Population Pharmacokinetic Model-Based Evaluation of Intact Oxaliplatin in Rats with Acute Kidney Injury
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
2.2. Renal Failure Models
2.3. PK Study of Intact L-OHP
2.4. Population PK Model Analysis
2.5. Population PK Model-Based Simulation
3. Results
3.1. Biochemical Parameters in Renal Failure Model Rats
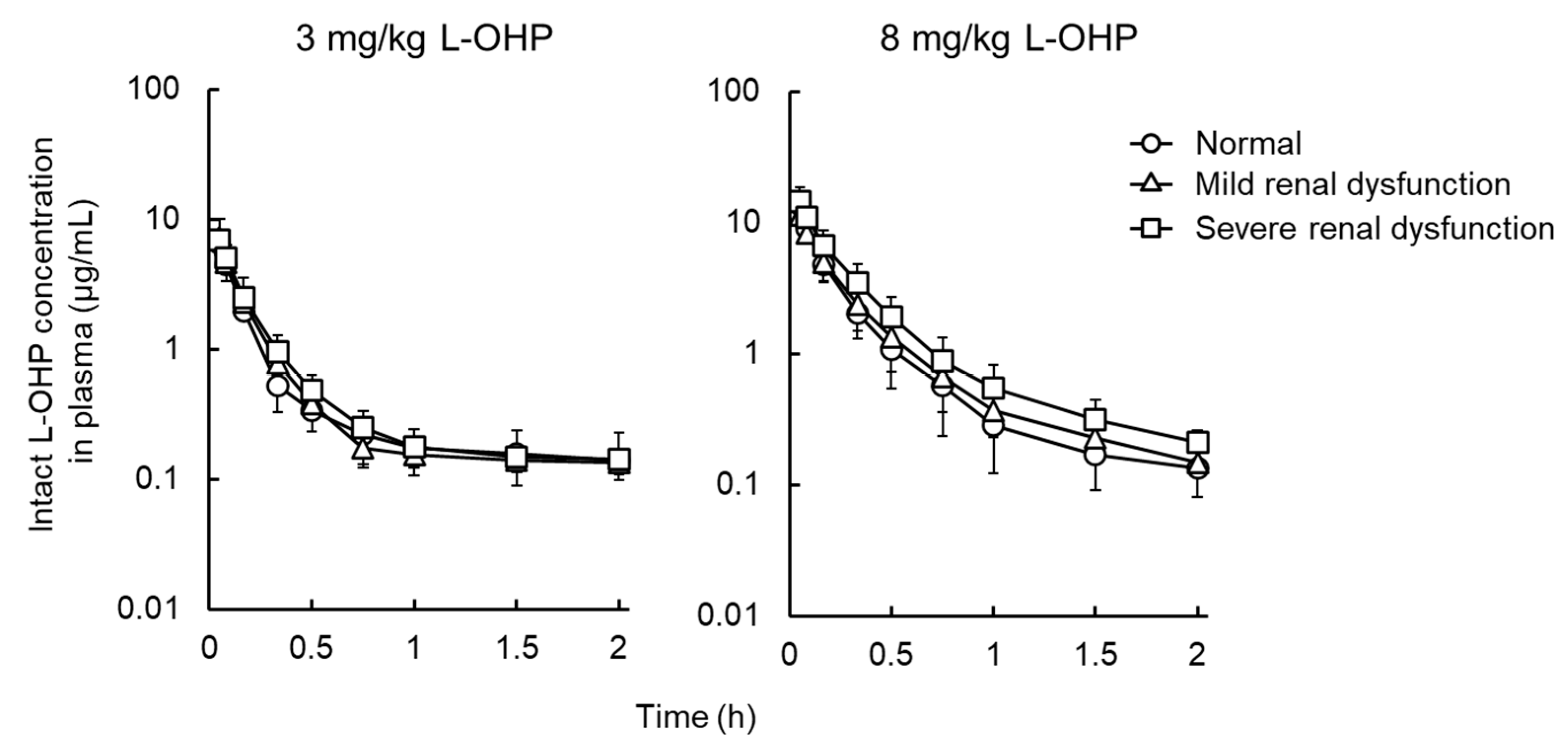
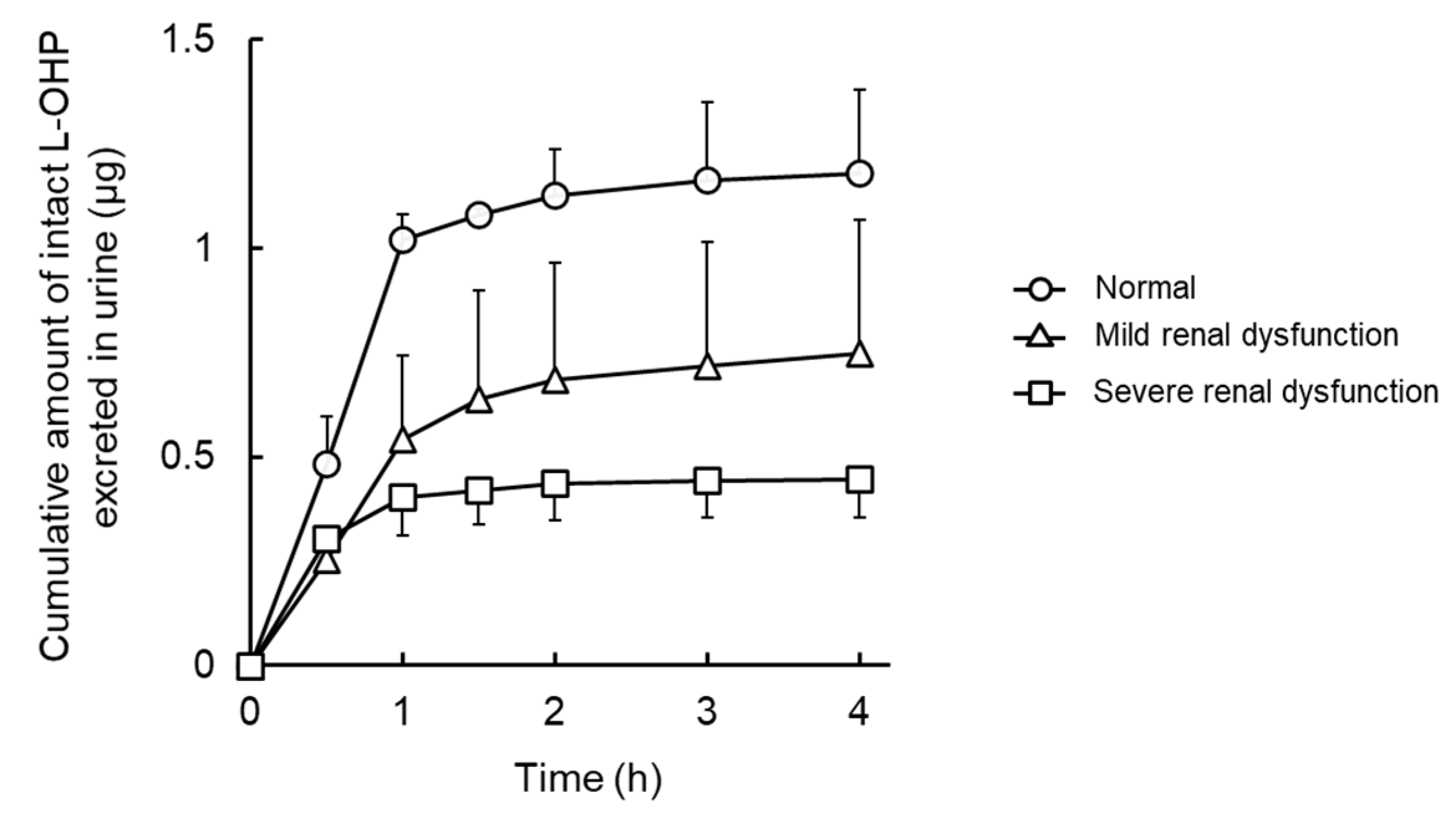
3.2. NCA Analysis and Urinary Excretion of Intact L-OHP in Renal Failure Model Rats
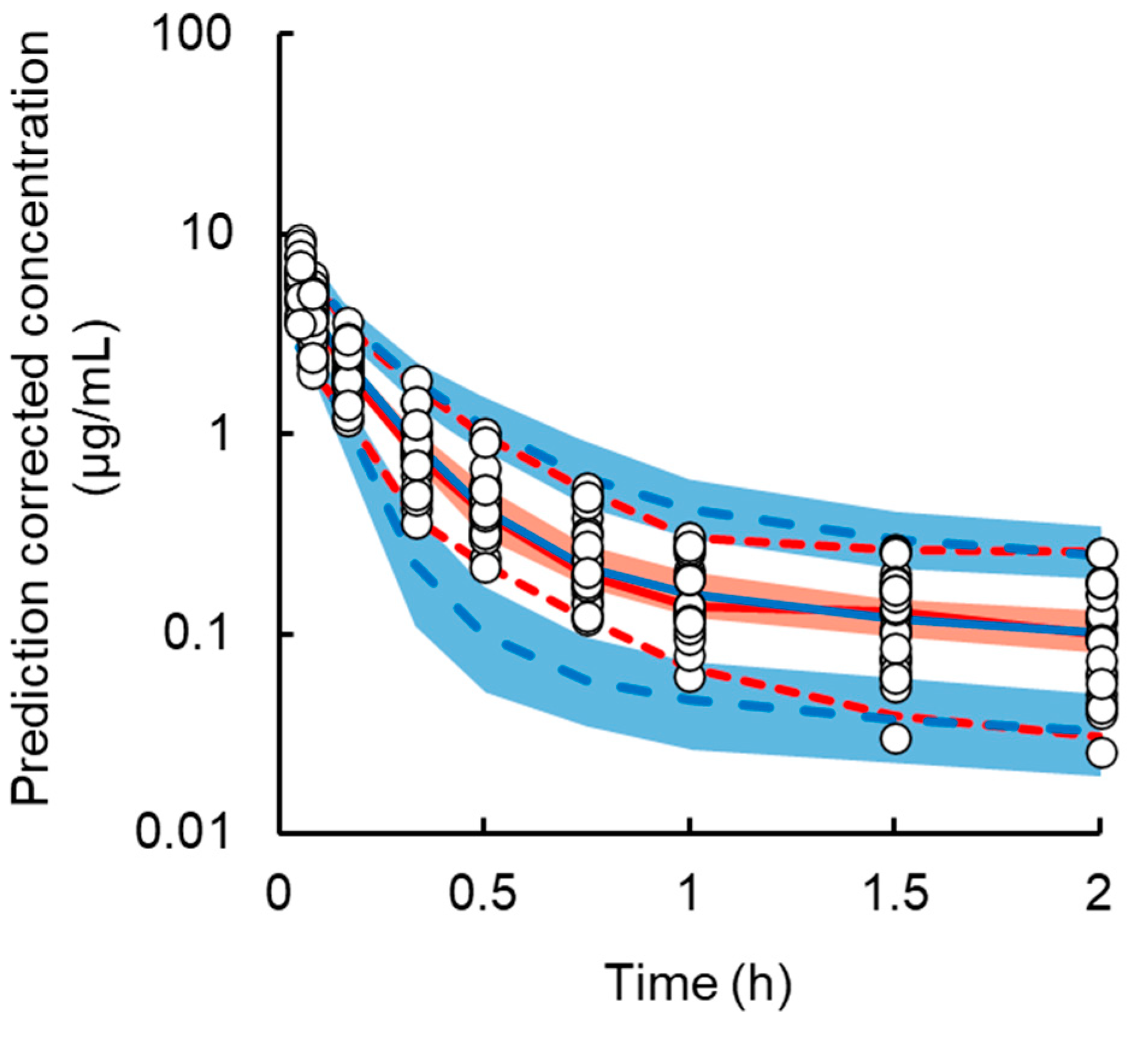
3.3. Population PK Analysis of Intact L-OHP in Normal and Renal Failure Model Rats
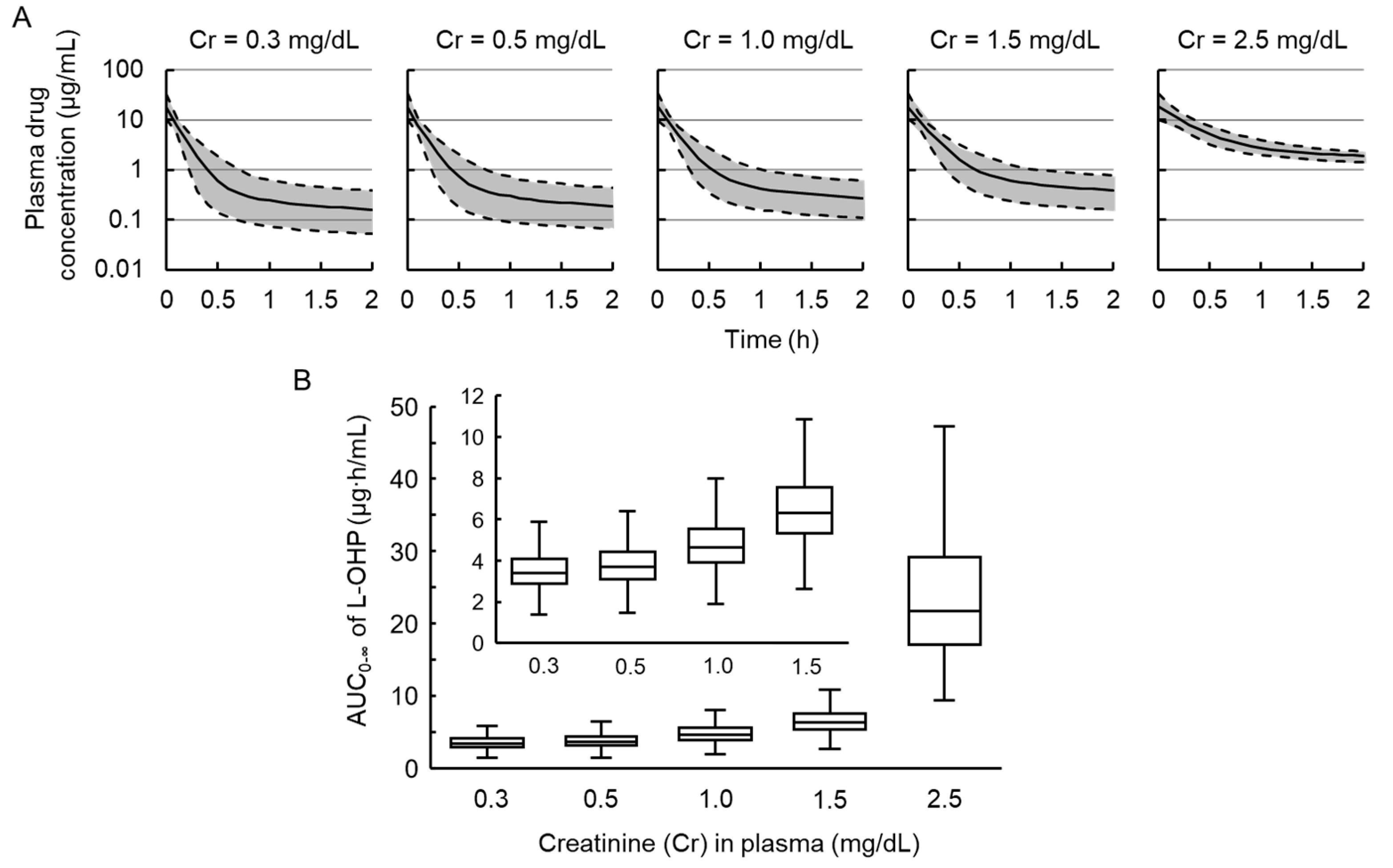
3.4. Simulations to Assess the Impact of Renal Failure on Plasma Concentration of L-OHP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosner, M.H.; Perazella, M.A. Acute Kidney Injury in Patients with Cancer. N. Engl. J. Med. 2017, 376, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute KidneyInjury Work Group. KDIGO Clinical Practice Guideline for Acute KidneyInjury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Fujii, T.; Uchino, S.; Takinami, M.; Bellomo, R. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Vilay, A.M.; Churchwell, M.D.; Mueller, B.A. Clinical review: Drug metabolism and nonrenal clearance in acute kidney injury. Crit. Care 2008, 12, 235. [Google Scholar] [CrossRef]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J., Jr.; Bennett, W.M.; Decker, B.S.; Eckardt, K.U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [CrossRef] [Green Version]
- Michaud, J.; Dubé, P.; Naud, J.; Leblond, F.A.; Desbiens, K.; Bonnardeaux, A.; Pichette, V. Effects of serum from patients with chronic renal failure on rat hepatic cytochrome P450. Br. J. Pharmacol. 2005, 144, 1067–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokunaga, A.; Miyamoto, H.; Fumoto, S.; Nishida, K. Effect of renal ischaemia/reperfusion-induced acute kidney injury on pharmacokinetics of midazolam in rats. J. Pharm. Pharmacol. 2019, 71, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Degirmencioglu, S.; Tanrıverdi, O.; Demiray, A.G.; Senol, H.; Dogu, G.G.; Yaren, A. Retrospective comparison of efficacy and safety of CAPOX and FOLFOX regimens as adjuvant treatment in patients with stage III colon cancer. J. Int. Med. Res. 2019, 47, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hao, Y.; Cong, X.; Zou, M.; Li, S.; Zhu, L.; Song, H.; Xue, Y. Peripheral Venous Blood Platelet-to-Lymphocyte Ratio (PLR) for Predicting the Survival of Patients With Gastric Cancer Treated With SOX or XELOX Regimen Neoadjuvant Chemotherapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819829485. [Google Scholar] [CrossRef] [PubMed]
- Go, S.I.; Lee, S.C.; Bae, W.K.; Zang, D.Y.; Lee, H.W.; Jang, J.S.; Ji, J.H.; Kim, J.H.; Park, S.; Sym, S.J.; et al. Modified FOLFIRINOX versus S-1 as second-line chemotherapy in gemcitabine-failed metastatic pancreatic cancer patients: A randomised controlled trial (MPACA-3). Eur. J. Cancer 2021, 157, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Reardon, J. Management of oxaliplatin-induced peripheral neuropathy. Ther. Clin. Risk Manag. 2005, 1, 249–258. [Google Scholar]
- Jamieson, S.M.; Liu, J.; Connor, B.; McKeage, M.J. Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother. Pharmacol. 2005, 56, 391–399. [Google Scholar] [CrossRef]
- Yonezawa, A.; Masuda, S.; Yokoo, S.; Katsura, T.; Inui, K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family). J. Pharmacol. Exp. Ther. 2006, 319, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoo, S.; Yonezawa, A.; Masuda, S.; Fukatsu, A.; Katsura, T.; Inui, K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 2007, 74, 477–487. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Organic cation transporter OCT/SLC22A and H(+)/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem. Pharmacol. 2011, 81, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Graham, M.A.; Bregman, D.B.; Ivy, P.; Chaney, S.G. Oxaliplatin: A review of evolving concepts. Cancer Investig. 2002, 20, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, C.H.; Remick, S.C.; Sharma, S.; Mani, S.; Ramanathan, R.K.; Doroshow, J.; Hamilton, A.; Mulkerin, D.; Graham, M.; Lockwood, G.F.; et al. National Cancer Institute Organ Dysfunction Working Group Study. Dose-escalating and pharmacological study of oxaliplatin in adult cancer patients with impaired renal function: A National Cancer Institute Organ Dysfunction Working Group Study. J. Clin. Oncol. 2003, 21, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, C.H.; Graham, M.A.; Lockwood, G.; Ng, C.M.; Goetz, A.; Greenslade, D.; Remick, S.C.; Sharma, S.; Mani, S.; Ramanathan, R.K.; et al. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin. Cancer Res. 2007, 13, 4832–4839. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Yazawa, M.; Yamamoto, M.; Koitabashi, K.; Ichikawa, D.; Koike, J.; Shibagaki, Y. A case of biopsy-proven oxaliplatin-induced acute tubulointerstitial nephritis with thrombocytopenia and anemia. CEN Case Rep. 2019, 8, 188–193. [Google Scholar] [CrossRef]
- Watanabe, D.; Fujii, H.; Yamada, Y.; Iihara, H.; Ishihara, T.; Matsuhashi, N.; Takahashi, T.; Yoshida, K.; Suzuki, A. Relationship Between Renal Function and the Incidence of Adverse Events in Patients With Colorectal Cancer Receiving Oxaliplatin. Anticancer Res. 2020, 40, 299–304. [Google Scholar] [CrossRef]
- Demachi, K.; Bando, H.; Nomura, H.; Shitara, K.; Yoshino, T.; Yamaguchi, M.; Kawasaki, T. Clinical impact of renal impairment on the safety and efficacy of S-1 plus oxaliplatin in patients with advanced gastric cancer: A single institutional study. Jpn. J. Clin. Oncol. 2020, 50, 129–137. [Google Scholar] [CrossRef]
- Lévi, F.; Metzger, G.; Massari, C.; Milano, G. Oxaliplatin: Pharmacokinetics and chronopharmacological aspects. Clin. Pharmacokinet. 2000, 38, 1–21. [Google Scholar]
- Arnould, S.; Hennebelle, I.; Canal, P.; Bugat, R.; Guichard, S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur. J. Cancer 2003, 39, 112–119. [Google Scholar] [CrossRef]
- Pinato, O.; Musetti, C.; Farrell, N.P.; Sissi, C. Platinum-based drugs and proteins: Reactivity and relevance to DNA adduct formation. J. Inorg. Biochem. 2013, 122, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koellensperger, G.; Hann, S. Ultra-fast HPLC-ICP-MS analysis of oxaliplatin in patient urine. Anal. Bioanal. Chem. 2010, 397, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ren, G.; Liu, Q.; Lu, X.; Zhang, Q.; Fan, A.; Lu, Y.; Li, N.; Chen, X.; Zhao, D. Hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the determination of intact oxaliplatin in cells: Validated and applied in colon cancer HCT-116 cell line. J. Pharm. Biomed. Anal. 2018, 155, 7–14. [Google Scholar] [CrossRef]
- Zhang, W.; Seymour, L.; Chen, E.X. Determination of intact oxaliplatin in human plasma using high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2008, 876, 277–282. [Google Scholar] [CrossRef]
- Ito, Y.; Kobuchi, S.; Takesada, W.; Takahashi, C. Assessment of Oxaliplatin-induced Chronic Neuropathy and Anticancer Efficacy Through Pharmacokinetic and Toxicodynamic Evaluation of a Rat Model of Colorectal Cancer. Anticancer Res. 2019, 39, 4207–4213. [Google Scholar] [CrossRef]
- Ito, H.; Yamaguchi, H.; Fujikawa, A.; Tanaka, N.; Furugen, A.; Miyamori, K.; Takahashi, N.; Ogura, J.; Kobayashi, M.; Yamada, T.; et al. A full validated hydrophilic interaction liquid chromatography-tandem mass spectrometric method for the quantification of oxaliplatin in human plasma ultrafiltrates. J. Pharm. Biomed. Anal. 2012, 71, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Kobuchi, S.; Takeda, M.; Morimoto, M. Effect of intact oxaliplatin in plasma on a cold allodynia after multiple administrations in colorectal cancer model rats. Ann. Palliat. Med. 2020, 9, 3000–3006. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Okada, A.; Sasaki, K.; Kishimoto, S.; Fukushima, S.; Hamori, M.; Nishimura, A.; Shibata, N.; Shirai, T.; Terauchi, R.; et al. Population Pharmacokinetic-Toxicodynamic Modeling and Simulation of Cisplatin-Induced Acute Renal Injury in Rats: Effect of Dosing Rate on Nephrotoxicity. J. Pharm. Sci. 2016, 105, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Golab, F.; Kadkhodaee, M.; Zahmatkesh, M.; Hedayati, M.; Arab, H.; Schuster, R.; Zahedi, K.; Lentsch, A.B.; Soleimani, M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009, 75, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Fuster, M.I.; Ramon-Lopez, A.; Lacueva, F.J.; Arroyo, A.; Más-Serrano, P.; Nalda-Molina, R. Population pharmacokinetics of oxaliplatin after intraperitoneal administration with hyperthermia in Wistar rats. Eur. J. Pharm. Sci. 2018, 119, 22–30. [Google Scholar] [CrossRef]
- Kobuchi, S.; Katsuyama, Y.; Ito, Y. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and simulation of oxaliplatin for hematological toxicity in rats. Xenobiotica 2020, 50, 223–230. [Google Scholar] [CrossRef]
- Kobuchi, S.; Shimizu, R.; Ito, A.Y. Semi-Mechanism-Based Pharmacokinetic-Toxicodynamic Model of Oxaliplatin-Induced Acute and Chronic Neuropathy. Pharmaceutics 2020, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Delord, J.P.; Umlil, A.; Guimbaud, R.; Grégoire, N.; Lafont, T.; Canal, P.; Bugat, R.; Chatelut, E. Population pharmacokinetics of oxaliplatin. Cancer Chemother. Pharmacol. 2003, 51, 127–131. [Google Scholar] [CrossRef]
- Nikanjam, M.; Stewart, C.F.; Takimoto, C.H.; Synold, T.W.; Beaty, O.; Fouladi, M.; Capparelli, E.V. Population pharmacokinetic analysis of oxaliplatin in adults and children identifies important covariates for dosing. Cancer Chemother. Pharmacol. 2015, 75, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, D.; Fujii, H.; Matsuhashi, N.; Iihara, H.; Yamada, Y.; Ishihara, T.; Takahashi, T.; Yoshida, K.; Suzuki, A. Dose Adjustment of Oxaliplatin Based on Renal Function in Patients With Metastatic Colorectal Cancer. Anticancer Res. 2020, 40, 2379–2386. [Google Scholar] [CrossRef]

| Biochemical Parameters | Normal | Mild Renal Failure | Severe Renal Failure |
|---|---|---|---|
| TP (g/dL) | 6.6 ± 0.4 | 5.6 ± 0.4 * | 6.1 ± 0.4 |
| Alb (g/dL) | 4.5 ± 0.2 | 3.5 ± 0.4 * | 3.7 ± 0.2 * |
| AST (IU/L) | 91.7 ± 31.3 | 473.8 ± 392.7 | 353.8 ± 150.0 * |
| ALT (IU/L) | 49.8 ± 21.0 | 88.3 ± 49.5 * | 99.5 ± 38.4 * |
| BUN (mg/dL) | 18.3 ± 1.4 | 27.9 ± 12.7 | 62.0 ± 12.8 * |
| Cr (mg/dL) | 0.27 ± 0.02 | 0.54 ± 0.21 * | 0.95 ± 0.17 * |
| CCr (mL/min/kg) | 4.2 ± 1.3 | 2.2 ± 0.7 * | 1.6 ± 0.7 * |
| Pharmacokinetic Parameters | Normal | Mild Renal Failure | Severe Renal Failure |
|---|---|---|---|
| 3 mg/kg L-OHP | |||
| t1/2 (h) | 2.6 ± 1.1 | 2.8 ± 0.8 | 2.3 ± 1.6 |
| AUC0–∞ (μg∙h/mL) | 2.0 ± 0.3 | 2.2 ± 0.2 | 2.4 ± 0.7 |
| CLtot (L/h/kg) | 1.6 ± 0.3 | 1.4 ± 0.1 | 1.3 ± 0.4 |
| Vd (L/kg) | 5.8 ± 2.4 | 5.7 ± 1.9 | 3.8 ± 1.7 |
| 8 mg/kg L-OHP | |||
| t1/2 (h) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.2 |
| AUC0–∞ (μg∙h/mL) | 3.4 ± 0.9 | 3.3 ± 1.0 | 4.4 ± 1.1 |
| CLtot (L/h/kg) | 2.5 ± 0.7 | 2.2 ± 0.8 | 1.9 ± 0.5 |
| Vd (L/kg) | 2.4 ± 0.9 | 2.9 ± 1.9 | 2.2 ± 1.0 |
| Parameters | Unit | Final Model | Bootstrap (n = 1000) | |||
|---|---|---|---|---|---|---|
| Estimate | CV% | Median | 2.5th–97.5th | |||
| Fixed effect parameters (θ) | ||||||
| V | L/kg | 0.44 | 7.4 | 0.44 | 0.39–0.49 | |
| V₂ | L/kg | 2.26 | 19.1 | 2.28 | 1.62–3.16 | |
| CL | L/h/kg | 1.76 | 8.8 | 1.76 | 1.50–2.03 | |
| CL₂ | L/h/kg | 1.0 | 10.0 | 1.0 | 0.84–1.10 | |
| Inter-individual variability (ω) | ||||||
| V | % | 37.5 | 19.3 | 37.1 | 29.6–43.9 | |
| CL | % | 30.5 | 26.0 | 29.8 | 21.6–37.5 | |
| CL₂ | % | 31.5 | 18.7 | 31.0 | 24.8–36.6 | |
| Residual variability (σ) | ||||||
| C | % | 14.9 | 6.9 | 14.9 | 13.1–17.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobuchi, S.; Kai, M.; Ito, Y. Population Pharmacokinetic Model-Based Evaluation of Intact Oxaliplatin in Rats with Acute Kidney Injury. Cancers 2021, 13, 6382. https://doi.org/10.3390/cancers13246382
Kobuchi S, Kai M, Ito Y. Population Pharmacokinetic Model-Based Evaluation of Intact Oxaliplatin in Rats with Acute Kidney Injury. Cancers. 2021; 13(24):6382. https://doi.org/10.3390/cancers13246382
Chicago/Turabian StyleKobuchi, Shinji, Miyu Kai, and Yukako Ito. 2021. "Population Pharmacokinetic Model-Based Evaluation of Intact Oxaliplatin in Rats with Acute Kidney Injury" Cancers 13, no. 24: 6382. https://doi.org/10.3390/cancers13246382
APA StyleKobuchi, S., Kai, M., & Ito, Y. (2021). Population Pharmacokinetic Model-Based Evaluation of Intact Oxaliplatin in Rats with Acute Kidney Injury. Cancers, 13(24), 6382. https://doi.org/10.3390/cancers13246382






