction coefficients at 260 nm (ε
260) were estimated for each PNA probe based on the PNA composition and the extinction coefficient of each PNA monomer (A: 13 700 M
−1cm
−1, C: 6 600 M
−1cm
−1, G: 11 700 M
−1cm
−1, and T: 8 600 M
−1cm
−1). Extinction coefficients used throughout all experiments for each probe are the following;
HP16: 98 000 M
−1cm
−1,
HP17: 126 900 M
−1cm
−1,
HP18: 155 800 M
−1cm
−1, and
HP15: 150 700 M
−1cm
−1.
4.2. Production of Affibody–PNA Conjugate
Expression and purification of Z
HER2:342-SR-H
6 were based on a previously described method [
20]. Briefly, Z
HER2:342-SR-H
6 was expressed in
E. coli BL21(DE3) cells and harvested after induction by IPTG and incubation overnight at RT. Harvested cells were resuspended in a Tris-based binding buffer and subsequently lysed by sonication. Purification was performed using an IMAC matrix (HisPur Cobalt Resin, Thermo Scientific, Rockford, IL, USA), and Z
HER2:342-SR-H
6 was eluted in 20 mM Tris-HCl, 300 mM NaCl, and 300 mM imidazole, pH 7.5. Eluted Z
HER2:342-SR-H
6 was buffer-exchanged to sortase A conjugation buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM CaCl
2, pH 7.5) on a PD-10 desalting column (GE Healthcare, Uppsala, Sweden). The purity and molecular weight of Z
HER2:342-SR-H
6 was confirmed using SDS-PAGE and MALDI-TOF (
Figures S5 and S6).
Z
HER2:342-SR-H
6 was site-specifically conjugated to
HP15 using sortase A-mediated ligation (SML). The SML method described below is based on a protocol for affibody–PNA conjugation previously published by our group [
13]. The glycine-modified
HP15 probe was dissolved in 10% DMSO and heated at 80 °C for 5 min before the concentration was estimated.
HP15, Z
HER2:342-SR-H
6, and NiCl
2 were mixed in sortase A conjugation buffer. The reaction proceeded for 30 min after the addition of Sortase A3*, and the mixture was subsequently subjected to a reverse IMAC step. The conjugation product, hydrolyzed protein byproducts, and unconjugated
HP15 could be collected in the flow-through. Flow-through was buffer-exchanged to 10 mM NaOAc pH 3.6 and lyophilized. The Z
HER2:342-SR-
HP15 conjugate was purified on RP-HPLC using the same column and solvents for purification of PNA probes but with a gradient going from 5% to 50% B in 25 min; the absorbance was monitored at 220 and 260 nm (
Figure S7). Fractions of the Z
HER2:342-SR-
HP15 conjugate were collected, lyophilized, and analyzed by MALDI-TOF. The purity of the conjugate was confirmed by analytical HPLC (Zorbax 300SB-C18, 3.5 µm particle size, 4.6 × 150 mm, Agilent) and electrospray ionization mass spectrometry (ESI-MS), (Thermo Ultimate3000, Thermo Fisher Scientific, Waltham, MA, USA, + Bruker Impact II, Bruker Daltonics, Billerica, MA, USA) (
Figures S7 and S8).
Final concentrations of the PNA probes and ZHER2:342-SR-HP15 were determined by measuring absorbance at 260 nm. The same extinction coefficient was used for both HP15 and ZHER2:342-SR-HP15 as the contribution from the protein part to the total absorbance at 260 nm was considered to be negligible.
4.3. Characterization of the PNA Pretargeting Probes
The kinetic parameters for hybridization of the PNA probes were analyzed using surface plasmon resonance (SPR) on a Biacore 8K instrument (GE Healthcare). A dextran chip Series S Sensor CM5 (GE Healthcare) was functionalized with ZHER2:342-SR-HP15 on three surfaces to 385, 194, and 185 resonance units (RU) using standard amine coupling procedures. The surface with 385 RU was used for kinetic measurements of HP16; 194 RU was used for HP17, and 185 RU was used for HP18. A reference surface was subjected to activation, followed by deactivation. The complementary PNA probes, HP16, HP17, and HP18, were injected at 5 concentrations—22.6, 45.3, 90.6, 181.25, and 362.5 nM—using a single-cycle injection. Association for each concentration was allowed for 300 s, followed by the next injection and association phase. After injection of the final concentration, dissociation was allowed for 10,000 s (2 h 47 min) before regeneration with 10 mM HCl for 30 s, followed by 15 mM NaOH for 30 s. All runs were performed in PBST (0.05% Tween-20) pH 7.4 using a flow rate of 50 µl/min at 25 °C. Kinetic parameters were calculated using the 1:1 binding model in Biacore Insight Evaluation software.
Melting temperatures for all three PNA hybridization complexes (
HP15:
HP16,
HP15:
HP17, and
HP15:
HP18) were determined by monitoring UV absorbance at 260 nm (Chirascan, Applied Photophysics) at a temperature range from 20 to 95 °C, using a temperature change of 1 °C/min. The PNA complexes were heated to 80 degrees for 5 min and were then allowed to hybridize at room temperature for 5 min prior to UV monitoring at room temperature for 5 min (
Figure 4). CD spectra of PNA:PNA hybridization complexes (
Figure S10) were collected before and after the determination of thermal denaturation.
4.4. Radiolabeling and In Vitro Stability
Radiolabeling of the primary and secondary PNA probes with
177Lu was performed using a previously described method [
21]. Briefly, 30 μg of peptide was dissolved in 100 μL of ascorbic acid (1 M, pH 5.5) by heating at 95 °C for 10 min, followed by sonication for 5 min to ensure total dissolving. Then, 3 μL (60–120 MBq) of [
177Lu]LuCl
3 was added, followed by vortexing. The mixture was incubated at 95 °C for 60 min. The reaction mixture was analyzed by radio-ITLC, eluted with 0.2 M citric acid, pH 2.0.
To remove loosely bound 177Lu, a treatment with an excess of ethylenediaminetetraacetic acid tetra sodium salt (EDTANa4) was performed initially. A freshly prepared solution of EDTANa4 (10 mg/mL in Milli-Q water) was added in 1000-fold molar excess to the reaction mixture and incubated at 95 °C for 10 min. This step was found unnecessary for new secondary probes due to the high radiochemical yield, purity, and stability of the label. [177Lu]Lu-HP16, [177Lu]Lu-HP17, and [177Lu]Lu-HP18 were used in biological in vitro and in vivo studies without any additional purification. For [177Lu]Lu-HP2 and [177Lu]Lu-ZHER2:342-SR-HP15, a purification was performed after EDTA treatment by size exclusion chromatography using disposable NAP-5 columns, pre-equilibrated and eluted with 1% BSA/PBS.
To evaluate stability, a fraction of the freshly radiolabeled conjugate (0.4 µg) was incubated with a 500-fold molar excess of EDTA for 60 min at 37 °C. Incubation was also performed in PBS as a control. The test was run in triplicates.
To validate the results of radio-ITLC, reversed phase-HPLC conducted on an Elite LaChrom system (Hitachi, VWR, Darmstadt, Germany) consisting of an L-2130 pump, a UV detector (L-2400), and a radiation flow detector (Bioscan, Washington, DC, USA) coupled in series was used. Purity analysis of 177Lu-labeled compounds was performed using an analytical column (Phenomenex, Aschaffenburg, Germany; Luna® 5 µm C18, 100 Å; 150 × 4.6 mm column). The HPLC conditions were as follows: A = 10 mM TFA/H2O; B = 10 mM TFA/acetonitrile; UV-detection at 220 nm; gradient elution: 0–15 min at 5% to 70% B, 15–18 min at 70% to 95% B, 19–20 min at 5% B; flow rate was 1.0 mL/min.
4.5. In Vitro Studies
Cells were seeded in cell-culture dishes with a density of 106 cells/dish. A set of three dishes was used for each data point.
The specificity of [177Lu]Lu-ZHER2:342-SR-HP15 binding to HER2-expressing cells was tested by the incubation of the cells with 1 nM of labeled conjugate for 1 h at 37 °C. To saturate the receptors, unlabeled ZHER2:342 (1000 nM) was added to a control set for 5 min before adding a radiolabeled probe.
An in vitro pretargeting specificity assay for novel [
177Lu]Lu-
HP16, [
177Lu]Lu-
HP17, and [
177Lu]Lu-
HP18 was performed using four sets of cell dishes, as described earlier [
16]. To demonstrate the pretargeting, one set of dishes was incubated with Z
HER2:342-SR-
HP15 (1 nM) for 1 h at 37 °C and washed. A
177Lu-labeled secondary probe (10 nM) was added, and cells were incubated for 1 h at 37 °C. To show that the pretargeting was HER2-mediated, the second set of dishes was incubated with an excess of affibody molecules Z
HER2:342 (1000 nM) for 5 min before adding Z
HER2:342-SR-
HP15. A
177Lu-labeled secondary probe (10 nM) was added, and cells were incubated for 1 h at 37 °C. To demonstrate that pretargeting was PNA-mediated, the third set of dishes was incubated with Z
HER2:342-SR-
HP15, followed by incubation with an excess of a nonlabeled secondary probe (300 nM) for 30 min, and then the
177Lu-labeled secondary probe was added, followed by 1 h incubation. In the fourth set, the cells were incubated only with the
177Lu-labeled secondary probe to assess nonspecific binding. At the end of the incubation, the cells were washed and detached by trypsin, and the radioactivity in cells was measured to calculate the percent of cell-bound radioactivity.
To evaluate the binding affinity of the radiolabeled conjugates to HER2 receptors and to a cell-bound primary probe, the kinetics of binding of 177Lu-labeled probes to, and their dissociation from, SKOV3 cells were measured using a LigandTracer yellow instrument (Ridgeview Instruments AB, Vänge, Sweden). SKOV3 cells (3 × 106 cells/dish) were seeded on a local area of a cell-culture dish (NunclonTM, Size 100620, NUNC A/S, Roskilde, Denmark). The SKOV3 cells were presaturated with ZHER2:342-SR-HP15 (1 nM) in two sets of dishes for 2 h and, thereafter, washed three times to remove the unbound primary agent. Two increasing concentrations of the radiolabeled molecules (for [177Lu]Lu-ZHER2:342-SR-HP15: 180 and 540 pM, and for 177Lu-labeled secondary probes: 1 and 5 nM) were added. The data was analyzed using Interaction Map software (Ridgeview Diagnostics AB, Uppsala, Sweden) to calculate the association rate constant (ka), the dissociation rate constant (kd), and the equilibrium dissociation constant (KD). The analysis was performed in duplicate.
Cellular processing and retention on SKOV3 and BT474 cells were studied during interrupted incubation by an acid-wash method [
14].
4.6. In Vivo Studies
Animal studies were planned in agreement with EU Directive 2010/63/EU for animal experiments and Swedish national legislation concerning the protection of laboratory animals and were approved by the Ethics Committee for Animal Research in Uppsala, Sweden (animal permission C4/16). For tumor implantation, 107 SKOV3 cells were subcutaneously injected on the right hind leg of female BALB/c nu/nu mice. The biodistribution experiments were performed two weeks after cell implantation. The average animal weight was 18 ± 1 g. The average tumor weight was 0.23 ± 0.11 g. For biodistribution measurement, the mice were euthanized at predetermined time points by an overdose of anesthesia (ketamine/xylazine), followed by heart puncture. The organs of interest and the tumor were collected and weighed, and their radioactivity was measured. The percentage of the total injected dose per gram of sample (% ID/g) was calculated.
The pretargeting protocol used in this study has previously been optimized for the affibody-based PNA-mediated therapy using [
177Lu]Lu-
HP2 [
21]. Upscaling experiments showed no significant changes in the biodistribution of [
177Lu]Lu-
HP2 when the injected mass of both primary and [
177Lu]Lu-
HP2 was doubled. Based on this study, 50 µg of primary agents and equimolar amounts of secondary agents (0.69, 0.85, 1, and 1 µg for
HP16,
HP17,
HP18, and
HP2, respectively) were injected per mouse.
For biodistribution studies, mice were randomized into groups of five. The 30 mice were intravenously injected with ZHER2:342-SR-HP15 (50 µg, 4 nmol in 100 μL PBS per mouse). Sixteen hours later, all mice were injected with [177Lu]Lu-HP16, [177Lu]Lu-HP17, or [177Lu]Lu-HP18 (194 pmol in 100 μL 2% BSA in PBS, 170 kBq). The biodistribution was measured at 4 and 144 h after injection of secondary probes. For comparison, the biodistribution of first-generation [177Lu]Lu-HP2 was measured in the same way using ZHER2:342-SR-HP1 as the primary agent.
To evaluate in vivo specificity, the biodistribution of [177Lu]Lu secondary probes was measured 4 h after injection, without the preinjection of a primary agent.
After the gamma-counter measurements were completed, tumors were embedded in a cryomedium (Neg-50, Thermo Scientific) and frozen at −80 °C. Frozen tumors were cut into serial sections (30 µm thick) using a cryomicrotome (CryoStar NX70, Thermo Scientific) and thaw-mounted on glass slides. For digital autoradiography, the slides with sections were put in a cassette and exposed to phosphor screens overnight. The phosphor screens were scanned by a Cyclone Storage Phosphor System at 600 dpi resolution and analyzed using OptiQuant software (PerkinElmer, Waltham, MA, USA).
To estimate a ratio of absorbed doses in tumor and kidneys, cumulated activity in kidney and tumor were assessed. The estimation was based on a clinically validated two-time point approach [
26]. The biodistribution data were nondecay-corrected, and areas under the time-activity plot were calculated using GraphPad Prism software. The assumptions were that the main absorbed dose would be due to betaparticles, as cross-doses would be negligible, and the absorbed fraction would be equal to 1.
4.7. SPECT/CT Imaging
Mice bearing SKOV3 xenografts were injected i.v. with 8 nmol of primary agent 16 h before injection of secondary 177Lu-labeled probes (680 pmol, 9–13 MBq). Immediately before imaging (4 h after injection), the animals were sacrificed by CO2 asphyxiation. SPECT imaging was performed using NanoScan SC (Mediso Medical Imaging Systems, Budapest, Hungary). CT acquisitions were carried out using the X-ray energy of 50 keV; 20-min SPECT helical scans were acquired using energy windows 50–62, 103–124, and 188–230 keV. The data were reconstructed using Tera-Tomo™ 3D SPECT Software.