Intraoperative HIFU Ablation of the Pancreas Using a Toroidal Transducer in a Porcine Model. The First Step towards a Clinical Treatment of Locally Advanced Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. HIFU Equipment

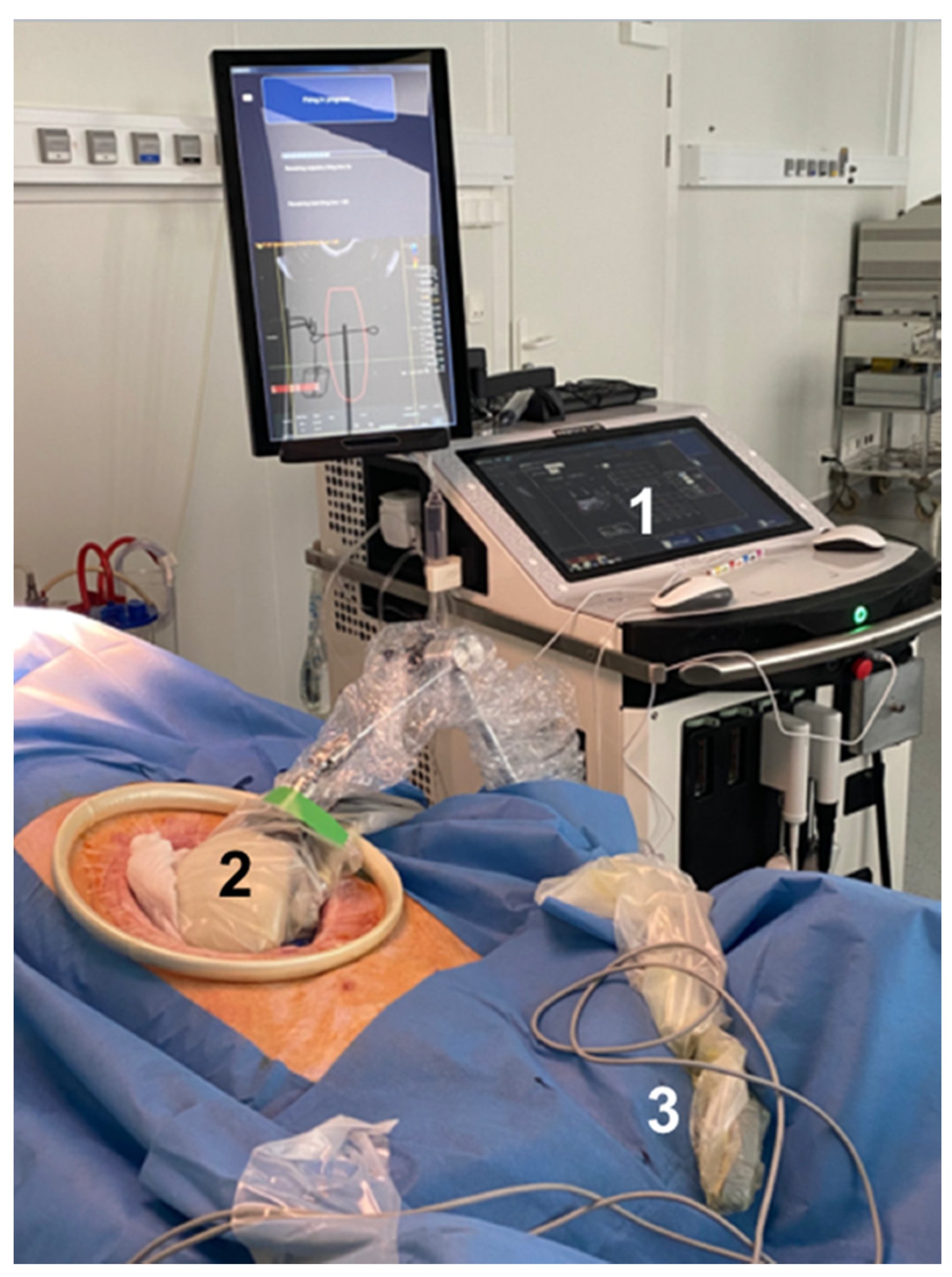
2.3. Treatment Procedure
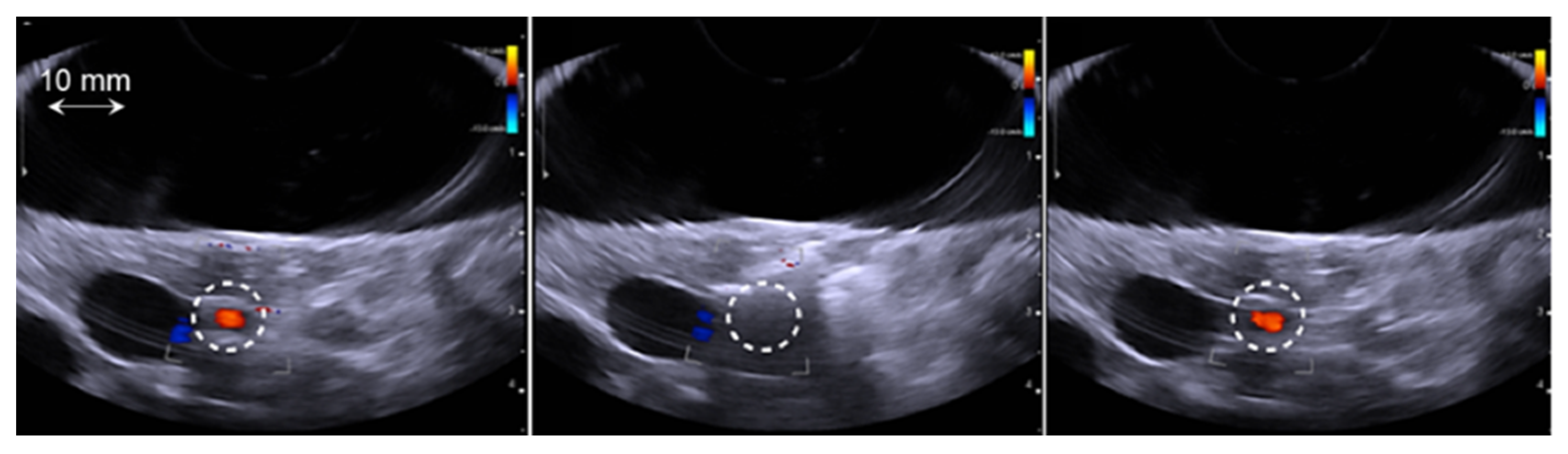
2.4. Data Analysis
3. Results
3.1. Overview
3.2. Clinical Tolerance
| Pig N° | Group | Emitted Energy (J) | Number of Sonications | Artery Spasm | Number of Sonications before the Artery Spasm |
|---|---|---|---|---|---|
| 1 | 1 | 30 | 3 | No | N/A |
| 2 | 3 | No | N/A | ||
| 3 | 3 | No | N/A | ||
| 4 | 3 | No | N/A | ||
| 5 | 3 | No | N/A | ||
| 6 | 3 | No | N/A | ||
| 7 | 1 | No | N/A | ||
| 8 | 2 | No | N/A | ||
| 9 | 2 | 40 | 3 | No | N/A |
| 10 | 3 | No | N/A | ||
| 11 | 3 | Yes | 3 | ||
| 12 | 3 | Yes | 3 | ||
| 13 | 3 | 45 | 2 | Yes | 1 |
| 14 | 3 | Yes | 1 | ||
| 15 | 1 | Yes | 1 | ||
| 16 | 3 | Yes | 1 | ||
| 17 | 4 | 52 | 2 | Yes | 1 |
| 18 | 2 | Yes | 1 | ||
| 19 | 2 | Yes | 1 | ||
| 20 | 2 | Yes | 1 |
| Pig N° | Group | Emitted Energy (kJ) | Treated Zone | Long Axis (mm) | Shot Axis (mm) |
|---|---|---|---|---|---|
| 1 | 1 | 30 | Necrotic | 18.2 | 18.9 |
| 2 | Cystic | 60.3 | 49.8 | ||
| 3 | Necrotic | 18.2 | 12.4 | ||
| 4 | Cystic | 31.8 | 25.5 | ||
| 5 | Cystic | 66.1 | 50.3 | ||
| 6 | Cystic | 74.9 | 36.6 | ||
| 7 | Cystic | 37.9 | 30.3 | ||
| 8 | Cystic | 41.5 | 34.0 | ||
| 9 | 2 | 40 | Necrotic | N/A * | N/A * |
| 10 | Necrotic | N/A * | N/A * | ||
| 11 | Cystic | 51.1 | 46.9 | ||
| 12 | Cystic | 46.8 | 18.7 | ||
| 13 | 3 | 45 | Necrotic | 27.5 | 25.4 |
| 14 | Necrotic | 23.7 | 18.3 | ||
| 15 | Cystic | 43.7 | 15.2 | ||
| 16 | Cystic | 31.6 | 19.6 | ||
| 17 | 4 | 52 | Necrotic | 33.8 | 18.6 |
| 18 | Necrotic | 35.8 | 23.9 | ||
| 19 | Necrotic | 31.9 | 18.5 | ||
| 20 | Cystic | 55.2 | 21.8 |
3.3. Analysis of HIFU Treatment
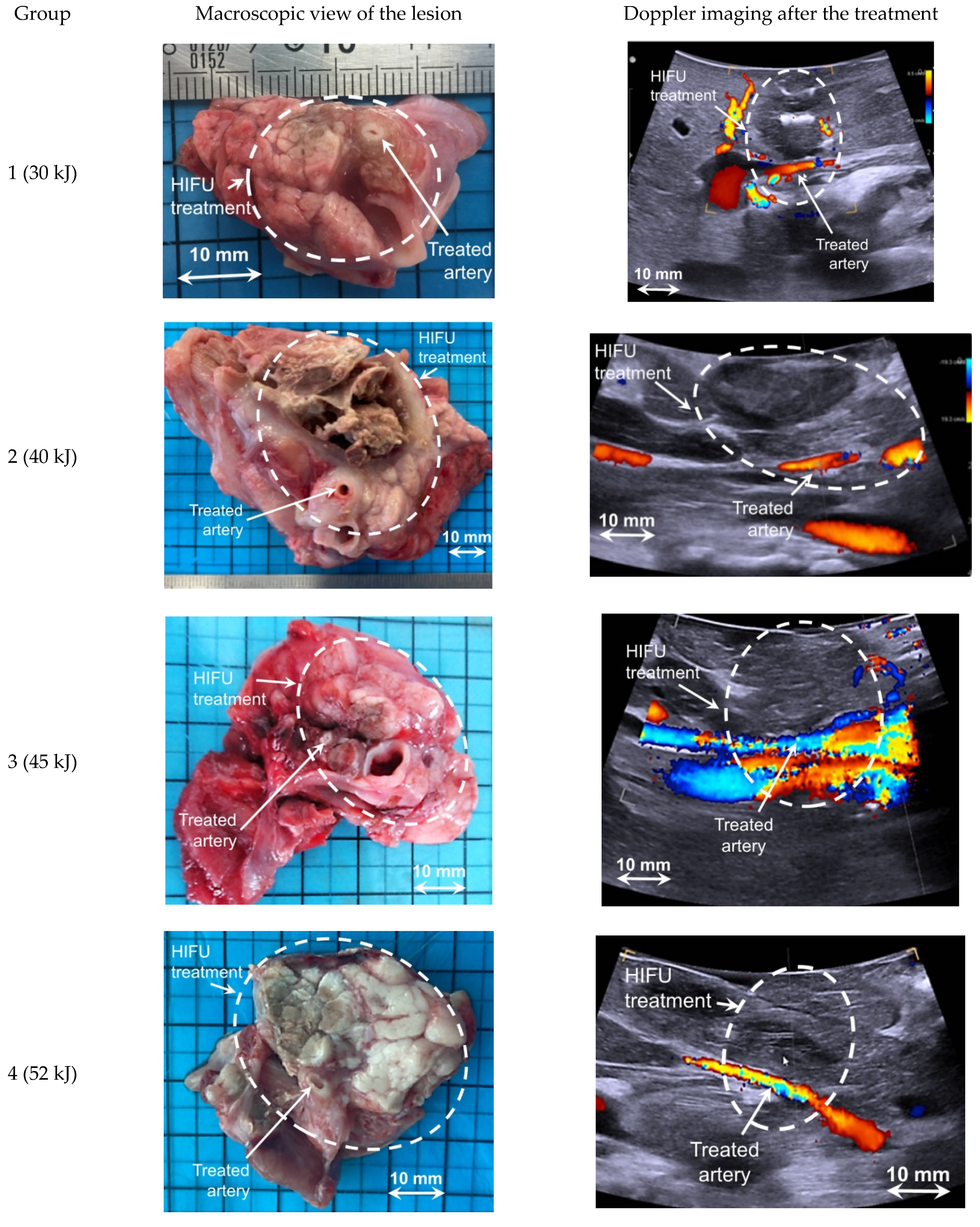
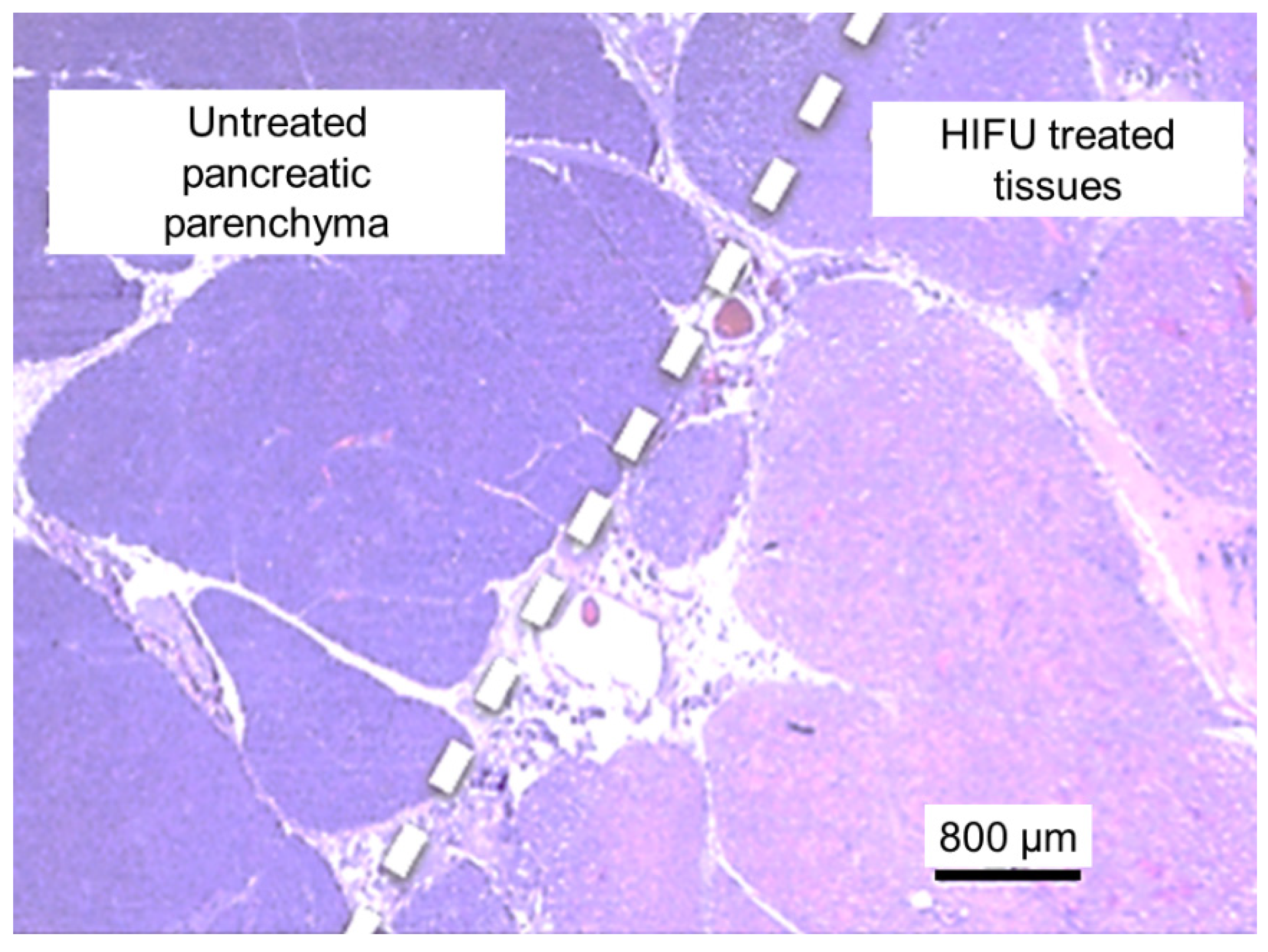
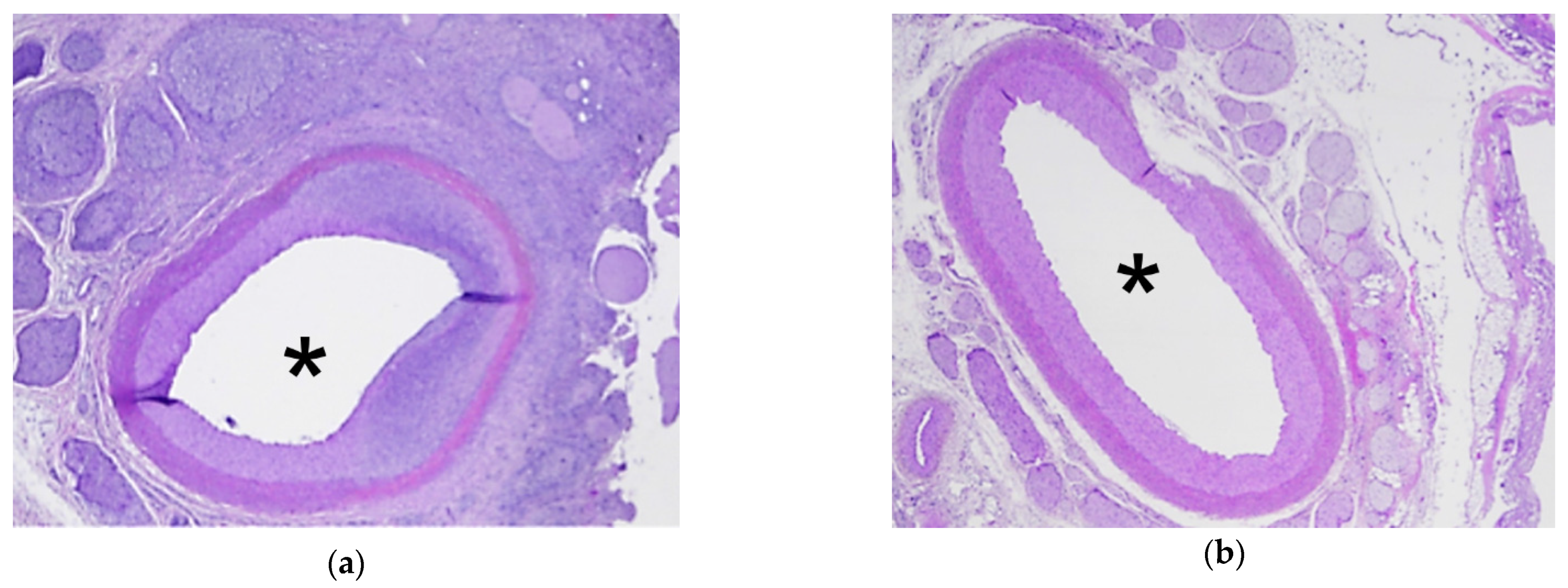
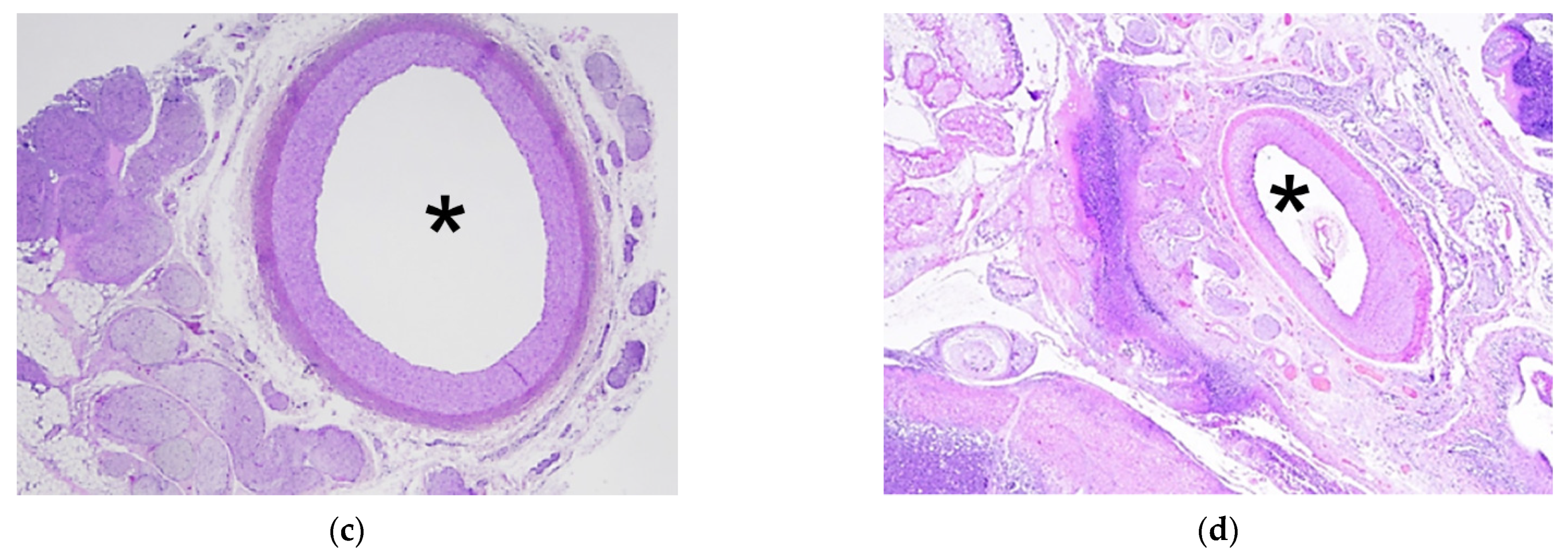
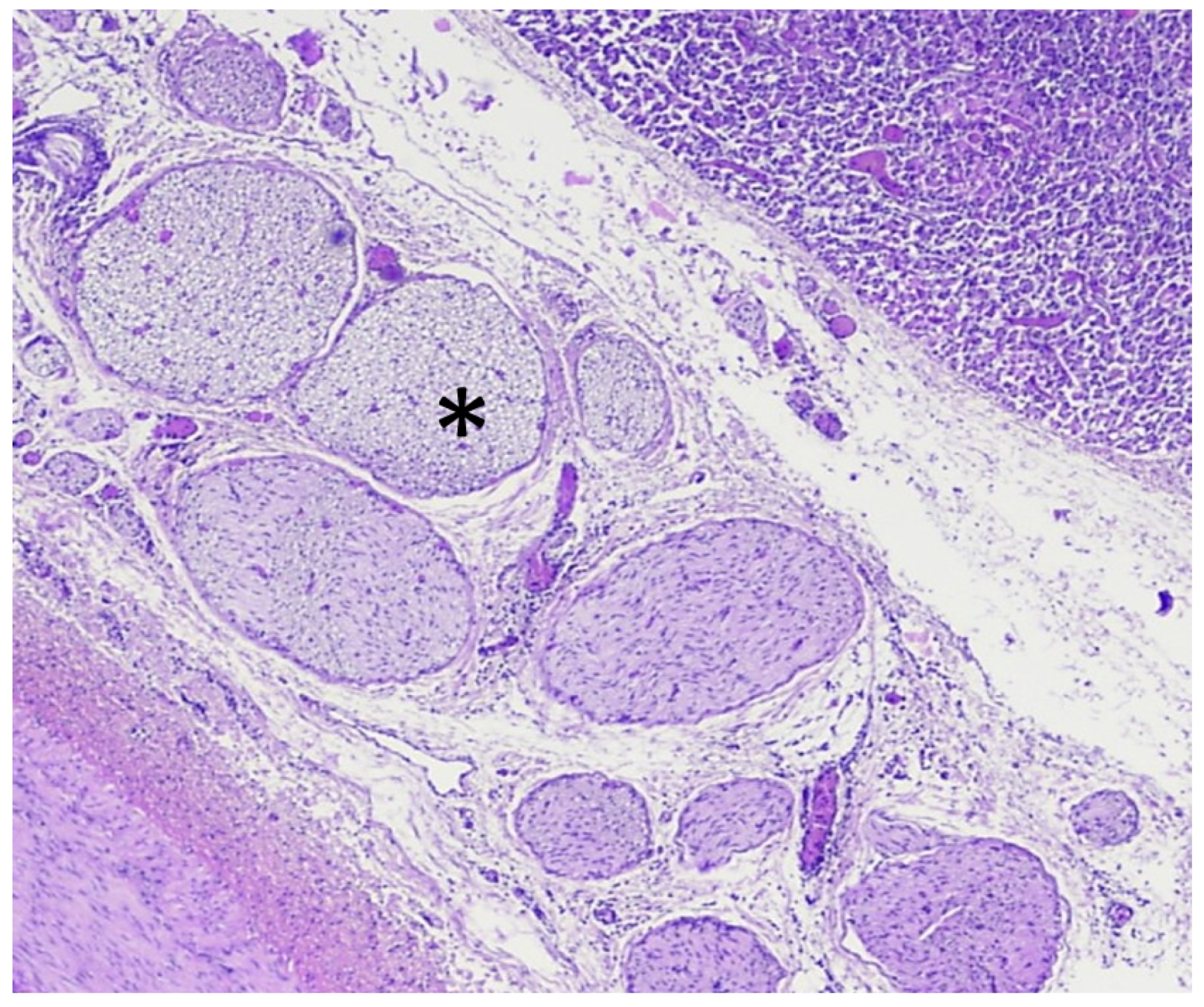
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Ishido, K.; Hakamada, K.; Kimura, N.; Miura, T.; Wakiya, T. Essential updates 2018/2019: Current topics in the surgical treatment of pancreatic ductal adenocarcinoma. Ann. Gastroenterol. Surg. 2021, 5, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Hackert, T. Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 1971. [Google Scholar] [CrossRef] [PubMed]
- van Veldhuisen, E.; Jenniskens, S.F.M.; van den Boezem, P.B.; Futterer, J.J.; de Wilt, J.H.W. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers 2019, 11, 976. [Google Scholar]
- Paiella, S.; de Pastena, M.; Faustini, F.; Landoni, L.; Pollini, T.; Bonamini, D.; Giuliani, T.; Bassi, C.; Esposito, A.; Tuveri, M.; et al. Central pancreatectomy for benign or low-grade malignant pancreatic lesions—A single-center retrospective analysis of 116 cases. Eur. J. Surg. Oncol. 2019, 45, 788–792. [Google Scholar] [CrossRef]
- Auclin, E.; Marthey, L.; Abdallah, R.; Mas, L.; Francois, E.; Saint, A.; Cunha, A.S.; Vienot, A.; Lecomte, T.; Hautefeuille, V.; et al. Role of FOLFIRINOX and chemoradiotherapy in locally advanced and borderline resectable pancreatic adenocarcinoma: Update of the AGEO cohort. Br. J. Cancer 2021, 124, 1941–1948. [Google Scholar] [CrossRef]
- Garnier, J.; Ewald, J.; Marchese, U.; Gilabert, M.; Moureau-Zabotto, L.; Giovannini, M.; Poizat, F.; Delpero, J.R.; Turrini, O. Borderline or locally advanced pancreatic adenocarcinoma: A single center experience on the FOLFIRINOX induction regimen. Eur. J. Surg. Oncol. 2020, 46, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Garnier, J.; Ewald, J.; Marchese, U.; Gilabert, M.; Launay, S.; Moureau-Zabotto, L.; Poizat, F.; Giovannini, M.; Delpero, J.R.; Turrini, O. Outcomes of patients with initially locally advanced pancreatic adenocarcinoma who did not benefit from resection: A prospective cohort study. BMC Cancer 2020, 20, 203. [Google Scholar]
- Philips, P.; Hays, D.; Martin, R.C. Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: Learning curve evaluation in the first 150 patients treated. PLoS ONE 2013, 8, e76260. [Google Scholar] [CrossRef]
- Girelli, R.; Frigerio, I.; Salvia, R.; Barbi, E.; Martini, P.T.; Bassi, C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br. J. Cancer 2010, 97, 220–225. [Google Scholar] [CrossRef]
- Paiella, S.; de Pastena, M.; D’Onofrio, M.; Crino, S.F.; Pan, T.L.; de Robertis, R.; Elio, G.; Martone, E.; Bassi, C.; Salvia, R. Palliative therapy in pancreatic cancer-interventional treatment with radiofrequency ablation/irreversible electroporation. Transl. Gastroenterol. Hepatol. 2018, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Dababou, S.; Marrocchio, C.; Rosenberg, J.; Bitton, R.; Pauly, K.B.; Napoli, A.; Hwang, J.H.; Ghanouni, P. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J. Ther. Ultrasound 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinova, M.; Rauch, M.; Mucke, M.; Rolke, R.; Gonzalez-Carmona, M.A.; Henseler, J.; Cuhls, H.; Radbruch, L.; Strassburg, C.P.; Zhang, L.; et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: Evaluation of feasibility, reduction of tumour volume and pain intensity. Eur. Radiol. 2016, 26, 4047–4056. [Google Scholar] [CrossRef]
- Strunk, H.M.; Lutzow, C.; Henseler, J.; Mucke, M.; Rauch, M.; Marx, C.; Schild, H.H.; Marinova, M. Mesenteric Vessel Patency Following HIFU Therapy in Patients with Locally Invasive Pancreatic Cancer. Ultraschall Med. 2018, 39, 650–658. [Google Scholar] [CrossRef]
- Marinova, M.; Strunk, H.M.; Rauch, M.; Henseler, J.; Clarens, T.; Brux, L.; Dolscheid-Pommerich, R.; Conrad, R.; Cuhls, H.; Radbruch, L.; et al. High-intensity focused ultrasound (HIFU) for tumor pain relief in inoperable pancreatic cancer: Evaluation with the pain sensation scale (SES). Schmerz 2017, 31, 31–39. [Google Scholar] [CrossRef]
- Strunk, H.M.; Henseler, J.; Rauch, M.; Mucke, M.; Kukuk, G.; Cuhls, H.; Radbruch, L.; Zhang, L.; Schild, H.H.; Marinova, M.; et al. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. Rofo 2016, 188, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Tanter, M.; Pernot, M.; Aubry, J.F.; Montaldo, G.; Marquet, F.; Fink, M. Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound. Int. J. Hyperth. 2007, 23, 141–151. [Google Scholar] [CrossRef]
- Dupre, A.; Melodelima, D.; Perol, D.; Chen, Y.; Vincenot, J.; Chapelon, J.Y.; Rivoire, M. First clinical experience of intra-operative high intensity focused ultrasound in patients with colorectal liver metastases: A phase I-IIa study. PLoS ONE 2015, 10, e0118212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupre, A.; Melodelima, D.; Perol, D.; Chen, Y.; Vincenot, J.; Chapelon, J.Y.; Rivoire, M. Evaluation of the Feasibility, Safety, and Accuracy of an Intraoperative High-intensity Focused Ultrasound Device for Treating Liver Metastases. J. Vis. Exp. 2019, 143. [Google Scholar] [CrossRef]
- Melodelima, D.; N’Djin, W.A.; Favre-Cabrera, J.; Parmentier, H.; Rivoire, M.; Chapelon, Y.J. Thermal ablation produced using a surgical toroidal high-intensity focused ultrasound device is independent from hepatic inflow occlusion. Phys. Med. Biol. 2009, 54, 6353–6368. [Google Scholar] [CrossRef]
- N’Djin, W.A.; Melodelima, D.; Parmentier, H.; Rivoire, M.; Chapelon, J.Y. In vivo preclinical evaluation of the accuracy of toroidal-shaped HIFU treatments using a tumor-mimic model. Phys. Med. Biol. 2010, 55, 2137–2154. [Google Scholar] [CrossRef]
- Melodelima, D.; N’Djin, W.A.; Parmentier, H.; Chesnais, S.; Rivoire, M.; Chapelon, J.Y. Thermal ablation by high-intensity-focused ultrasound using a toroid transducer increases the coagulated volume. Results of animal experiments. Ultrasound Med. Biol. 2009, 35, 425–435. [Google Scholar] [CrossRef]
- Parmentier, H.; Melodelima, D.; N’Djin, A.; Chesnais, S.; Chapelon, J.Y.; Rivoire, M. High-intensity focused ultrasound ablation for the treatment of colorectal liver metastases during an open procedure: Study on the pig. Ann. Surg. 2009, 249, 129–136. [Google Scholar]
- Dupre, A.; Melodelima, D.; Pflieger, H.; Chen, Y.; Vincenot, J.; Kocot, A.; Langonnet, S.; Rivoire, M. Thermal Ablation of the Pancreas With Intraoperative High-Intensity Focused Ultrasound: Safety and Efficacy in a Porcine Model. Pancreas 2017, 46, 219–224. [Google Scholar] [CrossRef]
- Reichling, C.; Nobile, L.; Pezzullo, M.; Navez, J.; Bachir, N.; D’Haene, N.; Maris, C.; Musala, C.; Fernandez, Y.; Viesca, M.; et al. Non-occlusive Mesenteric Ischemia as a Fatal Complication in Acute Pancreatitis: A Case Series. Dig. Dis. Sci. 2020, 65, 1212–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.S.; Cherr, G.S.; Craven, T.E.; Olsen, A.W.; Plonk, G.W.; Geary, R.L.; Ligush, J.L.; Hansen, K.J. Acute occlusive mesenteric ischemia: Surgical management and outcomes. Ann. Vasc. Surg. 2003, 17, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Salsano, G.; Salsano, A.; Sportelli, E.; Petrocelli, F.; Dahmane, M.; Spinella, G.; Pane, B.; Mambrini, S.; Palombo, D.; Santini, F. What is the Best Revascularization Strategy for Acute Occlusive Arterial Mesenteric Ischemia: Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2018, 41, 27–36. [Google Scholar] [CrossRef]
- Orsi, F.; Zhang, L.; Arnone, P.; Orgera, G.; Bonomo, G.; Vigna, P.D.; Monfardini, L.; Zhou, K.; Chen, W.; Wang, Z.; et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. Am. J. Roentgenol. 2010, 195, W245–W252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligresti, D.; Kuo, Y.T.; Baraldo, S.; Chavan, R.; Keane, M.G.; Seleem, S.; Seo, D.W. EUS anatomy of the pancreatobiliary system in a swine model: The WISE experience. Endosc. Ultrasound 2019, 8, 249–254. [Google Scholar]
- Ferrer, J.; Scott, W.E., III; Weegman, B.P.; Suszynski, T.M.; Sutherland, D.E.; Hering, B.J.; Papas, K.K. Pig pancreas anatomy: Implications for pancreas procurement, preservation, and islet isolation. Transplantation 2008, 86, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Khokhlova, T.D.; Hwang, J.H. HIFU for Palliative Treatment of Pancreatic Cancer. Adv. Exp. Med. Biol. 2016, 880, 83–95. [Google Scholar]
- Hwang, J.H.; Wang, Y.N.; Warren, C.; Upton, M.P.; Starr, F.; Zhou, Y.; Mitchell, S.B. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med. Biol. 2009, 35, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ye, X.; Yang, X.; Zheng, A.; Li, W.; Wang, J.; Han, X.; Wei, Z.; Meng, M.; Ni, Y.; et al. Experimental study in vivo ablation of swine pancreas using high-intensity focused ultrasound. J. Cancer Res. Ther. 2019, 15, 286–290. [Google Scholar]
- Xie, B.; Li, Y.Y.; Jia, L.; Nie, Y.Q.; Du, H.; Jiang, S.M. Experimental ablation of the pancreas with high intensity focused ultrasound (HIFU) in a porcine model. Int. J. Med. Sci. 2010, 8, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.; Lee, J.Y.; Lee, J.H.; Bae, J.S.; Cho, Y.J.; Kang, K.J.; Son, K.; Chung, Y.R.; Lee, K.B.; Han, J.K. A portable high-intensity focused ultrasound system for the pancreas with 3D electronic steering: A preclinical study in a swine model. Ultrasonography 2018, 37, 298–306. [Google Scholar] [CrossRef]
- Sebeke, L.C.; Rademann, P.; Maul, A.C.; Schubert-Quecke, C.; Annecke, T.; Yeo, S.Y.; Castillo-Gomez, J.D.; Schmidt, P.; Grull, H.; Heijman, E. Feasibility study of MR-guided pancreas ablation using high-intensity focused ultrasound in a healthy swine model. Int. J. Hyperth. 2020, 37, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Barrere, V.; Treilleux, I.; Chopin, N.; Melodelima, D. Development of a noninvasive HIFU treatment for breast adenocarcinomas using a toroidal transducer based on preliminary attenuation measurements. Ultrasonics 2021, 115, 106459. [Google Scholar] [CrossRef]
- Cilleros, C.; Dupre, A.; Vincenot, J.; Melodelima, D. Development of a Simple In Vitro Artery Model and an Evaluation of the Impact of Pulsed Flow on High-Intensity Focused Ultrasound Ablation. IRBM 2021, 42, 112–119. [Google Scholar] [CrossRef]
- Battais, A.; Barrere, V.; N’Djin, W.A.; Dupre, A.; Rivoire, M.; Melodelima, D. Fast and Selective Ablation of Liver Tumors by High-Intensity Focused Ultrasound Using a Toroidal Transducer Guided by Ultrasound Imaging: The Results of Animal Experiments. Ultrasound Med. Biol. 2020, 46, 3286–3295. [Google Scholar] [CrossRef]
- Caloone, J.; Huissoud, C.; Vincenot, J.; Kocot, A.; Dehay, C.; Chapelon, J.Y.; Rudigoz, R.C.; Melodelima, D. High-intensity focused ultrasound applied to the placenta using a toroidal transducer: A preliminary ex-vivo study. Ultrasound Obstet. Gynecol. 2015, 45, 313–319. [Google Scholar] [CrossRef] [Green Version]
- N’Djin, W.A.; Melodelima, D.; Schenone, F.; Rivoire, M.; Chapelon, J.Y. Assisted hepatic resection using a toroidal HIFU device: An in vivo comparative study in pig. Med. Phys. 2011, 38, 1769–1778. [Google Scholar] [CrossRef]
- Klaiber, U.; Schnaidt, E.S.; Hinz, U.; Gaida, M.M.; Heger, U.; Hank, T.; Strobel, O.; Neoptolemos, J.P.; Mihaljevic, A.L.; Buchler, M.W.; et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann. Surg. 2021, 273, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Baton, O.; Sideris, L.; Lasser, P.; Pocard, M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur. J. Surg. Oncol. 2004, 30, 85–87. [Google Scholar] [CrossRef]
- Girelli, R.; Frigerio, I.; Giardino, A.; Regi, P.; Gobbo, S.; Malleo, G.; Salvia, R.; Bassi, C. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbeck Arch. Surg. 2013, 398, 63–69. [Google Scholar] [CrossRef]
- Fegrachi, S.; Walma, M.S.; de Vries, J.J.J.; van Santvoort, H.C.; Besselink, M.G.; von Asmuth, E.G.; van Leeuwen, M.S.; Rinkes, I.H.B.; Bruijnen, R.C.; de Hingh, I.H.; et al. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: A phase II study. Eur. J. Surg. Oncol. 2019, 45, 2166–2172. [Google Scholar] [CrossRef]
- Ruarus, A.H.; Vroomen, L.; Geboers, B.; van Veldhuisen, E.; Puijk, R.S.; Nieuwenhuizen, S.; Besselink, M.G.; Zonderhuis, B.M.; Kazemier, G.; de Gruijl, T.D.; et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 2020, 294, 212–220. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Sun, S.; Zhang, Y.; Lin, X.; Lao, X.; Cui, B.; Li, S. Irreversible electroporation versus radiotherapy after induction chemotherapy on survival in patients with locally advanced pancreatic cancer: A propensity score analysis. BMC Cancer 2019, 19, 394. [Google Scholar]
- Stanislavova, N.; Karamanliev, M.; Ivanov, T.; Yotsov, T.; Zhou, K.; Dimitrov, D. Is high-intensity focused ultrasound (HIFU) an option for neoadjuvant therapy for borderline resectable pancreatic cancer patients?—A systematic review. Int. J. Hyperth. 2021, 38, 75–80. [Google Scholar] [CrossRef]
- Marinova, M.; Huxold, H.C.; Henseler, J.; Mucke, M.; Conrad, R.; Rolke, R.; Ahmadzadehfar, H.; Rauch, M.; Fimmers, R.; Luechters, G.; et al. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic Cancer. Ultraschall Med. 2019, 40, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, J.; Diao, L.; Ma, K.; Fan, Y.; Yang, W. High-intensity focused ultrasound ablation for advanced pancreatic cancer. J. Cancer Res. Ther. 2019, 15, 831–835. [Google Scholar] [PubMed]
- Delpero, J.R.; Bachellier, P.; Regenet, N.; le Treut, Y.P.; Paye, F.; Carrere, N.; Sauvanet, A.; Autret, A.; Turrini, O.; Monges-Ranchin, G.; et al. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB 2014, 16, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’Djin, W.A.; Chapelon, J.Y.; Melodelima, D. An Ultrasound Image-Based Dynamic Fusion Modeling Method for Predicting the Quantitative Impact of In Vivo Liver Motion on Intraoperative HIFU Therapies: Investigations in a Porcine Model. PLoS ONE 2015, 10, e0137317. [Google Scholar]
- Vincenot, J.; Melodelima, D.; Chavrier, F.; Vignot, A.; Kocot, A.; Chapelon, J.Y. Electronic beam steering used with a toroidal HIFU transducer substantially increases the coagulated volume. Ultrasound Med. Biol. 2013, 39, 1241–1254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilleros, C.; Dupré, A.; Chen, Y.; Vincenot, J.; Rivoire, M.; Melodelima, D. Intraoperative HIFU Ablation of the Pancreas Using a Toroidal Transducer in a Porcine Model. The First Step towards a Clinical Treatment of Locally Advanced Pancreatic Cancer. Cancers 2021, 13, 6381. https://doi.org/10.3390/cancers13246381
Cilleros C, Dupré A, Chen Y, Vincenot J, Rivoire M, Melodelima D. Intraoperative HIFU Ablation of the Pancreas Using a Toroidal Transducer in a Porcine Model. The First Step towards a Clinical Treatment of Locally Advanced Pancreatic Cancer. Cancers. 2021; 13(24):6381. https://doi.org/10.3390/cancers13246381
Chicago/Turabian StyleCilleros, Celia, Aurélien Dupré, Yao Chen, Jeremy Vincenot, Michel Rivoire, and David Melodelima. 2021. "Intraoperative HIFU Ablation of the Pancreas Using a Toroidal Transducer in a Porcine Model. The First Step towards a Clinical Treatment of Locally Advanced Pancreatic Cancer" Cancers 13, no. 24: 6381. https://doi.org/10.3390/cancers13246381
APA StyleCilleros, C., Dupré, A., Chen, Y., Vincenot, J., Rivoire, M., & Melodelima, D. (2021). Intraoperative HIFU Ablation of the Pancreas Using a Toroidal Transducer in a Porcine Model. The First Step towards a Clinical Treatment of Locally Advanced Pancreatic Cancer. Cancers, 13(24), 6381. https://doi.org/10.3390/cancers13246381






