Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Case Definitions
2.3. Statistical Analyses
2.4. Human Research Ethics
3. Results
3.1. Patient and Cancer Characteristics in The PCNSL Cohort
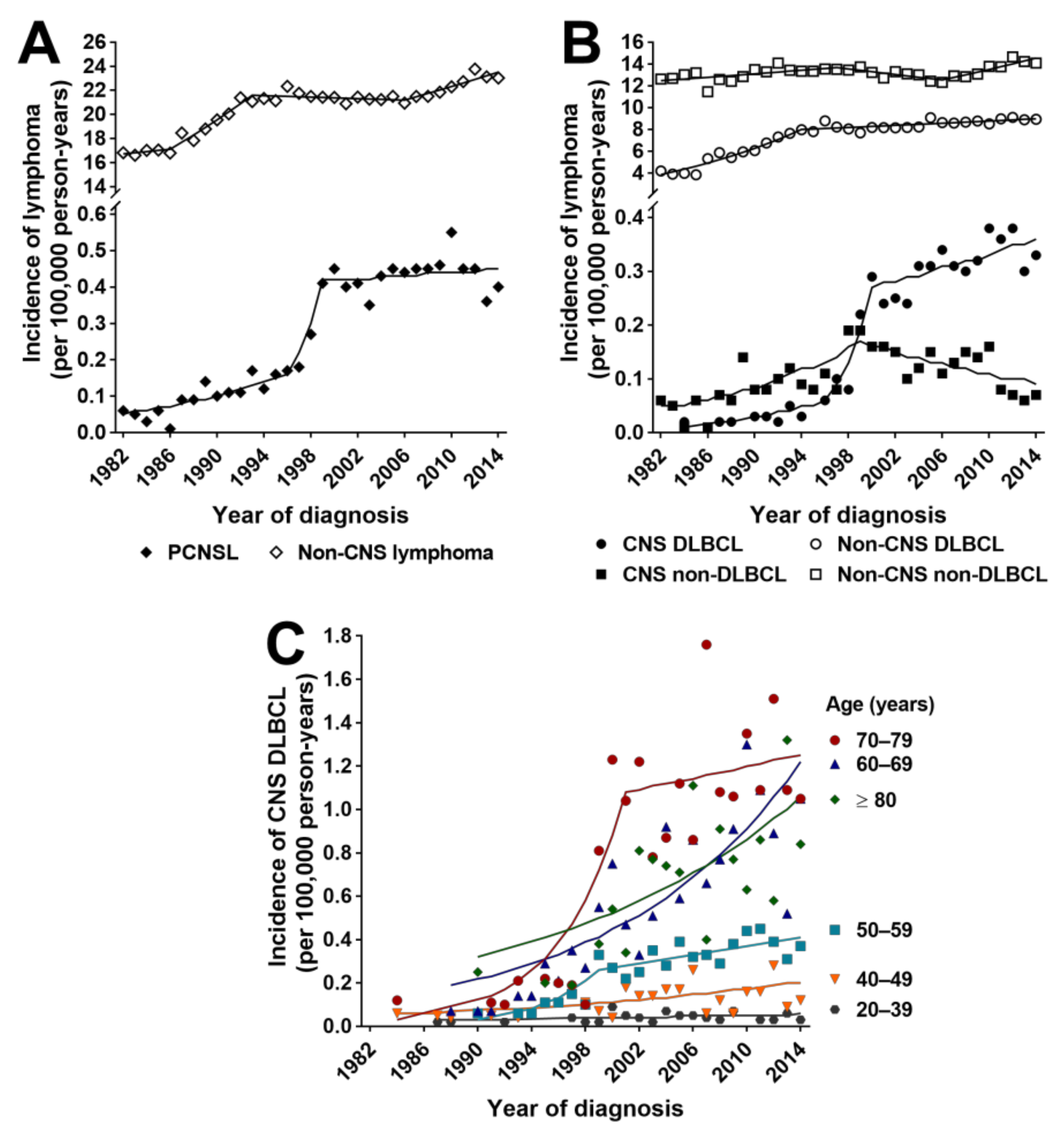
3.2. Incidence Rates and Trends for PCNSL, Non-CNS Lymphoma and Non-Hematological Primary CNS Cancers
3.3. Incidence Rates and Trends for CNS and Non-CNS DLBCL
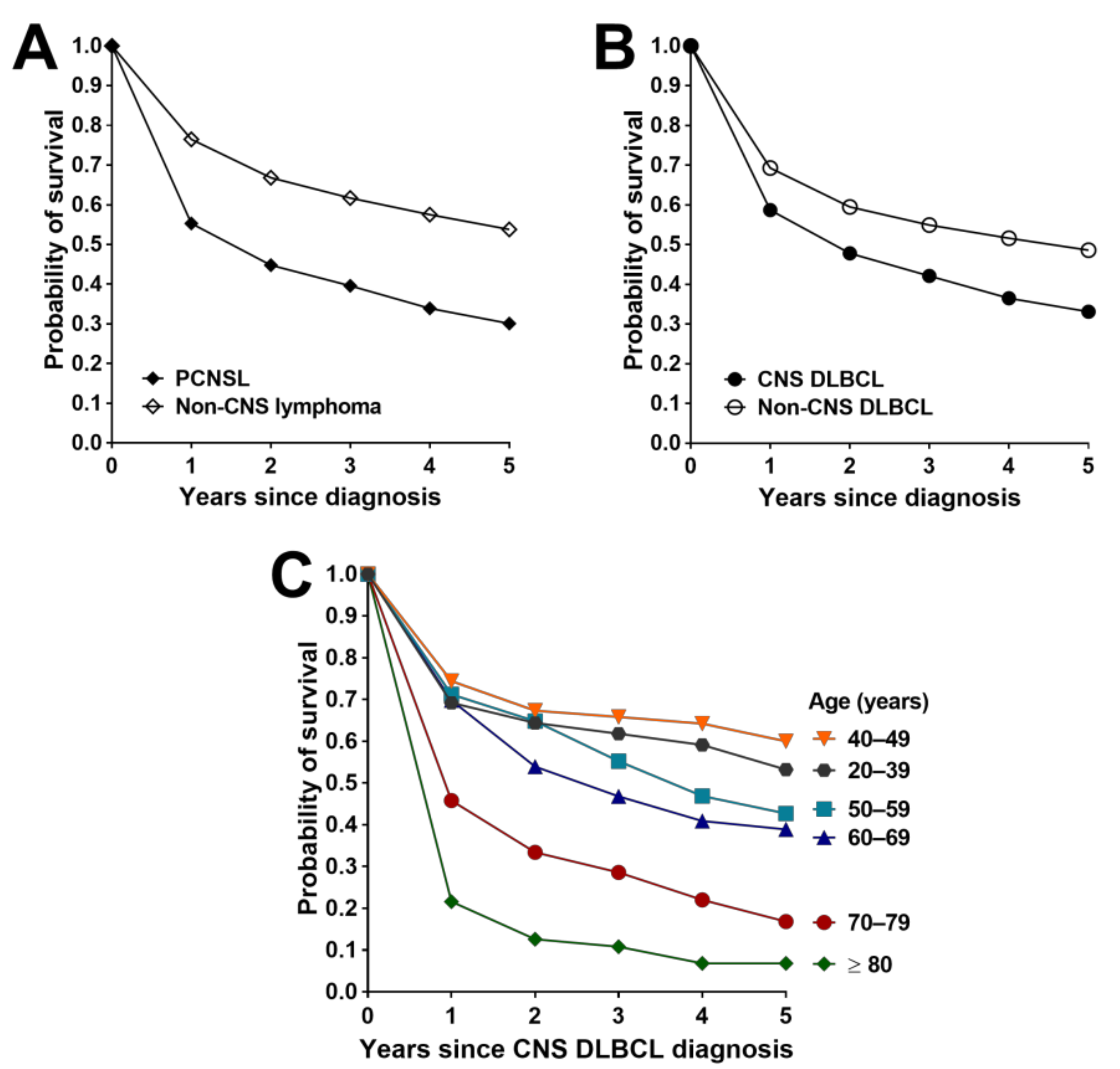
3.4. Survival Outcomes for PCNSL, Non-CNS Lymphoma and Non-Hematological Primary CNS Cancers
3.5. Survival Outcomes for CNS and Non-CNS DLBCL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Rigau, V.; Zouaoui, S.; Mathieu-Daudé, H.; Darlix, A.; Maran, A.; Trétarre, B.; Bessaoud, F.; Bauchet, F.; Attaoua, R.; Fabbro-Peray, P.; et al. French brain tumor database: 5-year histological results on 25,756 cases. Brain Pathol. 2011, 21, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.L.; Smith, J.R. Eye involvement in primary central nervous system lymphoma. Surv. Ophthalmol. 2020, 65, 548–561. [Google Scholar] [CrossRef]
- Kasamon, Y.L.; Ambinder, R.F. AIDS-related primary central nervous system lymphoma. Hematol. Oncol. Clin. N. Am. 2005, 19, 665–687. [Google Scholar] [CrossRef]
- Shan, Y.; Hu, Y. Prognostic factors and survival in primary central nervous system lymphoma: A population-based study. Dis. Markers 2018, 2018, 7860494. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Besson, C.; Clarke, C.A.; Morton, L.M.; Nogueira, L.; Pawlish, K.; Yanik, E.L.; Suneja, G.; Engels, E.A. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br. J. Haematol. 2016, 174, 417–424. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Kråkenes, J.; Goplen, A.K.; Dunlop, O.; Mella, O.; Espeland, A. AIDS-related primary central nervous system lymphoma: A Norwegian national survey 1989–2003. BMC Cancer 2008, 8, 225. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Krossnes, B.K.; Aarseth, J.H.; Scheie, D.; Johannesen, T.B.; Mella, O.; Espeland, A. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989–2003: Time trends in a 15-year national survey. Cancer 2007, 110, 1803–1814. [Google Scholar] [CrossRef]
- Shin, S.H.; Jung, K.W.; Ha, J.; Lee, S.H.; Won, Y.J.; Yoo, H. Population-based incidence and survival for primary central nervous system lymphoma in Korea, 1999–2009. Cancer Res. Treat. 2015, 47, 569–574. [Google Scholar] [CrossRef]
- Van der Meulen, M.; Dinmohamed, A.G.; Visser, O.; Doorduijn, J.K.; Bromberg, J.E.C. Improved survival in primary central nervous system lymphoma up to age 70 only: A population-based study on incidence, primary treatment and survival in the Netherlands, 1989–2015. Leukemia 2017, 31, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, S.; Branvall, E.; Celsing, F.; Papworth, K.; Ljungqvist, M.; Enblad, G.; Ekstrom-Smedby, K. Increasing incidence of primary central nervous system lymphoma but no improvement in survival in Sweden 2000–2013. Eur. J. Haematol. 2018, 100, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Nakamura, H.; Kino, T.; Takeshima, H.; Kuratsu, J. Rising incidence of primary central nervous system lymphoma in Kumamoto, Japan. Surg. Neurol. 2006, 66, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Census of Population and Housing: Australia Revealed. 2016. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/2024.0 (accessed on 12 October 2020).
- Minimum Agreed Dataset of the Australian Cancer Registry. Available online: https://www.aihw.gov.au/about-our-data/our-data-collections/australian-cancer-database/about-australian-cancer-database (accessed on 12 October 2020).
- Australian Cancer Database, 2015. Quality Statement. Available online: https://meteor.aihw.gov.au/content/index.phtml/itemId/716147 (accessed on 29 June 2020).
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; 1st rev.; Sobin, L., Parkin, D.M., Whelan, S., Eds.; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organisation. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Available online: https://icd.who.int/browse10/2010/en#/ (accessed on 29 June 2020).
- JoinPoint Regression Program. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 18 March 2019).
- Australian Bureau of Statistics National, State and Territory Population Data. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#data-downloads-data-cubes (accessed on 22 June 2020).
- WHO Standard Population 2000–2025 Data. Available online: https://apps.who.int/healthinfo/statistics/mortality/whodpms/definitions/pop.htm (accessed on 17 August 2020).
- Ahmad, O.; Boschi-Pinto, C.; Lopez, A.D.; Murray, C.J.L.; Lozano, R.; Inoue, M. Age Standardization of Rates: A New WHO Standard; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2015; National Cancer Institute: Bethesda, MD, USA, 2018. [Google Scholar]
- Australian Bureau of Statistics Life Tables, States Territories and Australia, 2012–2014. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3302.0.55.0012012-2014?OpenDocument (accessed on 4 January 2021).
- Dickman, P.W.; Adami, H.-O. Interpreting trends in cancer patient survival. J. Intern. Med. 2006, 260, 103–117. [Google Scholar] [CrossRef]
- Fuentes-Raspall, R.; Solans, M.; Aunon-Sanz, C.; Saez, M.; Marcos-Gragera, R. Incidence and survival of primary central nervous system lymphoma (PCNSL): Results from the Girona cancer registry (1994–2013). Clin. Transl. Oncol. 2018, 20, 1628–1630. [Google Scholar] [CrossRef]
- Baldi, I.; Gruber, A.; Alioum, A.; Berteaud, E.; Lebailly, P.; Huchet, A.; Tourdias, T.; Kantor, G.; Maire, J.P.; Vital, A.; et al. Descriptive epidemiology of CNS tumors in France: Results from the Gironde Registry for the period 2000–2007. Neuro Oncol. 2011, 13, 1370–1378. [Google Scholar] [CrossRef]
- O’Connell, K.; Looby, S.; Gou, P.; Flavin, R.; Farrell, M.; Cryan, J.B.; Beausang, A.; Brett, F.M. CNS lymphoma, the Irish experience: A retrospective review of neuropathologically confirmed cases over 10 years. Clin. Neuropathol. 2020, 212–220. [Google Scholar] [CrossRef]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef]
- Hao, D.; DiFrancesco, L.M.; Brasher, P.M.; deMetz, C.; Fulton, D.S.; DeAngelis, L.M.; Forsyth, P.A. Is primary CNS lymphoma really becoming more common? A population-based study of incidence, clinicopathological features and outcomes in Alberta from 1975 to 1996. Ann. Oncol. 1999, 10, 65–70. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Pineros, M.; Znaor, A.; Marcos-Gragera, R.; Steliarova-Foucher, E.; Bray, F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control. 2019, 30, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- The Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia Annual Surveillance Report 2014; HIV Supplement; The Kirby Institute, UNSW: Sydney, NSW, Australia, 2014. [Google Scholar]
- Olson, J.E.; Janney, C.A.; Rao, R.D.; Cerhan, J.R.; Kurtin, P.J.; Schiff, D.; Kaplan, R.S.; O’Neill, B.P. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: A surveillance, epidemiology, and end results analysis. Cancer 2002, 95, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Cook, J.R. As the world turns, evolving lymphoma classifications-past, present and future. Human Pathol. 2020, 95, 55–77. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 2017, 31, 833–843.e5. [Google Scholar] [CrossRef]
- Braggio, E.; Van Wier, S.; Ojha, J.; McPhail, E.; Asmann, Y.W.; Egan, J.; da Silva, J.A.; Schiff, D.; Lopes, M.B.; Decker, P.A.; et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin. Cancer Res. 2015, 21, 3986–3994. [Google Scholar] [CrossRef]
- Makino, K.; Nakamura, H.; Shinojima, N.; Kuroda, J.I.; Yano, S.; Mikami, Y.; Mukasa, A. BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse large B-cell lymphoma. J. Neuro Oncol. 2018, 140, 115–121. [Google Scholar] [CrossRef]
- Camilleri-Broet, S.; Criniere, E.; Broet, P.; Delwail, V.; Mokhtari, K.; Moreau, A.; Kujas, M.; Raphael, M.; Iraqi, W.; Sautes-Fridman, C.; et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood 2006, 107, 190–196. [Google Scholar] [CrossRef]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Molina, A. A decade of rituximab: Improving survival outcomes in non-Hodgkin’s lymphoma. Annu. Rev. Med. 2008, 59, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Wight, J.C.; Yue, M.; Keane, C.; Johnston, A.; Linton, K.; Chin, C.; Wai, S.H.; Talaulikar, D.; Gasiorowski, R.; Cheah, C.Y.; et al. Outcomes of synchronous systemic and central nervous system (CNS) involvement of diffuse large B-cell lymphoma are dictated by the CNS disease: A collaborative study of the Australasian Lymphoma Alliance. Br. J. Haematol. 2019, 187, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Reni, M. Prognostic factors in primary central nervous system lymphomas. Hematol. Oncol. Clin. N. Am. 2005, 19, 629–649. [Google Scholar] [CrossRef]
- Fox, C.P.; Phillips, E.H.; Smith, J.; Linton, K.; Gallop-Evans, E.; Hemmaway, C.; Auer, D.P.; Fuller, C.; Davies, A.J.; McKay, P.; et al. Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br. J. Haematol. 2018, 184, 348–363. [Google Scholar] [CrossRef]
| Characteristics | N (%) or Median (Range) |
|---|---|
| Median age at diagnosis (years) | 66 (20–100) |
| Sex | ‒ |
| Males | 734 (55.2) |
| Females | 595 (44.8) |
| Basis of diagnosis | ‒ |
| Histopathology | 1189 (89.4) |
| Cytology | 41 (3.1) |
| Clinical diagnosis without tissue specimen | 86 (6.5) |
| Other § | 13 (1.0) |
| Prevalent types: ICD-O-3.1 Morphology Code | ‒ |
| DLBCL | ‒ |
| Strict definition: 9680, 9684 | 852 (64.1) |
| Broad definition: 9590, 9591, 9675, 9680, 9684 | 1259 (94.7) |
| T-cell, NK-cell lymphoma: 9702, 9714, 9719 | 24 (1.8) |
| Other: 9590 | 239 (17.9) |
| Distribution by initial site of CNS involvement | ‒ |
| Brain | 1202 (90.4) |
| Meninges | 23 (1.7) |
| Spinal cord, cranial nerve, other | 104 (7.8) |
| Cancer Subset | Age-Standardized § Incidence Per 100,000 Person-Years | |||||||
|---|---|---|---|---|---|---|---|---|
| Australian Standard Population | World Standard Population | |||||||
| Observed Mean (95% CI) | AAPC (%) (95% CI) | Observed Mean (95% CI) | AAPC (%) (95% CI) | |||||
| 1982–2014 | 2000–2014 | 1982–2014 | 2000–2014 | 1982–2014 | 2000–2014 | 1982–2014 | 2000–2014 | |
| PCNSL | 0.27 (0.21–0.33) | 0.43 (0.41–0.46) | 6.8 (1.7–12.2) * | 0.5 (−0.9–1.9) | 0.23 (0.18–0.28) | 0.36 (0.34–0.39) | 6.5 (3.0–10.1) * | −0.1 (−1.7–1.5) |
| Non-CNS lymphoma | 20.58 (19.87–21.30) | 21.89 (21.41–22.38) | 1.1 (0.7–1.5) * | 0.7 (0.4–1) * | 16.91 (16.36–17.46) | 17.88 (17.48–18.27) | 1.0 (0.6–1.4) * | 0.7 (0.4–1) * |
| CNS DLBCL | 0.16 (0.11–0.22) | 0.31 (0.2–0.33) | 11.3 (5.7–17.2) *,‡ | 2.1 (0.4–3.8) * | 0.14 (0.09–0.18) | 0.26 (0.24–0.28) | 10.1 (4.6–15.9) *,‡ | 2.1 (0.4–3.9) * |
| Non-CNS DLBCL | 7.39 (6.80–7.98) | 8.60 (8.4–8.80) | 2.7 (2.2–3.1) * | 0.6 (0.3–0.9) * | 6.03 (5.56–6.49) | 6.94 (6.81–7.08) | 2.7 (2.2–3.1) * | 0.5 (0.2–0.8) * |
| CNS non-DLBCL | 0.10 (0.09–0.12) | 0.12 (0.10–0.14) | 2.2 (−0.3–4.8) | −3.9 (−7–−0.7) * | 0.09 (0.08–0.10) | 0.10 (0.08–0.12) | 1.5 (−0.9–3.9) | −4.8 (−8.2–−1.3) * |
| Non-CNS non-DLBCL | 13.19 (12.97–13.42) | 13.29 (12.91–13.66) | 0.5 (0.1–0.9) * | 0.6 (0–1.3) * | 10.88 (10.70–11.06) | 10.93 (10.61–11.25) | 0.4 (0–0.8) * | 0.7 (0.1–1.4) * |
| Non-hematological CNS cancers | 9.03 (8.88–9.17) | 9.26 (9.07–9.45) | 0.0 (−0.3–0.3) | −0.5 (−1.2–0.3) | 7.96 (7.86–8.06) | 8.09 (7.93–8.25) | −0.1 (−0.4–0.3) | −0.5 (−1.2–0.2) |
| Age (Years) | Age-Standardized § Incidence Per 100,000 Person-Years | |
|---|---|---|
| Observed Mean (95% CI) | AAPC (%) (95% CI) | |
| 20–29 | 0.03 (0.01–0.05) | –0.1 (-5.3–5.5) |
| 30–39 | 0.06 (0.04–0.08) | 2.1 (−1.9–6.4) |
| 40–49 | 0.14 (0.10–0.18) | 4.4 (1.0–7.8) * |
| 50–59 | 0.34 (0.29–0.37) | 3.0 (1.0–5.1) * |
| 60–69 | 0.77 (0.63–0.92) | 7.5 (4.8–10.2) * |
| 70–79 | 1.14 (1.00–1.28) | 2.6 (−1.1–6.4) |
| ≥80 | 0.76 (0.62–0.89) | 5.1 (1.8–8.6) * |
| Cancer Subset | Time Interval | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1982–2014 | 1982–1999 | 2000–2014 | p-Value * | |||||||
| N | Median Survival (Days) (95% CI) | 5-Year Survival (Probability) (95% CI) | N | Median Survival (Days) (95% CI) | 5-Year Survival (Probability) (95% CI) | N | Median Survival (Days) (95% CI) | 5-Year Survival (Probability) (95% CI) | Overall Survival | |
| PCNSL | 1329 | 522 (424–620) | 0.30 (0.27–0.33) | 283 | 318 (200–436) | 0.21 (0.16–0.26) | 1046 | 600 (465–734) | 0.33 (0.30–0.36) | <0.001 |
| Non-CNS lymphoma | 92,464 | 2263 (2185–2267) | 0.54 (0.53–0.54) | 39,193 | 1318 (1278–1358) | 0.45 (0.44–0.45) | 53,271 | 3388 (3310–3466) | 0.62 (0.62–0.62) | <0.001 |
| p-value § | <0.0001 | NA | NA | <0.0001 | NA | NA | <0.0001 | NA | NA | NA |
| CNS DLBCL | 852 | 639 (482–796) | 0.33 (0.30–0.37) | 93 | 461 (297–625) | 0.24 (0.15–0.32) | 759 | 690 (500–880) | 0.34 (0.31–0.38) | 0.043 |
| Non-CNS DLBCL | 33,997 | 1643 (1533–1667) | 0.49 (0.48–0.49) | 13,063 | 674 (632–716) | 0.38 (0.37–0.38) | 20,934 | 2679 (2565–2792) | 0.56 (0.56–0.57) | <0.001 |
| p-value § | <0.0001 | NA | NA | <0.0001 | NA | NA | <0.0001 | NA | NA | NA |
| CNS non-DLBCL | 477 | 337 (227–447) | 0.25 (0.21–0.29) | 190 | 244 (85–403) | 0.20 (0.14–0.25) | 287 | 425 (271–579) | 0.30 (0.24–0.35) | 0.01 |
| Non-CNS non-DLBCL | 58,467 | 2553 (2500–2606) | 0.57 (0.57–0.57) | 26,130 | 1640 (1588–1692) | 0.48 (0.47–0.48) | 32,337 | 3773 (3666–3880) | 0.66 (0.65–0.66) | <0.001 |
| p-value § | <0.0001 | NA | NA | <0.0001 | NA | NA | <0.0001 | NA | NA | NA |
| Non-hematological CNS cancers | 40,326 | 277 (272–282) | 0.19 (0.19–0.20) | 18,151 | 222 (215–229) | 0.18 (0.17–0.18) | 22,175 | 329 (321–336) | 0.20 (0.20–0.21) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrall, A.L.; Smith, J.R. Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study. Cancers 2021, 13, 403. https://doi.org/10.3390/cancers13030403
Farrall AL, Smith JR. Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study. Cancers. 2021; 13(3):403. https://doi.org/10.3390/cancers13030403
Chicago/Turabian StyleFarrall, Alexandra L., and Justine R. Smith. 2021. "Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study" Cancers 13, no. 3: 403. https://doi.org/10.3390/cancers13030403
APA StyleFarrall, A. L., & Smith, J. R. (2021). Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study. Cancers, 13(3), 403. https://doi.org/10.3390/cancers13030403





