Identification of Lynch Syndrome Carriers among Patients with Small Bowel Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Statistical Analysis
3. Results
3.1. General Characteristics
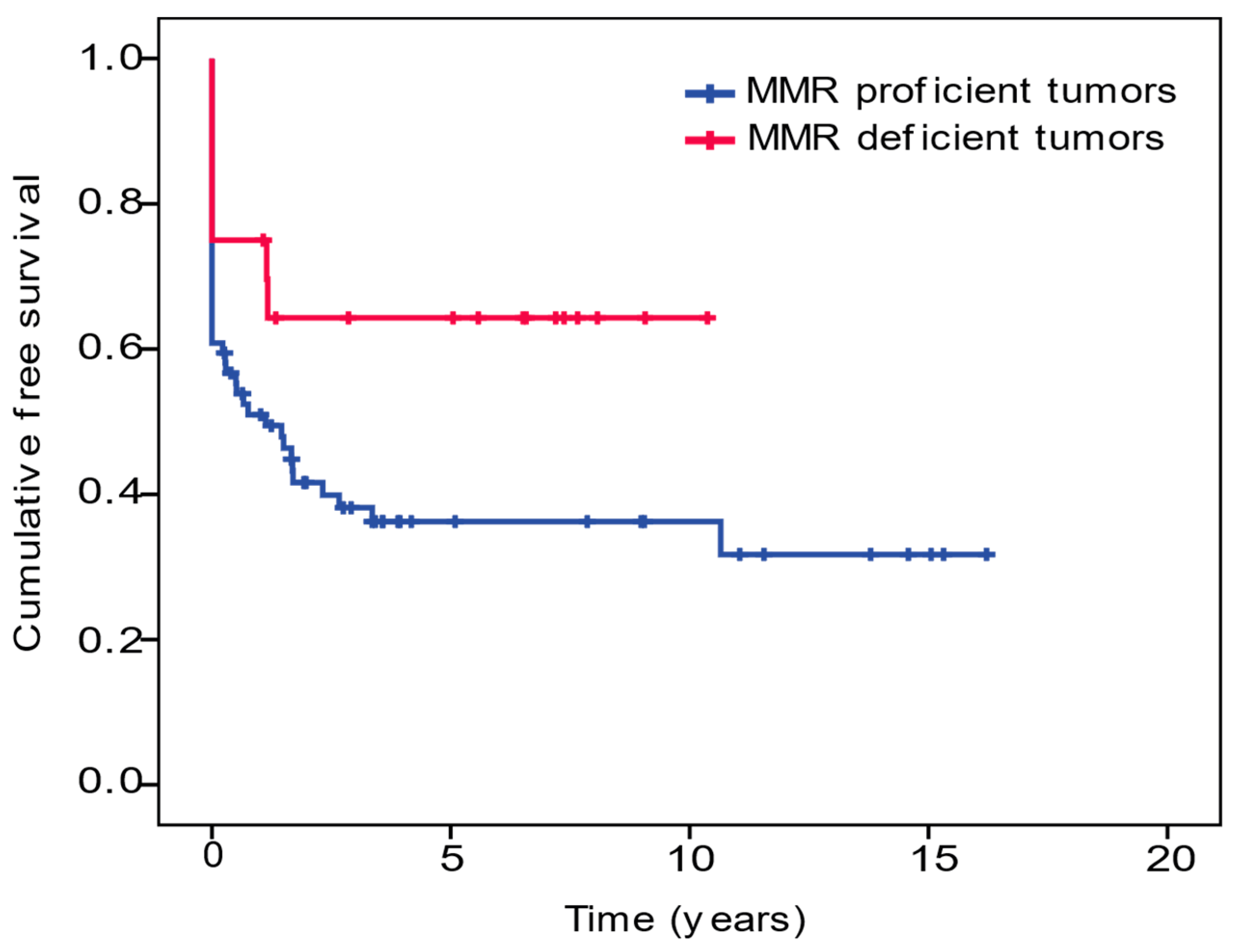
3.2. Tumor Mismatch Repair Analysis
3.3. MMR Germline Genetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aparicio, T.; Zaanan, A.; Mary, F.; Afchain, P.; Manfredi, S.; Evans, T.R.J. Small Bowel Adenocarcinoma. Gastroenterol. Clin. N. Am. 2016, 45, 447–457. [Google Scholar] [CrossRef]
- Neugut, A.I.; Jacobson, J.S.; Suh, S.; Mukherjee, R.; Arber, N. The epidemiology of cancer of the small bowel. Cancer Epidemiol. Biomark. Prev. 1998, 7, 243–251. [Google Scholar]
- Brueckl, W.M.; Heinze, E.; Milsmann, C.; Wein, A.; Koebnick, C.; Jung, A.; Croner, R.S.; Brabletz, T.; Kirchner, T.; Hahn, E.G.; et al. Prognostic significance of microsatellite instability in curatively resected adenocarcinoma of the small intestine. Cancer Lett. 2004, 203, 181–190. [Google Scholar] [CrossRef]
- Chirita-Emandi, A.; Andreescu, N.; Zimbru, C.G.; Tutac, P.; Arghirescu, S.; Serban, M.; Puiu, M. Challenges in reporting pathogenic/potentially pathogenic variants in 94 cancer predisposing genes-in pediatric patients screened with NGS panels. Sci. Rep. 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, S. Genetic risks and familial associations of small bowel carcinoma. World J. Gastrointest. Oncol. 2016, 8, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef]
- Moreira, L.; Balaguer, F.; Lindor, N.; De La Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Moreira, L.; Muñoz, J.; Cuatrecasas, M.; Quintanilla, I.; Leoz, M.L.; Carballal, S.; Ocaña, T.; López-Cerón, M.; Pellise, M.; Castellví-Bel, S.; et al. Prevalence of Somatic MutL Homolog 1 Promoter Hypermethylation in Lynch Syndrome Colorectal Cancer. Cancer 2015, 121, 1395–1404. [Google Scholar] [CrossRef]
- Ten Kate, G.L.; Kleibeuker, J.H.; Nagengast, F.M.; Craanen, M.; Cats, A.; Menko, F.H.; Vasen, H.F.A. Is surveillance of the small bowel indicated for Lynch syndrome families? Gut 2007, 56, 1198–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haanstra, J.F.; Al-Toma, A.; Dekker, E.; Vanhoutvin, S.A.L.W.; Nagengast, F.M.; Mathus-Vliegen, E.M.; Van Leerdam, M.E.; De Vos Tot Nederveen Cappel, W.H.; Sanduleanu, S.; Veenendaal, R.A.; et al. Prevalence of small-bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Gut 2015, 64, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Planck, M.; Ericson, K.; Piotrowska, Z.; Halvarsson, B.; Rambech, E.; Nilbert, M. Microsatellite instability and expression of MLH1 and MSH2 in carcinomas of the small intestine. Cancer 2003, 97, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Svrcek, M.; Zaanan, A.; Beohou, E.; Laforest, A.; Afchain, P.; Mitry, E.; Taieb, J.; Di Fiore, F.; Gornet, J.M.; et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br. J. Cancer 2013, 109, 3057–3066. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.M.D.; Warren, B.F.; Mortensen, N.J.M.C.; Kim, H.C.; Biddolph, S.C.; Elia, G.; Beck, N.E.; Williams, G.T.; Shepherd, N.A.; Bateman, A.C.; et al. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut 2002, 50, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Warth, A.; Kloor, M.; Schirmacher, P.; Bla, H. Genetics and epigenetics of small bowel adenocarcinoma: The interactions of CIN, MSI, and CIMP. Mod. Pathol. 2011, 24, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulmann, K.; Brasch, F.E.; Kunstmann, E.; Engel, C.; Pagenstecher, C.; Vogelsang, H.; Krüger, S.; Vogel, T.; Knaebel, H.P.; Rüschoff, J.; et al. HNPCC-associated small bowel cancer: Clinical and molecular characteristics. Gastroenterology 2005, 128, 590–599. [Google Scholar] [CrossRef]
- Pérez-Carbonell, L.; Ruiz-Ponte, C.; Guarinos, C.; Alenda, C.; Payá, A.; Brea, A.; Egoavil, C.M.; Castillejo, A.; Barberá, V.M.; Bessa, X.; et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut 2012, 61, 865–872. [Google Scholar] [CrossRef]
- Thompson, B.A.; Spurdle, A.B.; Plazzer, J.P.; Greenblatt, M.S.; Akagi, K.; Al-Mulla, F.; Bapat, B.; Bernstein, I.; Capellá, G.; Den Dunnen, J.T.; et al. Application of a 5-tiered scheme for standardized classification of 2360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat. Genet. 2014, 46, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Nagy, R.; Sweet, K.; Eng, C. Highly penetrant hereditary cancer syndromes. Oncogene 2004, 23, 6445–6470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2015, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Balaguer, F.; Dekker, E.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Ricciardiello, L.; Rupinska, M.; et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 1082–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haanstra, J.F.; Kleibeuker, J.H.; Koornstra, J.J. Role of new endoscopic techniques in Lynch syndrome. Fam. Cancer 2013, 12, 267–272. [Google Scholar] [CrossRef]
- Saurin, J.C.; Pilleul, F.; Soussan, E.B.; Maniere, T.; D’Halluin, P.N.; Gaudric, M.; Cellier, C.; Heresbach, D.; Gaudin, J.L.; Capsule Commission of the French Society of Digestive Endoscopy (SFED). Small-bowel capsule endoscopy diagnoses early and advanced neoplasms in asymptomatic patients with Lynch syndrome. Endoscopy 2010, 42, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.J.; Hong, S.M.; Jun, S.Y.; Eom, D.W. Prognostic implications of immune classification in a multicentre cohort of patients with small intestinal adenocarcinoma. Pathology 2020, 52, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/ Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1–10. [Google Scholar] [CrossRef]
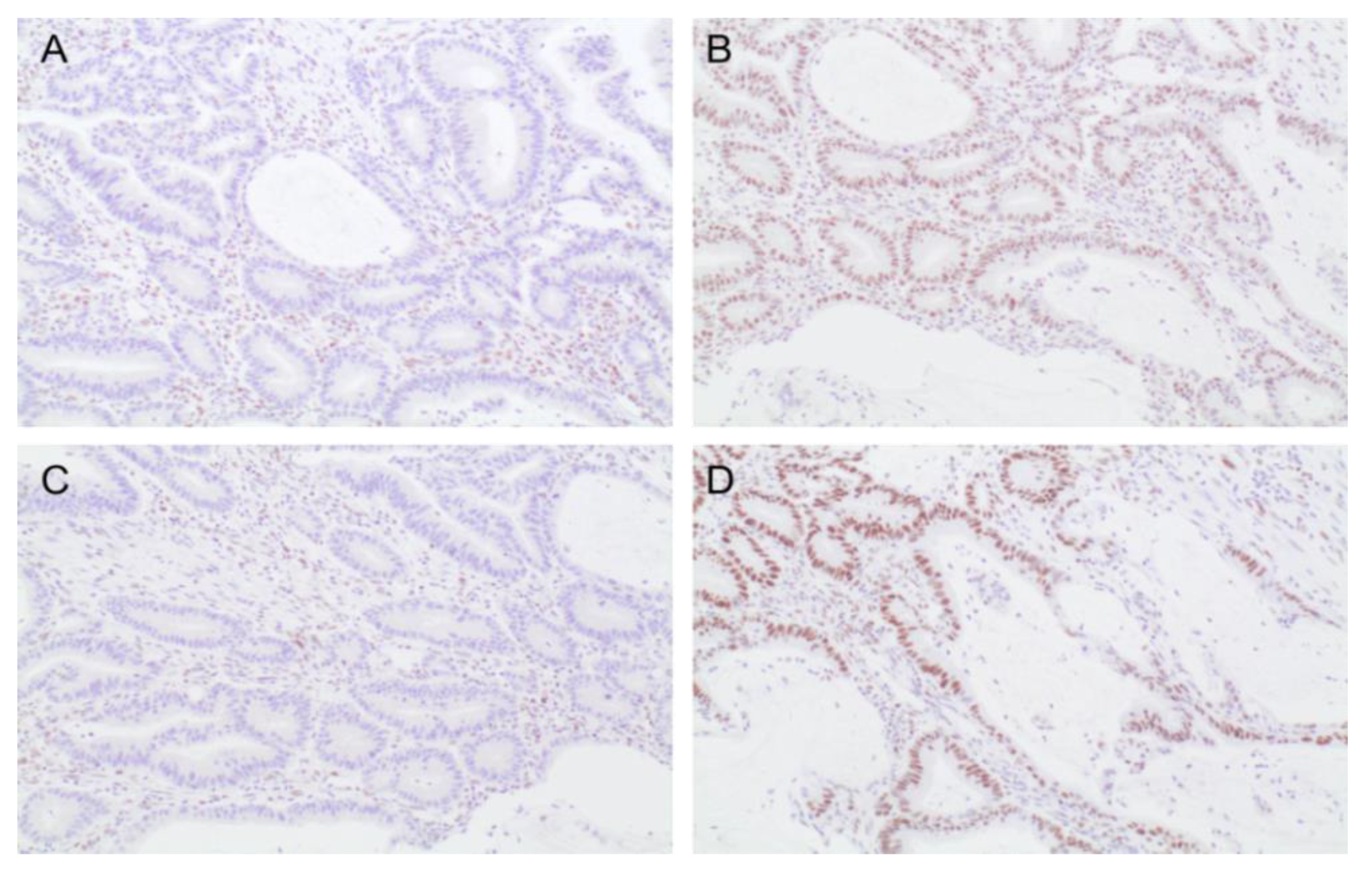
| Characteristics | MMR Proficient Tumor (n = 74) | MMR Deficient Tumor (n = 20) | p Value | Total Patients (n = 94) |
|---|---|---|---|---|
| Male sex, n (%) | 44 (59.5) | 12 (60) | 0.136 | 52 (55.3) |
| Clinical diagnostic criteria for LS *, n (%) | 2 (2.7) | 4 (20) | 0.018 | 6 (6.4) |
| Median age at diagnosis, y (IQR) | 68.5 (54.8–77) | 58 (44.5–69) | 0.047 | 65.5 (53.8–75.3) |
| Stage | 1 | |||
| I–II, n (%) | 30 (45.5) | 9 (45) | 39 (45.3) | |
| III–IV, n (%) | 36 (54.5) | 11 (55) | 47 (54.7) | |
| Histological grade | 0.915 | |||
| G1, n (%) | 18 (27.3) | 5 (25) | 23 (26.7) | |
| G2, n (%) | 25 (37.9) | 7 (35) | 32 (37.2) | |
| G3, n (%) | 23 (34.8) | 8 (40) | 31 (36) | |
| Location | 0.020 | |||
| Duodenum, n (%) | 31 (42.5) | 10 (50) | 41 (44.1) | |
| Jejunum, n (%) | 19 (26) | 9 (45) | 28 (30.1) | |
| Ileum, n (%) | 23 (31.5) | 1 (5) | 24 (25.8) | |
| Surgical Intervention, n (%) | 65 (87.8) | 20 (100) | 0.197 | 65 (69.5) |
| With curative intention, n (%) | 47 (63.5) | 18 (90) | 0.028 | |
| Chemotherapy, n (%) | 36 (48.6) | 11 (55) | 0.802 | 47 (50) |
| SBAmortality, n (%) | 39 (52.7) | 5 (25) | 0.042 | 44 (46.8) |
| Median ageSBA mortality, y (IQR) | 72 (58–80) | 60 (58.5–85.5) | 0.956 | 71.5 (58.25–80) |
| Five-year overall survival (%) | 15 (20.3) | 11 (55) | 0.004 | 26 (27.7) |
| Mean overall survival, y (95% CI) | 7.121 (5.20–9.04) | 6.57 (4.84–8.30) | 0.073 | 7.286 (5.69–8.88) |
| Five-year SBA-free survival (%) | 12 (16.2) | 10 (50) | 0.005 | 22 (23.4) |
| Mean SBA-free survival, y (95% CI) | 5.96 (4.18–7.75) | 6.79 (4.66–8.93) | 0.048 | 6.86 (5.21–8.51) |
| Sex | SBA Risk Factors | Family History of Cancer | Metachronous Neoplasm | Age at Diagnosis (y) | Location | TNM | Stage | Deficient MMR Proteins | Germline MMR Study | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | No | Two 1st-degree relatives: bladder 40 y-o and pancreatic cancer 38 y-o | CRC 43 y-o | 51 | Jejunum | T3N1M0 | III | MLH1/PMS2 | MLH1 (c.1644 C>G; p. Tyr548Ter) | Alive |
| Female | No | 1st-degree relative CRC 33 y-o; 3rd-degree relative gastric cancer 45 y-o | No | 67 | Jejunum | T3N0M0 | II | MLH1/PMS2 | MLH1 (c.350C>T; p. Thr117Met) | Alive |
| Female | No | No | CRC 49 y-o | 69 | Duodenum | T3N0M0 | II | MLH1/PMS2 | MLH1 (c.306+5G>A) | Alive |
| Male | No | Two 1st-degree relatives CRC: 23 and 50 y-o | CRC 41 y-o | 42 | Jejunum | T4N1M0 | III | MSH2/MSH6 | MSH2 (c.1861C>T; p. Arg621Ter) | Alive |
| Male | No | No | No | 44 | Duodenum | T4N1M0 | III | MSH2/MSH6 | MSH2 (c.927dupAG) | Dead |
| Male | No | Amsterdam II Criteria: >3 relatives with CRC and/or other LS spectrum cancers | CRC 32 y-o, ureter cancer 44 y-o prostate cancer 65 y-o | 69 | Duodenum | T3N1M0 | III | MSH2/MSH6 | MSH2 (c.1387-?_661+?del) | Dead |
| Male | No | 1st-degree relative CRC 67 y-o | No | 46 | Ileum | T4N1M0 | III | MSH2/MSH6 | MSH2 (c. 842C>G; p. Ser281Ter) | Alive |
| Female | No | 1st-degree relative CRC 60 y-o | Intestinal lymphoma | 82 | Jejunum | T3N0M0 | II | MSH2/MSH6 | MSH6 (c.2188dupT; p. Tyr730LeufsTer26) | Alive |
| Female | No | 1st-degree relative bladder cancer 66 y-o; 2nd-degree relative ureter cancer 67a | No | 39 | Jejunum | T3N0M0 | II | PMS2 | PMS2 (c.1831dup; p. Ile611fs) | Alive |
| Female | Celiac Disease | Four 2nd-degree relatives CRC > 85 y-o | No | 60 | Jejunum | T3N0M0 | II | MLH1/PMS2 | Negative | Alive |
| Female | No | No | No | 71 | Jejunum | T3N0M0 | II | MLH1 | Negative | Alive |
| Female | No | 1st-degree relative endometrium 63 y-o | Follicular lymphoma | 37 | Duodenum | T3N0M0 | II | MLH1/PMS2 | Negative | Alive |
| Male | No | Two 1st-degree relatives: CRC 87 y-o, laryngeal cancer 65 y-o | No | 64 | Duodenum | T3N2M0 | III | MLH1/PMS2 | Negative | Alive |
| Female | No | No | No | 57 | Duodenum | T4N1M1 | IV | MSH2/MSH6 | Negative | Dead |
| Female | No | Three 2nd-degree relatives: leukemia, ovarian cancer, renal cancer | No | 43 | Duodenum | T4N0M0 | II | MSH2/MSH6 | Negative | Alive |
| Female | No | Relative prostate cancer 62 y-o | No | 75 | Jejunum | T4N2M0 | III | MLH1 | NA | Dead |
| Female | No | No | CRC 42 y-o, Hodgkin lymphoma 55 y-o | 59 | Duodenum | T3N1M0 | III | MLH1/PMS2 | NA | Dead |
| Male | No | Relative prostate cancer | No | 90 | Jejunum | T3N1M0 | III | MSH2 | NA | Dead |
| Male | No | 3rd-degree relative hepatic cancer | No | 53 | Duodenum | T3N1M0 | III | MSH2/MSH6 | NA | Alive |
| Female | No | 1st-degree relative unknown cancer 54 y-o; two 2nd-degree relatives: gastric and intestinal cancer | No | 56 | Duodenum | T3N0M0 | II | PMS2 | NA | Dead |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, A.; Bujanda, L.; Cuatrecasas, M.; Bofill, A.; Alvarez-Urturi, C.; Hernandez, G.; Aguilera, L.; Carballal, S.; Llach, J.; Herrera-Pariente, C.; et al. Identification of Lynch Syndrome Carriers among Patients with Small Bowel Adenocarcinoma. Cancers 2021, 13, 6378. https://doi.org/10.3390/cancers13246378
Sánchez A, Bujanda L, Cuatrecasas M, Bofill A, Alvarez-Urturi C, Hernandez G, Aguilera L, Carballal S, Llach J, Herrera-Pariente C, et al. Identification of Lynch Syndrome Carriers among Patients with Small Bowel Adenocarcinoma. Cancers. 2021; 13(24):6378. https://doi.org/10.3390/cancers13246378
Chicago/Turabian StyleSánchez, Ariadna, Luis Bujanda, Miriam Cuatrecasas, Alex Bofill, Cristina Alvarez-Urturi, Goretti Hernandez, Lara Aguilera, Sabela Carballal, Joan Llach, Cristina Herrera-Pariente, and et al. 2021. "Identification of Lynch Syndrome Carriers among Patients with Small Bowel Adenocarcinoma" Cancers 13, no. 24: 6378. https://doi.org/10.3390/cancers13246378
APA StyleSánchez, A., Bujanda, L., Cuatrecasas, M., Bofill, A., Alvarez-Urturi, C., Hernandez, G., Aguilera, L., Carballal, S., Llach, J., Herrera-Pariente, C., Iglesias, M., Rivero-Sánchez, L., Jung, G., Moreno, L., Ocaña, T., Bayarri, C., Pellise, M., Castells, A., Castellví-Bel, S., ... Moreira, L. (2021). Identification of Lynch Syndrome Carriers among Patients with Small Bowel Adenocarcinoma. Cancers, 13(24), 6378. https://doi.org/10.3390/cancers13246378









