In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Rapid Patient-Derived AGCT Cell Line Establishment and Systematic Drug Screening
2.2. Treatment with Chemotherapeutic, Anti-Hormonal, or Targeted Monotherapy Shows Inefficacy at Maximum Plasma Concentrations in All AGCT Cell Lines
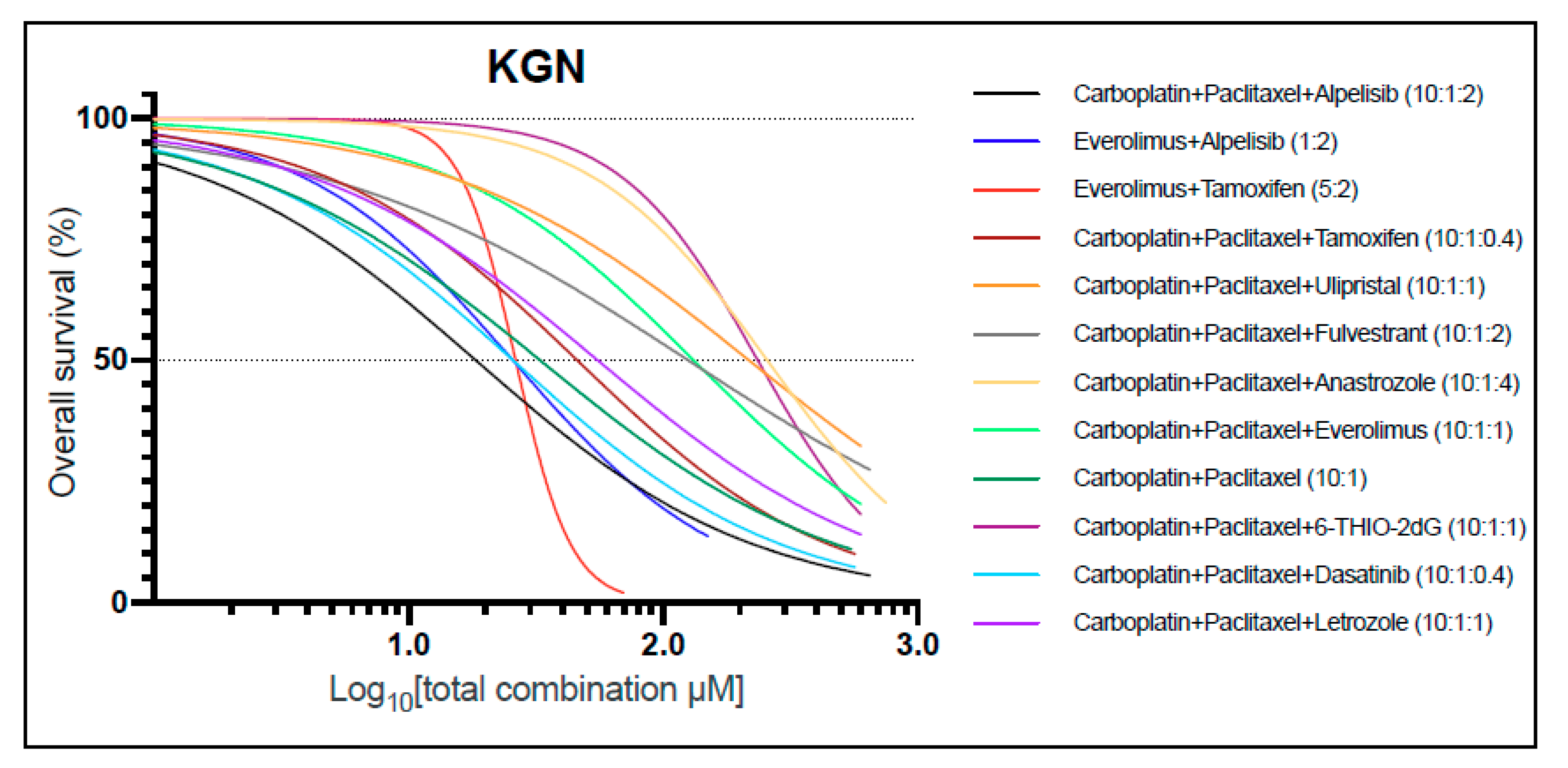
2.3. Combination Treatment in KGN Shows Synergistic Effects and Allows for Drug Dose Reduction
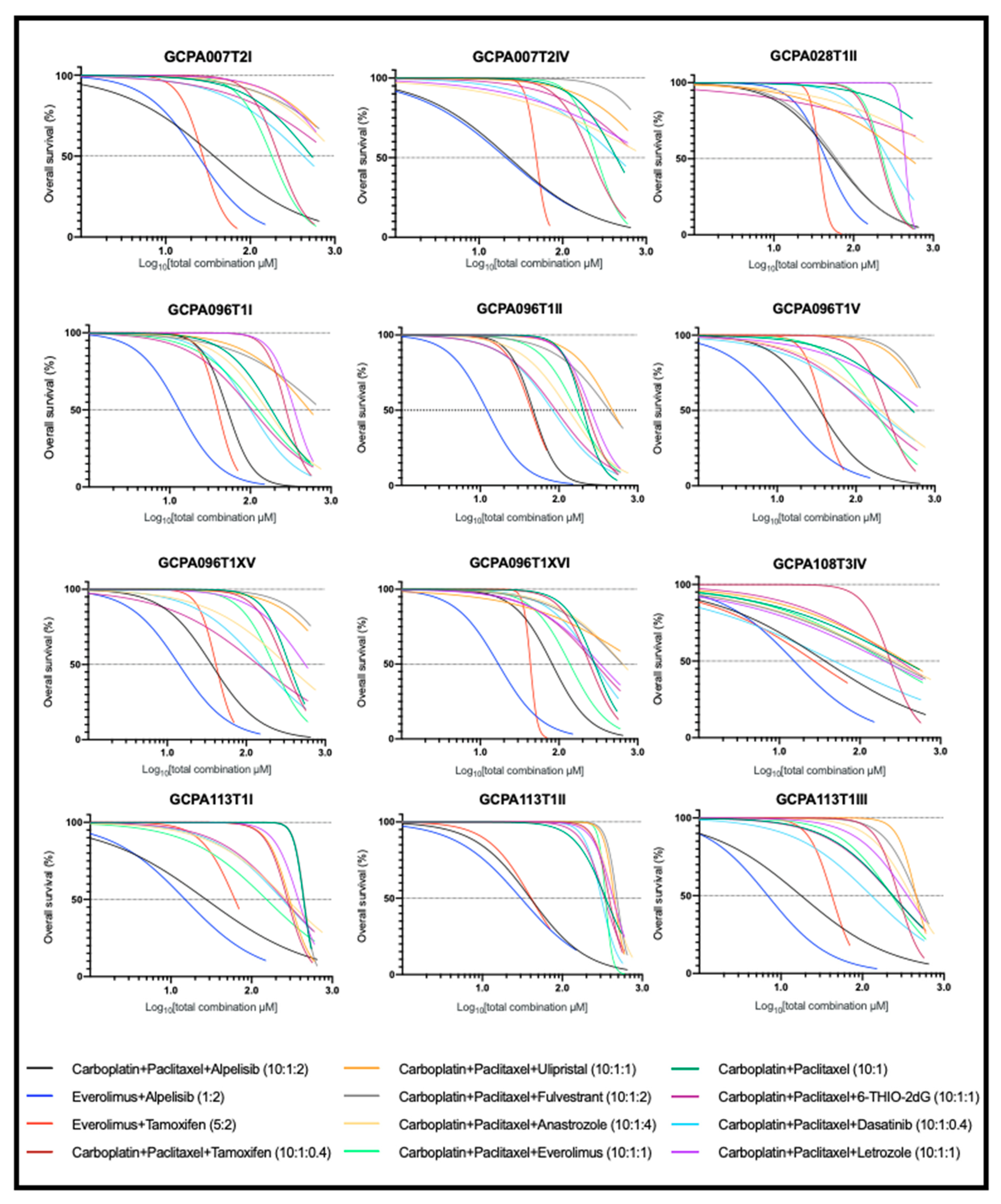
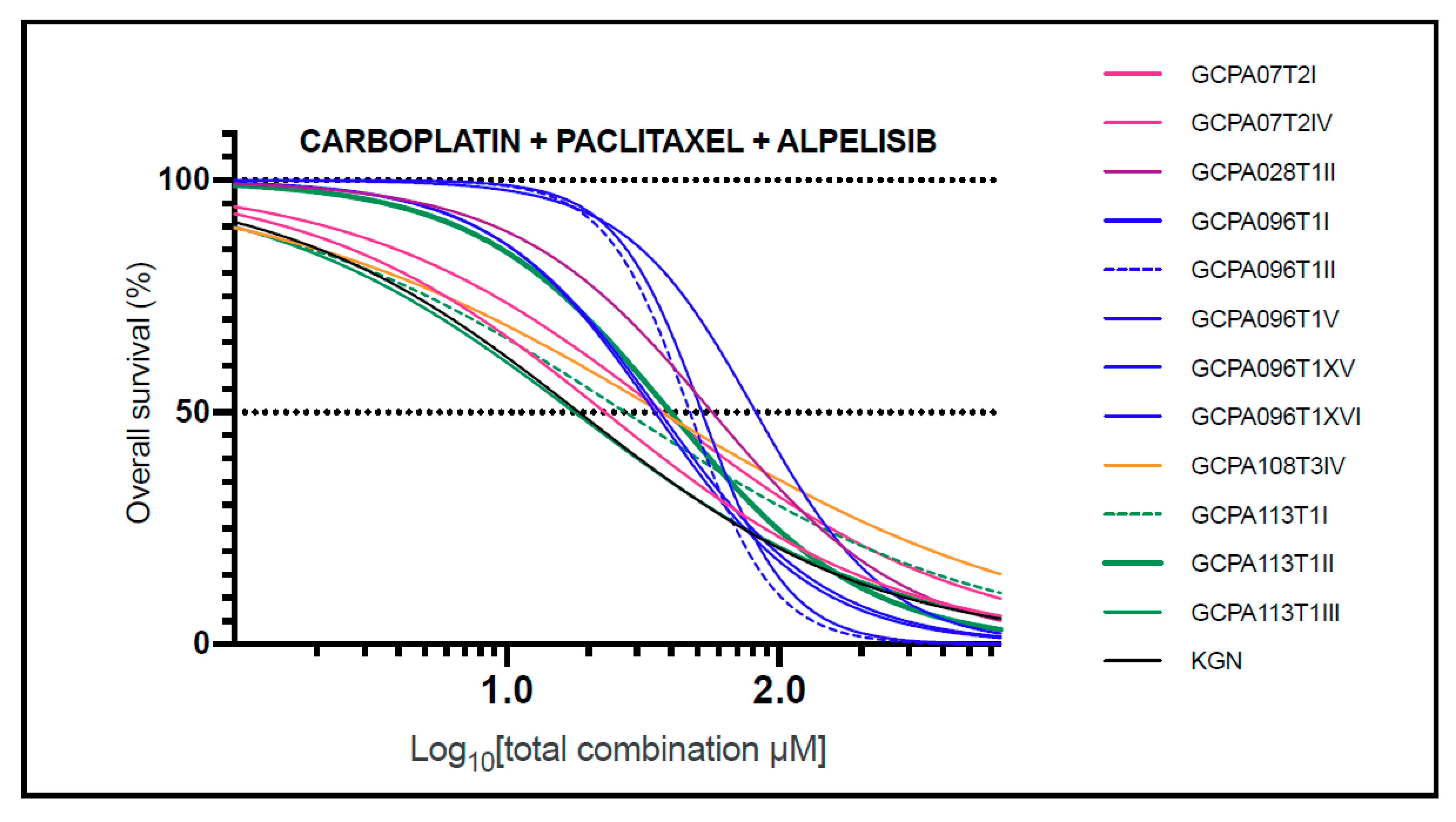
2.4. The Combination of Carboplatin, Paclitaxel, and Alpelisib Is Also Consistently Effective in AGCT-Patient-Derived Cell Lines
2.5. FOXL2 Mutation Status Does Not Affect Response to Effective Drug Combinations
3. Discussion
3.1. The PI3K Inhibitor Alpelisib
3.2. PI3K Inhibition in Combination with Chemotherapy
3.3. Combined PI3K/mTOR Inhibition
3.4. PI3K-mTOR Inhibition Combined with Anti-Hormonal Treatment
3.5. Previous Drug Screen Studies
3.6. The Limited Effects of Monotherapies
3.7. FOXL2 Mutation Status in Patient-Derived Cell Lines
3.8. Estimating Efficacy of Drug Combinations
3.9. A Robust Drug Screen Model
4. Materials and Methods
4.1. Patient Recruitment and Tumor Tissue Acquisition
4.2. Tumor Tissue Processing and 2D Cell Line Establishment
4.3. Targeted Pathway Sequencing
4.4. Control Cell Models
4.5. AGCT Viability Assessment in Response to Monotherapy and Combination Treatment
4.6. Efficacy and Safety of Monotherapies
4.7. Combination Treatment for AGCTs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryk, S.; Pukkala, E.; Martinsen, J.-I.; Unkila-Kallio, L.; Tryggvadottir, L.; Sparen, P.; Kjaerheim, K.; Weiderpass, E.; Riska, A. Incidence and occupational variation of ovarian granulosa cell tumours in Finland, Iceland, Norway and Sweden during 1953-2012: A longitudinal cohort study. BJOG 2017, 124, 143–149. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; Bleeker, M.C.G.; van der Velden, J.; Overbeek, L.I.H.; Kenter, G.G.; Buist, M.R. The incidence of endometrial hyperplasia and cancer in 1031 patients with a granulosa cell tumor of the ovary: Long-term follow-up in a population-based cohort study. Int. J. Gynecol. Cancer 2013, 23, 1417–1422. [Google Scholar] [CrossRef]
- Schumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef]
- Ohel, G.; Kaneti, H.; Schenker, J.G. Granulosa cell tumors in Israel: A study of 172 cases. Gynecol. Oncol. 1983, 15, 278–286. [Google Scholar] [CrossRef]
- Fox, H.; Agrawal, K.; Langley, F.A. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer 1975, 35, 231–241. [Google Scholar] [CrossRef]
- Stenwig, J.T.; Hazekamp, J.T.; Beecham, J.B. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol. Oncol. 1979, 7, 136–152. [Google Scholar] [CrossRef]
- Björkholm, E.; Silfverswärd, C. Prognostic factors in granulosa-cell tumors. Gynecol. Oncol. 1981, 11, 261–274. [Google Scholar] [CrossRef]
- Shah, S.P.; Kobel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E.; et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef]
- McConechy, M.K.; Farkkila, A.; Horlings, H.M.; Talhouk, A.; Unkila-Kallio, L.; Van Meurs, H.S.; Winnie, Y.; Rozenberg, N.; Andersson, N.; Zaby, K.; et al. Molecularly defined adult granulosa cell tumor of the ovary: The clinical phenotype. J. Natl. Cancer Inst. 2016, 108, 1–5. [Google Scholar] [CrossRef]
- Jamieson, S.; Fuller, P.J. Management of granulosa cell tumour of the ovary. Curr. Opin. Oncol. 2008, 20, 560–564. [Google Scholar] [CrossRef]
- Van Meurs, H.S.; Schuit, E.; Horlings, H.M.; van der Velden, J.; van Driel, W.J.; Mol, B.W.J.; Kenter, G.G.; Buist, M.R. Development and internal validation of a prognostic model to predict recurrence free survival in patients with adult granulosa cell tumors of the ovary. Gynecol. Oncol. 2014, 134, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; Buist, M.R.; Westermann, A.M.; Sonke, G.S.; Kenter, G.G.; van der Velden, J. Effectiveness of Chemotherapy in Measurable Granulosa Cell Tumors: A Retrospective Study and Review of Literature. Int. J. Gynecol. Cancer 2014, 24, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, H.S.; Van Der Velden, J.; Buist, M.R.; Van Driel, W.J.; Kenter, G.G.; Van Lonkhuijzen, L.R.C.W. Evaluation of response to hormone therapy in patients with measurable adult granulosa cell tumors of the ovary. Acta Obstet. Gynecol. Scand. 2015, 94, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Mangili, G.; Ottolina, J.; Cormio, G.; Loizzi, V.; De Iaco, P.; Pellegrini, D.A.; Candiani, M.; Giorda, G.; Scarfone, G.; Cecere, S.C.; et al. Adjuvant chemotherapy does not improve disease-free survival in FIGO stage IC ovarian granulosa cell tumors: The MITO-9 study. Gynecol. Oncol. 2016, 143, 276–280. [Google Scholar] [CrossRef]
- Yang, A.D.; Curtin, J.; Muggia, F. Ovarian adult-type granulosa cell tumor: Focusing on endocrine-based therapies. Int. J. Endocr. Oncol. 2018, 5, IJE08. [Google Scholar] [CrossRef]
- Seagle, B.-L.L.; Ann, P.; Butler, S.; Shahabi, S. Ovarian granulosa cell tumor: A National Cancer Database study. Gynecol. Oncol. 2017, 146, 285–291. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, Y.; Wu, M.; Shen, K.; Yang, J.; Huang, H.; Ren, T. Is adjuvant chemotherapy beneficial for patients with FIGO stage IC adult granulosa cell tumor of the ovary? J. Ovarian Res. 2018, 11, 25. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Gennarelli, R.L.; Leitao, M.M.; Aghajanian, C.A.; Iasonos, A.; Zivanovic, O.; Zamarin, D. Adjuvant chemotherapy in patients with operable granulosa cell tumors of the ovary: A surveillance, epidemiology, and end results cohort study. Cancer Med. 2018, 7, 2280–2287. [Google Scholar] [CrossRef]
- Nasioudis, D.; Ko, E.M.; Haggerty, A.F.; Giuntoli, R.L.; Burger, R.A.; Morgan, M.A.; Latif, N.A. Role of adjuvant chemotherapy in the management of stage IC ovarian granulosa cell tumors. Gynecol. Oncol. Rep. 2019, 28, 145–148. [Google Scholar] [CrossRef]
- Haltia, U.-M.; Andersson, N.; Yadav, B.; Färkkilä, A.; Kulesskiy, E.; Kankainen, M.; Tang, J.; Bützow, R.; Riska, A.; Leminen, A.; et al. Systematic drug sensitivity testing reveals synergistic growth inhibition by dasatinib or mTOR inhibitors with paclitaxel in ovarian granulosa cell tumor cells. Gynecol. Oncol. 2017, 144, 621–630. [Google Scholar] [CrossRef]
- Jamieson, S.; Fuller, P.J. Tyrosine Kinase Inhibitors as Potential Therapeutic Agents in the Treatment of Granulosa Cell Tumors of the Ovary. Int. J. Gynecol. Cancer 2015, 25, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Laguë, M.-N.; Lefèvre, P.; Tsoi, M.; Dodelet-Devillers, A.; Kumar, V.; Lapointe, E.; Paquet, M.; Nadeau, M.-È.; Boerboom, D. Pharmacological targeting of mammalian target of rapamycin inhibits ovarian granulosa cell tumor growth. Carcinogenesis 2012, 33, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Pilsworth, J.A.; Cochrane, D.R.; Xia, Z.; Aubert, G.; Färkkilä, A.E.M.; Horlings, H.M.; Yanagida, S.; Yang, W.; Lim, J.L.P.; Wang, Y.K.; et al. TERT promoter mutation in adult granulosa cell tumor of the ovary. Mod. Pathol. 2018, 31, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.; Monroe, G.; Kutzera, J.; Groeneweg, J.; Stelloo, E.; Paijens, S.; Nijman, H.; van Meurs, H.; van Lonkhuijzen, L.; Piek, J.; et al. Whole genome analysis of ovarian granulosa cell tumors reveals tumor heterogeneity and a high- grade tp53-specific subgroup. Cancers 2020, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Puechl, A.M.; Edwards, J.; Suri, A.; Nakayama, J.; Bean, S.; Gehrig, P.; Saks, E.; Duska, L.; Broadwater, G.; Ehrisman, J.; et al. The association between progesterone receptor expression and survival in women with adult granulosa cell tumors. Gynecol. Oncol. 2019, 153, 74–79. [Google Scholar] [CrossRef]
- McEvoy, G.K. AHFS Drug Information 1996; American Society of Health-Systems Pharmacists: Besthesda, MD, USA, 1996. [Google Scholar]
- Sioufi, A.; Gauducheau, N.; Pineau, V.; Marfil, F.; Jaouen, A.; Cardot, J.M.; Godbillon, J.; Czendlik, C.; Howald, H.; Pfister, C.; et al. Absolute bioavailability of letrozole in healthy postmenopausal women. Biopharm. Drug Dispos. 1997, 18, 779–789. [Google Scholar] [CrossRef]
- Tredway, D.R.; Buraglio, M.; Hemsey, G.; Denton, G. A phase I study of the pharmacokinetics, pharmacodynamics, and safety of single- and multiple-dose anastrozole in healthy, premenopausal female volunteers. Fertil. Steril. 2004, 82, 1587–1593. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Harrison, M. Fulvestrant: Pharmacokinetics and pharmacology. Br. J. Cancer 2004, 90, S7–S10. [Google Scholar] [CrossRef]
- Pohl, O.; Osterloh, I.; Gotteland, J.-P. Ulipristal acetate—Safety and pharmacokinetics following multiple doses of 10–50 mg per day. J. Clin. Pharm. Ther. 2013, 38, 314–320. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Stevenson, J.P.; Johnson, S.W. Clinical Pharmacokinetics and Administration of Established Platinum Drugs. Drugs 2000, 59, 19–27. [Google Scholar] [CrossRef]
- Kearns, C.M.; Gianni, L.; Egorin, M.J. Paclitaxel pharmacokinetics and pharmacodynamics. In Seminars in Oncology; W.B. Saunders Co-Elsevier Inc.: Philadelphia, PA, USA, 1995; Volume 22. [Google Scholar]
- Chen, R.; Cui, J.; Wang, Q.; Li, P.; Liu, X.; Hu, H.; Wei, W. Antiproliferative effects of anastrozole on MCF-7 human breast cancer cells in vitro are significantly enhanced by combined treatment with testosterone undecanoate. Mol. Med. Rep. 2015, 12, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; El-Hiti, G.; Abd-Allateef, M.; Yousif, E. Cytotoxicity anticancer activities of anastrozole against breast, liver hepatocellular, and prostate cancer cells. Saudi Med. J. 2017, 38, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Laguë, M.-N.; Paquet, M.; Fan, H.-Y.; Kaartinen, M.J.; Chu, S.; Jamin, S.P.; Behringer, R.R.; Fuller, P.J.; Mitchell, A.; Doré, M.; et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 2008, 29, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Ebbert, K.; Cordeiro, M.H.; Romero, M.M.; Whelan, K.A.; Suarez, A.A.; Woodruff, T.K.; Kurita, T. Constitutive Activation of PI3K in Oocyte Induces Ovarian Granulosa Cell Tumors. Cancer Res. 2016, 76, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.; Gatalica, Z. Comprehensive Molecular Profiling of Adult Ovarian Granulosa Cell Tumors (GCT) Identifies Candidate Actionable Targets. In Proceedings of the ESGO State of the Art 2018 Conference, Lyon, France, 4–6 October 2018. [Google Scholar]
- Damodaran, S.; Litton, J.K.; Hess, K.R.; Eppig, C.T.; Grzegorzewski, K.J.; Meric-Bernstam, F.; Wistuba, I.I.; White, J.B.; Rauch, G.M.; Candelaria, R.P.; et al. Abstract OT2-06-01: A phase-2 trial of neoadjuvant alpelisib and nab-paclitaxel in anthracycline refractory triple negative breast cancers with PIK3CA or PTEN alterations. In Proceedings of the Ongoing Clinical Trials, San Antonio, TX, USA, 10–14 December 2019; American Association for Cancer Research: Philadelphia, PA, USA, 2020; p. OT2-06-01. [Google Scholar]
- Rodon, J.; Curigliano, G.; Delord, J.-P.; Harb, W.; Azaro, A.; Han, Y.; Wilke, C.; Donnet, V.; Sellami, D.; Beck, T. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Kim, J.-W.; Sung, J.H.; Suh, K.J.; Lee, J.Y.; Kim, S.H.; Lee, J.-O.; Kim, J.W.; Kim, Y.J.; Kim, J.H.; et al. PI3K-targeting strategy using alpelisib to enhance the antitumor effect of paclitaxel in human gastric cancer. Sci. Rep. 2020, 10, 12308. [Google Scholar] [CrossRef]
- Corona, S.P.; Sobhani, N.; Ianza, A.; Roviello, G.; Mustacchi, G.; Bortul, M.; Zanconati, F.; Generali, D. Advances in systemic therapy for metastatic breast cancer: Future perspectives. Med. Oncol. 2017, 34, 119. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA -Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized Phase II Trial of Everolimus in Combination With Tamoxifen in Patients With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer With Prior Exposure to Aromatase Inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef]
- Leung, D.T.H.; Nguyen, T.; Oliver, E.M.; Matti, J.; Alexiadis, M.; Silke, J.; Jobling, T.W.; Fuller, P.J.; Chu, S. Combined PPARγ Activation and XIAP Inhibition as a Potential Therapeutic Strategy for Ovarian Granulosa Cell Tumors. Mol. Cancer Ther. 2019, 18, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Bridgham, J.T.; Swenson, J.A. Activation of the Akt/Protein Kinase B Signaling Pathway Is Associated with Granulosa Cell Survival1. Biol. Reprod. 2001, 64, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Fridborg, H.; Nygren, P.; Larsson, R. Relationship between pharmacokinetic parameters in patients and cytotoxicity in vitro of standard and investigational anticancer drugs. Anticancer. Drugs 1995, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Xue, H.; Sutcliffe, M.; Gout, P.W.; Huntsman, D.G.; Miller, D.M.; Gilks, C.B.; Wang, Y.Z. Establishment of subrenal capsule xenografts of primary human ovarian tumors in SCID mice: Potential models. Gynecol. Oncol. 2005, 96, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 Cells—Changing the Course of Breast Cancer Research and Care for 45 Years. JNCI J. Natl. Cancer Inst. 2015, 107, djv073. [Google Scholar] [CrossRef]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Nishi, Y.; Yanase, T.; Mu, Y.-M.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and Characterization of a Steroidogenic Human Granulosa-Like Tumor Cell Line, KGN, That Expresses Functional Follicle-Stimulating Hormone Receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef]
- Lie, B.-L.; Leung, E.; Leung, P.C.K.; Auersperg, N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol. Cell. Endocrinol. 1996, 120, 169–176. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin, and Other Platinum Complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am. J. Cancer Res. 2011, 1, 925–954. [Google Scholar] [PubMed]
- Chou, T.-C. The mass-action law based algorithms for quantitative econo-green bio-research. Integr. Biol. 2011, 3, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
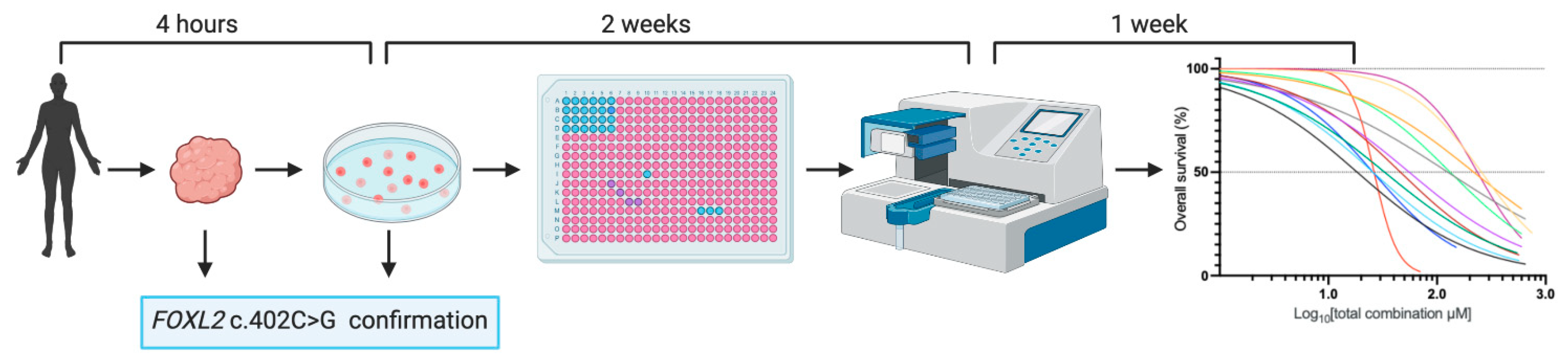

| Cell Line | Tumor Origin | Tumor Type | Previous Systemic Treatment | Tumo FOXL2 c.402C > G Mutational Status | Cell Line FOXL2 c.402C > G Mutational Status |
|---|---|---|---|---|---|
| Direct Patient-Derived Cell Lines | |||||
| GCPA007 | AGCT | Recurrence | Radiotherapy, chemotherapy 1 | ||
| T2.I | +/− | +/− | |||
| T2.IV | +/− | +/− | |||
| GCPA028 | AGCT | Recurrence | No | ||
| T1.II | +/− | +/− | |||
| GCPA096 | AGCT | Recurrence | No | ||
| T1.I | +/− | +/− | |||
| T1.II | +/− | −/− 3 | |||
| T1.V | +/− | +/− | |||
| T1.XV | +/− | +/− | |||
| T1.XVI | +/− | +/− | |||
| GCPA108 | AGCT | Recurrence | Anti-hormonal treatment, chemotherapy, RFA 2 | ||
| T3.IV | +/− | +/− | |||
| GCPA113 | AGCT | Recurrence | No | ||
| T1.I | +/− | −/− 3 | |||
| T1.II | +/− | +/+ 3 | |||
| T1.III | +/− | +/− | |||
| Control cell lines | |||||
| KGN | AGCT | Primary | No | +/− | +/− |
| SVOG-3e | Granulosa cells | N/A | No | N/A | N/A |
| MCF-7 | Breast cancer | Recurrence | Radiotherapy, anti-hormonal treatment | N/A | N/A |
| SH-SY5Y | Neuroblastoma | Recurrence | Radiotherapy, chemotherapy | N/A | N/A |
| Drug | Mechanism | Concentration Range (μM) | Solvent |
|---|---|---|---|
| Carboplatin | Intra- and inter-strand cross-linkage of DNA | 500–0 | MQ + 0.01% Tween |
| Paclitaxel | Microtubule stabilizer, induces mitotic arrest | 50–0 | DMSO |
| Tamoxifen | Estrogen receptor blocker | 20–0 | DMSO |
| Letrozole | Aromatase inhibitor | 50–0 | DMSO |
| Fulvestrant | Estrogen receptor blocker | 100–0 | DMSO |
| Ulipristal | Progesterone receptor blocker | 50–0 | DMSO |
| Anastrozole | Aromatase inhibitor | 200–0 | DMSO |
| Everolimus | mTOR inhibitor | 50–0 | DMSO |
| Alpelisib | PI3K inhibitor | 100–0 | DMSO |
| Dasatinib | Tyrosin kinase inhibitor | 20–0 | DMSO |
| 6-THIO-2dG | Telomerase blocker | 50–0 | DMSO |
| Drug Combination | Combination Ratio |
|---|---|
| Carboplatin + Paclitaxel | 10:1 |
| Carboplatin + Paclitaxel + Tamoxifen | 10:1:0.4 |
| Carboplatin + Paclitaxel + Letrozole | 10:1:1 |
| Carboplatin + Paclitaxel + Fulvestrant | 10:1:2 |
| Carboplatin + Paclitaxel + Ulipristal | 10:1:1 |
| Carboplatin + Paclitaxel + Anastrozole | 10:1:4 |
| Carboplatin + Paclitaxel + Everolimus | 10:1:1 |
| Carboplatin + Paclitaxel + Alpelisib | 10:1:2 |
| Carboplatin + Paclitaxel + Dasatinib | 10:1:0.4 |
| Carboplatin + Paclitaxel + 6-THIO-2dG | 10:1:1 |
| Everolimus + Tamoxifen | 5:2 |
| Everolimus + Alpelisib | 1:2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roze, J.; Sendino Garví, E.; Stelloo, E.; Stangl, C.; Sereno, F.; Duran, K.; Groeneweg, J.; Paijens, S.; Nijman, H.; van Meurs, H.; et al. In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors. Cancers 2021, 13, 368. https://doi.org/10.3390/cancers13030368
Roze J, Sendino Garví E, Stelloo E, Stangl C, Sereno F, Duran K, Groeneweg J, Paijens S, Nijman H, van Meurs H, et al. In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors. Cancers. 2021; 13(3):368. https://doi.org/10.3390/cancers13030368
Chicago/Turabian StyleRoze, Joline, Elena Sendino Garví, Ellen Stelloo, Christina Stangl, Ferdinando Sereno, Karen Duran, Jolijn Groeneweg, Sterre Paijens, Hans Nijman, Hannah van Meurs, and et al. 2021. "In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors" Cancers 13, no. 3: 368. https://doi.org/10.3390/cancers13030368
APA StyleRoze, J., Sendino Garví, E., Stelloo, E., Stangl, C., Sereno, F., Duran, K., Groeneweg, J., Paijens, S., Nijman, H., van Meurs, H., van Lonkhuijzen, L., Piek, J., Lok, C., Jonges, G., Witteveen, P., Verheijen, R., van Haaften, G., Zweemer, R., & Monroe, G. (2021). In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors. Cancers, 13(3), 368. https://doi.org/10.3390/cancers13030368







