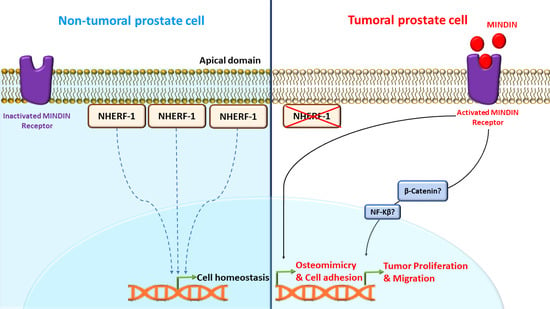
MINDIN Exerts Protumorigenic Actions on Primary Prostate Tumors via Downregulation of the Scaffold Protein NHERF-1
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
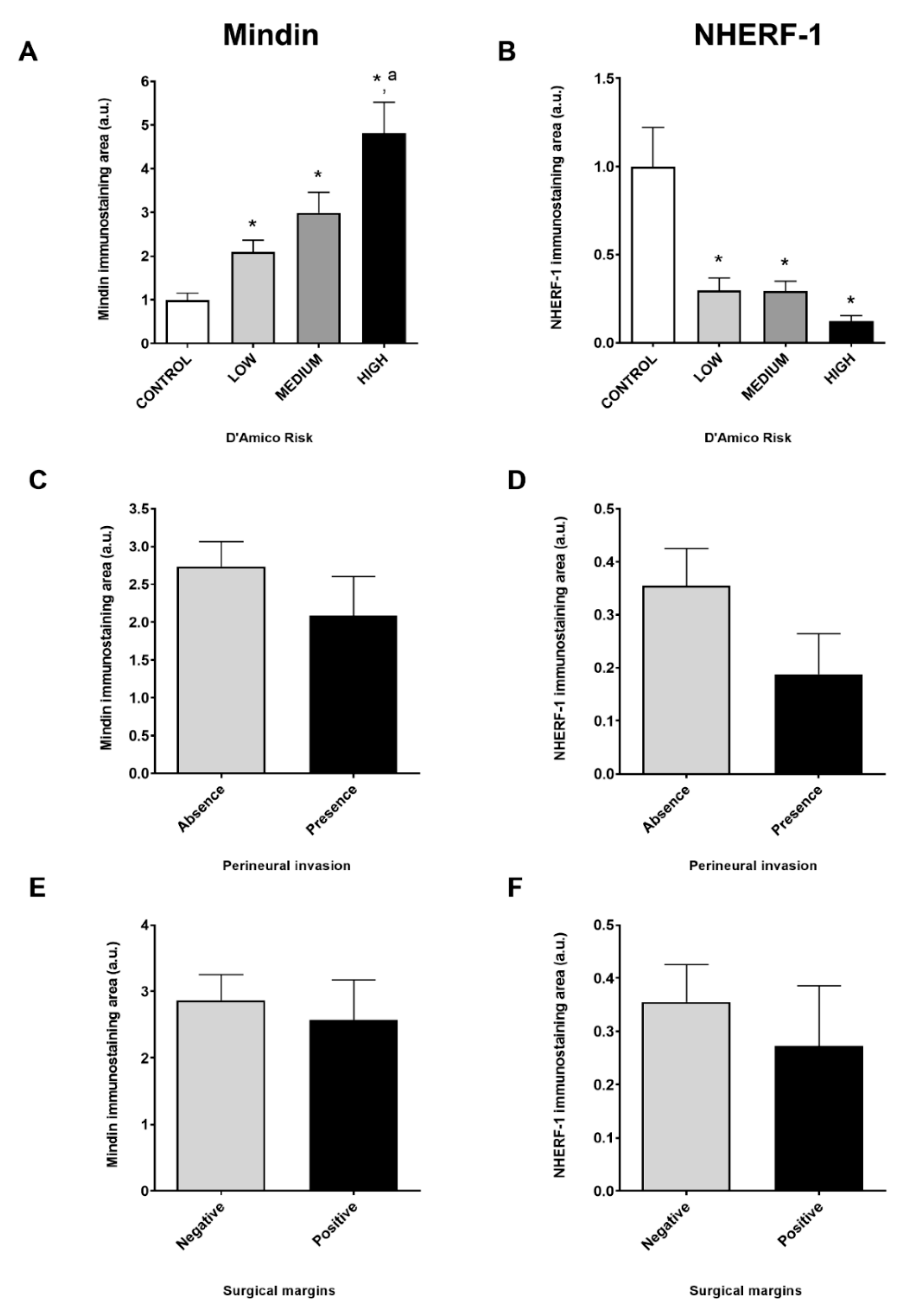
2.1. MINDIN and NHERF-1 Show Opposite Patterns of Expression in Human Prostate Tumors
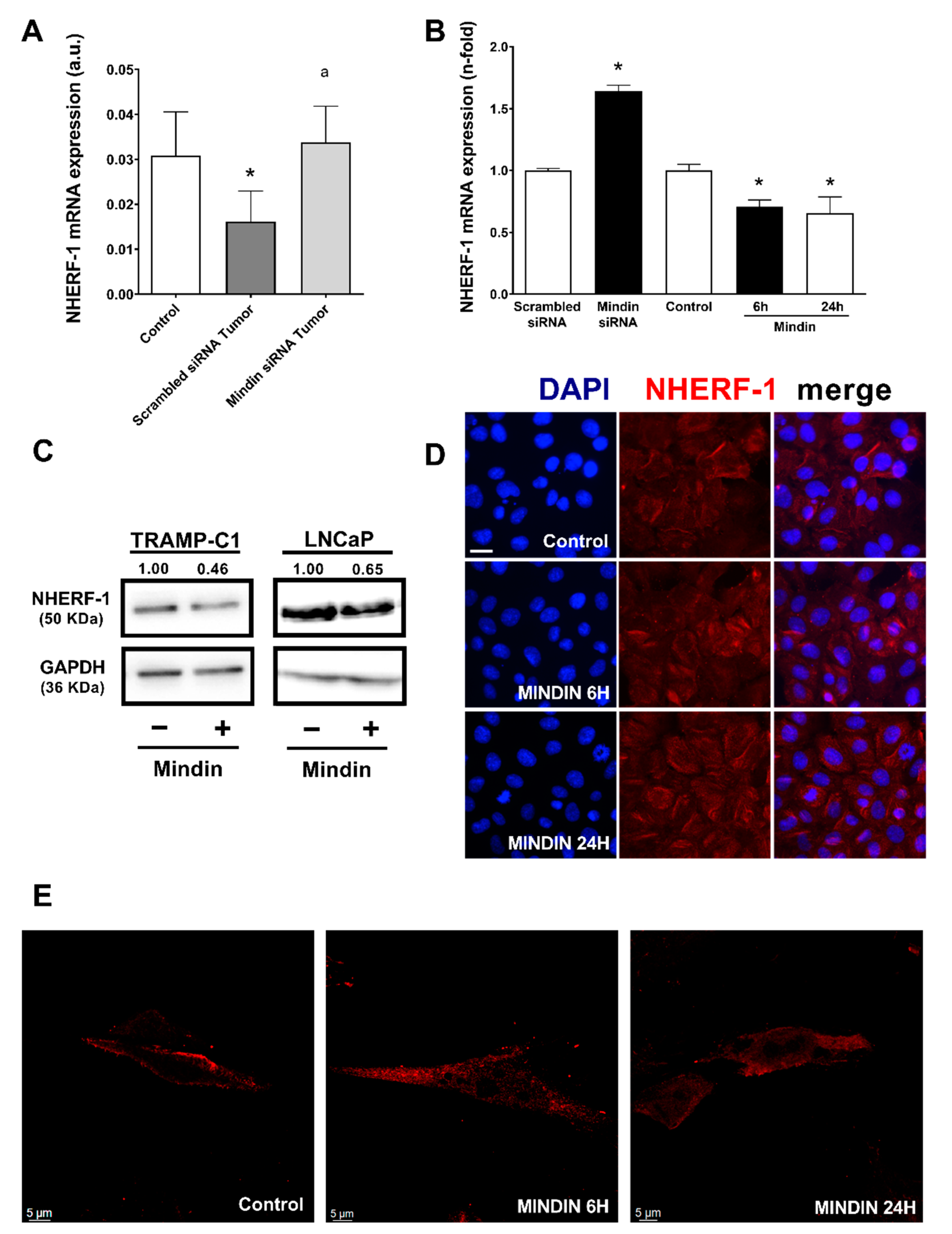
2.2. MINDIN Reduces NHERF-1 Expression and Triggers Its Mobilization to the Cytoplasm in Prostate Tumor Cells
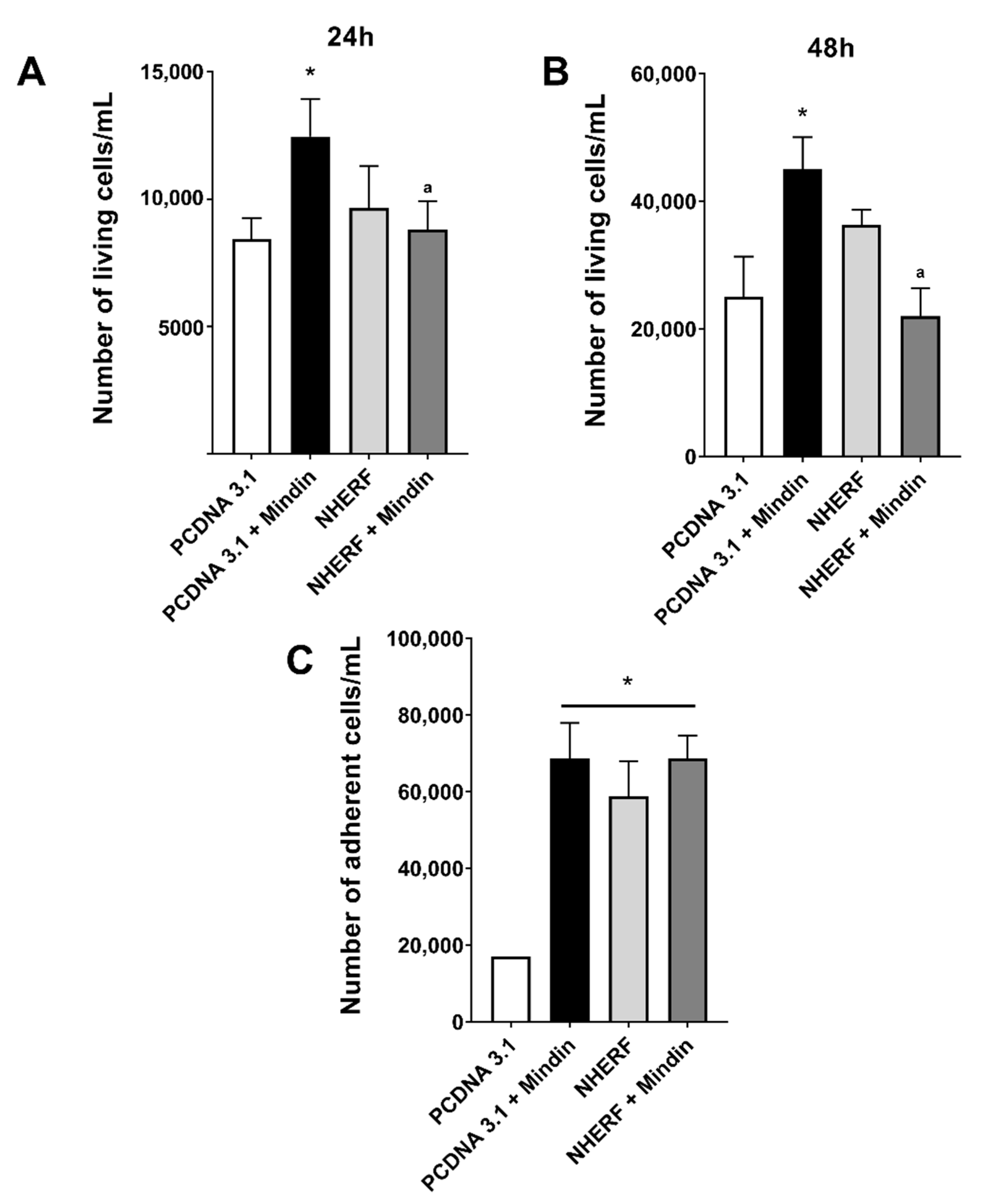
2.3. Downregulation of NHERF-1 Expression Mediates MINDIN Effects on Prostate Adenocarcinoma Cell Migration and Proliferation
3. Discussion
4. Materials and Methods
4.1. Human Tissue Specimens
4.2. Animal Model
4.3. Cell Culture
4.4. Immunohistochemistry and Immunofluorescence
4.5. Cell Silencing and Transfection
4.6. Western Blot Analysis
4.7. Real Time PCR
4.8. Proliferation, Migration and Adhesion Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, M.C.S.; Goggins, W.B.; Wang, H.H.X.; Fung, F.D.H.; Leung, C.; Wong, S.Y.S.; Ng, C.F.; Sung, J.J.Y. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef]
- Logothetis, C.J.; Lin, S.H. Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 2005, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Borsari, V.; Brogini, S.; Giavaresi, G.; Parrilli, A.; Cepollaro, S.; Cadossi, M.; Martini, L.; Mazzotti, A.; Fini, M. An in vitro 3D bone metastasis model by using a human bone tissue culture and human sex-related cancer cells. Oncotarget 2016, 7, 76966–76983. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Lipton, A.; Roodman, G.D.; Guise, T.A.; Boyce, B.F.; Brufsky, A.M.; Clézardin, P.; Croucher, P.I.; Gralow, J.R.; Hadji, P.; et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat. Rev. 2010, 36, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Kan, C.; Vargas, G.; Le Pape, F.; Clézardin, P. Cancer cell colonisation in the bone microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Khalid, O.; Gabet, Y.; Shah, R.R.; Purcell, D.J.; Mav, D.; Kohn-Gabet, A.E.; Shi, Y.; Coetzee, G.A.; Frenkel, B. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol. Cancer 2010, 9, 258. [Google Scholar] [CrossRef]
- Koeneman, K.S.; Yeung, F.; Chung, L.W.K. Osteomimetic properties of prostate cancer cells: A hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 1999, 39, 246–261. [Google Scholar] [CrossRef]
- Kingsley, L.A.; Fournier, P.G.J.; Chirgwin, J.M.; Guise, T.A. Molecular Biology of Bone Metastasis. AACR Educ. B 2008, 2008, 443–457. [Google Scholar] [CrossRef]
- Dougall, W.C. Molecular pathways: Osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 2012, 18, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Akech, J.; Wixted, J.J.; Bedard, K.; Van Der Deen, M.; Hussain, S.; Guise, T.A.; Van Wijnen, A.J.; Stein, J.L.; Languino, L.R.; Altieri, D.C.; et al. Runx2 association with progression of prostate cancer in patients: Mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 2010, 29, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12, 6213–6217. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Bettocchi, C.; Palazzo, S.; Vavallo, A.; Galleggiante, V.; Trabucco, S.; Di Clemente, D.; Selvaggi, F.P.; Battaglia, M.; et al. Spondin-2, a secreted extracellular matrix protein, is a novel diagnostic biomarker for prostate cancer. J. Urol. 2013, 190, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, C.; Pang, B.; Xue, M.; Wang, J.; Zhou, J. Spondin-2 (SPON2), a more prostate-cancer-specific diagnostic biomarker. PLoS ONE 2012, 7, e37225. [Google Scholar] [CrossRef]
- Zhu, B.-P.; Guo, Z.-Q.; Lin, L.; Liu, Q. Serum BSP, PSADT, and Spondin-2 levels in prostate cancer and the diagnostic significance of their ROC curves in bone metastasis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 61–67. [Google Scholar]
- Ardura, J.A.; Gutiérrez-Rojas, I.; Álvarez-Carrión, L.; Rodríguez-Ramos, M.R.; Pozuelo, J.M.; Alonso, V. The secreted matrix protein mindin increases prostate tumor progression and tumor-bone crosstalk via ERK 1/2 regulation. Carcinogenesis 2019, 40, 828–839. [Google Scholar] [CrossRef]
- Ardura, J.A.; Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Friedman, P.A.; Gortázar, A.R.; Alonso, V. MINDIN secretion by prostate tumors induces premetastatic changes in bone via β-catenin. Endocr. Relat. Cancer 2020, 127, 441–456. [Google Scholar] [CrossRef]
- Bartholow, T.L.; Becich, M.J.; Chandran, U.R.; Parwani, A.V. Immunohistochemical analysis of ezrin-radixin-moesin-binding phosphoprotein 50 in prostatic adenocarcinoma. BMC Urol. 2011, 11, 12. [Google Scholar] [CrossRef]
- Centonze, M.; Saponaro, C.; Mangia, A. NHERF1 between Promises and Hopes: Overview on Cancer and Prospective Openings. Transl. Oncol. 2018, 11, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.A.; Friedman, P.A. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol. Rev. 2011, 63, 882–900. [Google Scholar] [CrossRef] [PubMed]
- Donowitz, M.; Cha, B.; Zachos, N.C.; Brett, C.L.; Sharma, A.; Tse, C.M.; Li, X. NHERF family and NHE3 regulation. J. Physiol. 2005, 567, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.; Von Zastrow, M.; Friedman, P.A. Role of PDZ Proteins in Regulating Trafficking, Signaling, and Function of GPCRs. Means, Motif, and Opportunity. Adv. Pharmacol. 2011, 62, 279–314. [Google Scholar] [PubMed]
- Seidler, U.; Singh, A.K.; Cinar, A.; Chen, M.; Hillesheim, J.; Hogema, B.; Riederer, B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport: Lessons learned from knockout mice. Ann. N. Y. Acad. Sci. 2009, 1165, 249–260. [Google Scholar] [CrossRef]
- Reczek, D.; Bretscher, A. The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J. Biol. Chem. 1998, 273, 18452–18458. [Google Scholar] [CrossRef]
- Yun, C.H.C.; Lamprecht, G.; Forster, D.V.; Sidor, A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J. Biol. Chem. 1998, 273, 25856–25863. [Google Scholar] [CrossRef]
- Weinman, E.J.; Shenolikar, S. The Na-H exchanger regulatory factor. Exp. Nephrol. 1997, 5, 449–452. [Google Scholar] [PubMed]
- Wade, J.B.; Welling, P.A.; Donowitz, M.; Shenolikar, S.; Weinman, E.J. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am. J. Physiol. Cell Physiol. 2001, 280. [Google Scholar] [CrossRef]
- Shenolikar, S.; Voltz, J.W.; Cunningham, R.; Weinman, E.J. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology 2004, 19, 362–369. [Google Scholar] [CrossRef][Green Version]
- Pan, Y.; Wang, L.; Dai, J.L. Suppression of breast cancer cell growth by Na+/H+exchanger regulatory factor 1 (NHERF1). Breast Cancer Res. 2006, 8, R63. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Agarwal, N.K.; Morales, F.C.; Hayashi, Y.; Aldape, K.D.; Cote, G.; Georgescu, M.M. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene 2012, 31, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.F.; Sun, L.C.; Liu, H.; Huang, Y.; Li, Y.; He, J. EBP50 exerts tumor suppressor activity by promoting cell apoptosis and retarding extracellular signal-regulated kinase activity. Amino Acids 2010, 38, 1261–1268. [Google Scholar] [CrossRef]
- Cardone, R.A.; Bellizzi, A.; Busco, G.; Weinman, E.J.; Dell’Aquila, M.E.; Casavola, V.; Azzariti, A.; Mangia, A.; Paradiso, A.; Reshkin, S.J. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol. Biol. Cell 2007, 18, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Kislin, K.L.; McDonough, W.S.; Eschbacher, J.M.; Armstrong, B.A.; Berens, M.E. Nherf-i: Modulator of glioblastoma cell migration and invasion 1,2. Neoplasia 2009, 11, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Morales, F.C.; Hayashi, Y.; Aldape, K.D.; Georgescu, M.M. Loss of PTEN binding adapter protein NHERF1 from plasma membrane in glioblastoma contributes to PTEN inactivation. Cancer Res. 2010, 70, 6697–6703. [Google Scholar] [CrossRef]
- Georgescu, M.-M.; Morales, F.; Molina, J.; Hayashi, Y. Roles of NHERF1/EBP50 in Cancer. Curr. Mol. Med. 2008, 8, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.C.; Takahashi, Y.; Kreimann, E.L.; Georgescu, M.M. Ezrin-Radixin-Moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA 2004, 101, 17705–17710. [Google Scholar] [CrossRef]
- Kreimann, E.L.; Morales, F.C.; De Orbeta-Cruz, J.; Takahashi, Y.; Adams, H.; Liu, T.J.; McCrea, P.D.; Georgescu, M.M. Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 2007, 26, 5290–5299. [Google Scholar] [CrossRef]
- Ma, Q.; Jiao, Y.; Hao, Y.; Yan, S.; Lyu, N.; Gao, H.; Li, D.; Liu, Q.; Zheng, J.; Song, N. Targeting of NHERF1 through RNA interference inhibits the proliferation and migration of metastatic prostate cancer cells. Oncol. Lett. 2016, 11, 1149–1154. [Google Scholar] [CrossRef]
- Shibata, T.; Chuma, M.; Kokubu, A.; Sakamoto, M.; Hirohashi, S. EBP50, a β-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 2003, 38, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Molina, J.R.; Hamilton, S.R.; Georgescu, M.M. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia 2010, 12, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Stemmer-Rachamimov, A.O.; Wiederhold, T.; Nielsen, G.P.; James, M.; Pinney-Michalowski, D.; Roy, J.E.; Cohen, W.A.; Ramesh, V.; Louis, D.N. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am. J. Pathol. 2001, 158, 57–62. [Google Scholar] [CrossRef]
- Bellizzi, A.; Malfettone, A.; Cardone, R.A.; Mangia, A. NHERF1/EBP50 in breast cancer: Clinical perspectives. Breast Care 2010, 5, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, A.D.; Kalloger, S.E.; Köbel, M.; Cipollone, J.; Roskelley, C.D.; Mehl, E.; Gilks, C.B. Primary ovarian mucinous carcinoma of intestinal type: Significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int. J. Gynecol. Pathol. 2010, 29, 99–107. [Google Scholar] [CrossRef]
- Karn, T.; Ruckhäberle, E.; Hanker, L.; Müller, V.; Schmidt, M.; Solbach, C.; Gätje, R.; Gehrmann, M.; Holtrich, U.; Kaufmann, M.; et al. Gene expression profiling of luminal B breast cancers reveals NHERF1 as a new marker of endocrine resistance. Breast Cancer Res. Treat. 2011, 130, 409–420. [Google Scholar] [CrossRef]
- Liu, L.; Alonso, V.; Guo, L.; Tourkova, I.; Henderson, S.E.; Almarza, A.J.; Friedman, P.A.; Blair, H.C. Na+/H+ exchanger regulatory factor 1 (NHERF1) directly regulates osteogenesis. J. Biol. Chem. 2012, 287, 43312–43321. [Google Scholar] [CrossRef]
- Sardana, G.; Jung, K.; Stephan, C.; Diamandis, E.P. Proteomic analysis of conditioned media from the PC3, LNCaP, and 22Rv1 prostate cancer cell lines: Discovery and validation of candidate prostate cancer biomarkers. J. Proteome Res. 2008, 7, 3329–3338. [Google Scholar] [CrossRef]
- Parry, R.; Schneider, D.; Hudson, D.; Parkes, D.; Xuan, J.A.; Newton, A.; Toy, P.; Lin, R.; Harkins, R.; Alicke, B.; et al. Identification of a novel prostate tumor target, mindin/RG-1, for antibody-based radiotherapy of prostate cancer. Cancer Res. 2005, 65, 8397–8405. [Google Scholar] [CrossRef]
- Wang, L.F.; Liu, Y.S.; Yang, B.; Li, P.; Cheng, X.S.; Xiao, C.X.; Liu, J.J.; Li, S.; Ren, J.L.; Guleng, B. The extracellular matrix protein mindin attenuates colon cancer progression by blocking angiogenesis via Egr-1-mediated regulation. Oncogene 2018, 37, 601–615. [Google Scholar] [CrossRef]
- Simon, I.; Liu, Y.; Krall, K.L.; Urban, N.; Wolfert, R.L.; Kim, N.W.; McIntosh, M.W. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol. Oncol. 2007, 106, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Chen, Z.; Xu, L.; Nolley, R.; Chang, E.; Mitchell, B.; Brooks, J.D.; Gambhir, S.S.; Kumar-Sinha, C. A magnetic bead-based sensor for the quantification of multiple prostate cancer biomarkers. PLoS ONE 2015, 10, e0139484. [Google Scholar] [CrossRef]
- Hanousková, L.; Řezáč, J.; Veselý, Š.; Průša, R.; Kotaška, K. Diagnostic benefits of mindin as a prostate cancer biomarker. J. Med. Biochem. 2019, 39, 108–111. [Google Scholar]
- He, Y.W.; Li, H.; Zhang, J.; Hsu, C.L.; Lin, E.; Zhang, N.; Guo, J.; Forbush, K.A.; Bevan, M.J. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat. Immunol. 2004, 5, 88–97. [Google Scholar] [CrossRef]
- Reczek, D.; Berryman, M.; Bretscher, A. Identification of EPB50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 1997, 139, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Chen, Y.; Yung Gee, H.; Stec, E.; Melowic, H.R.; Blatner, N.R.; Tun, M.P.; Kim, Y.; Källberg, M.; Fujiwara, T.K.; et al. Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nat. Commun. 2012, 3, 1249. [Google Scholar] [CrossRef] [PubMed]
- Hanono, A.; Garbett, D.; Reczek, D.; Chambers, D.N.; Bretscher, A. EPI64 regulates microvillar subdomains and structure. J. Cell Biol. 2006, 175, 803–813. [Google Scholar] [CrossRef]
- Chiba, H.; Sakai, N.; Murata, M.; Osanai, M.; Ninomiya, T.; Kojima, T.; Sawada, N. The nuclear receptor hepatocyte nuclear factor 4α acts as a morphogen to induce the formation of microvilli. J. Cell Biol. 2006, 175, 971–980. [Google Scholar] [CrossRef]
- Vaquero, J.; Nguyen Ho-Bouldoires, T.H.; Clapéron, A.; Fouassier, L. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: From signaling regulation to clinical relevance. Oncogene 2017, 36, 3067–3079. [Google Scholar] [CrossRef]
- Jiang, Y.G.; Luo, Y.; He, D.L.; Li, X.; Zhang, L.L.; Peng, T.; Li, M.C.; Lin, Y.H. Role of Wnt/β-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1α. Int. J. Urol. 2007, 14, 1034–1039. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Barrick, S.R.; Grubisha, M.J.; Brufsky, A.M.; Friedman, P.A.; Romero, G. Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene 2011, 30, 32–42. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Hsu, Y.H.; Huang, H.Y.; Shann, Y.J.; Huang, C.Y.F.; Wei, S.C.; Chen, C.L.; Jou, T.S. Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions. J. Clin. Invest. 2012, 122, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.; Scarpi, E.; Malfettone, A.; Addati, T.; Giotta, F.; Simone, G.; Amadori, D.; Mangia, A. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis. 2013, 4, e904. [Google Scholar] [CrossRef] [PubMed]
- Guleng, B.; Lian, Y.M.; Ren, J.L. Mindin is upregulated during colitis and may activate NF-κB in a TLR-9 mediated manner. World J. Gastroenterol. 2010, 16, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Sterling, J.A.; Edwards, J.R.; DeGraff, D.J.; Lee, C.; Park, S.I.; Matusik, R.J. Activation of NF-κ B Signaling Promotes Growth of Prostate Cancer Cells in Bone. PLoS ONE 2013, 8, e60983. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.L.; Song, G.J.; Barrick, S.; Wehbi, V.L.; Vilardaga, J.P.; Bauer, P.M.; Bisello, A. Ezrin-Radixin-Moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-κB (NF-κB). J. Biol. Chem. 2013, 288, 36426–36436. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, Y.; Borrell, V.; Garcia, C.; Burstyn-Cohen, T.; Tzarfaty, V.; Frumkin, A.; Nose, A.; Okamoto, H.; Higashijima, S.I.; Soriano, E.; et al. F-spondin and mindin: Two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development 1999, 126, 3637–3648. [Google Scholar] [PubMed]
- Jia, W.; Li, H.; He, Y.W. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood 2005, 106, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.R.; Barrios, R.J.; Morton, R.A.; Boyce, B.F.; DeMayo, F.J.; Finegold, M.J.; Angelopoulou, R.; Rosen, J.M.; Greenberg, N.M. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996, 56, 4096–4102. [Google Scholar]
- Parra-Cabrera, C.; Samitier, J.; Homs-Corbera, A. Multiple biomarkers biosensor with just-in-time functionalization: Application to prostate cancer detection. Biosens. Bioelectron. 2016, 77, 1192–1200. [Google Scholar] [CrossRef]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.A.; Coy, H.M.; Nerenz, R.D.; Meyer, M.B.; Pike, J.W. Mouse Rankl expression is regulated in T Cells by c-Fos through a cluster of distal regulatory enhancers designated the T cell control region. J. Biol. Chem. 2011, 286, 20880–20891. [Google Scholar] [CrossRef]
- Wang, B.; Jin, H.; Shu, B.; Mira, R.R.; Chen, D. Chondrocytes-specific expression of osteoprotegerin modulates osteoclast formation in metaphyseal bone. Sci. Rep. 2015, 5, 13667. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]


| Primer [Reference] | Forward (5′--->3′) | Reverse (5′--->3′) |
|---|---|---|
| Na+/H+ exchanger regulatory factor or NHERF-1 (NHERF-1) [47] | TCGGGGTTGTTGGCTGGAGAC | GAGCTCGCGCAAGTGGCTCT |
| Tartrate-resistant acid phosphatase (TRAP) [18,19] | CACGAGAGTCCTGCTTGTC | AGTTGGTGTGGGCATACTTC |
| Osterix [18,19] | CTGCCTGACTCCTTGGGACC | GCCATAGTGAGCTTCTTCCTCAA |
| RANK [18,71] | GCAACCTCCAGTCAGCA | GAAGTCACAGCCCTCAGAATC |
| Receptor activator of nuclear factor kappa-Β ligand (RANKL) [18,72] | TGTACTTTCGAGCGCAGATG | AGGCTTGTTTCATCCTCCTG |
| Osteoprotegerin (OPG) [18,73] | CAGAGCGAAACACAGTTTG | CACACAGGGTGACATCTATTC |
| Osteocalcin [18,73] | GCAATAAGGTAGTGAACAGACTCC | CCATAGATGCGTTTGTAGGCGG |
| Alkaline phosphatase [18,73] | CCAGAAAGACACCTTGACTGTGG | TCTTGTCCGTGTCGCTCACCAT |
| Runt-related transcription factor 2 (RUNX2) [18,73] | CCTGAACTCTGCACCAAGTCCT | TCATCTGGCTCAGATAGGAGGG |
| Beta Actin [73] | GAACCCTAAGGCCAACCGTG | ACCAGAGGCATACAGGGACAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Rodríguez-Ramos, M.R.; Ardura, J.A.; Alonso, V. MINDIN Exerts Protumorigenic Actions on Primary Prostate Tumors via Downregulation of the Scaffold Protein NHERF-1. Cancers 2021, 13, 436. https://doi.org/10.3390/cancers13030436
Álvarez-Carrión L, Gutiérrez-Rojas I, Rodríguez-Ramos MR, Ardura JA, Alonso V. MINDIN Exerts Protumorigenic Actions on Primary Prostate Tumors via Downregulation of the Scaffold Protein NHERF-1. Cancers. 2021; 13(3):436. https://doi.org/10.3390/cancers13030436
Chicago/Turabian StyleÁlvarez-Carrión, Luis, Irene Gutiérrez-Rojas, María Rosario Rodríguez-Ramos, Juan A. Ardura, and Verónica Alonso. 2021. "MINDIN Exerts Protumorigenic Actions on Primary Prostate Tumors via Downregulation of the Scaffold Protein NHERF-1" Cancers 13, no. 3: 436. https://doi.org/10.3390/cancers13030436
APA StyleÁlvarez-Carrión, L., Gutiérrez-Rojas, I., Rodríguez-Ramos, M. R., Ardura, J. A., & Alonso, V. (2021). MINDIN Exerts Protumorigenic Actions on Primary Prostate Tumors via Downregulation of the Scaffold Protein NHERF-1. Cancers, 13(3), 436. https://doi.org/10.3390/cancers13030436