Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures
Abstract
:Simple Summary
Abstract
1. Introduction
2. NEN Liver Metastases
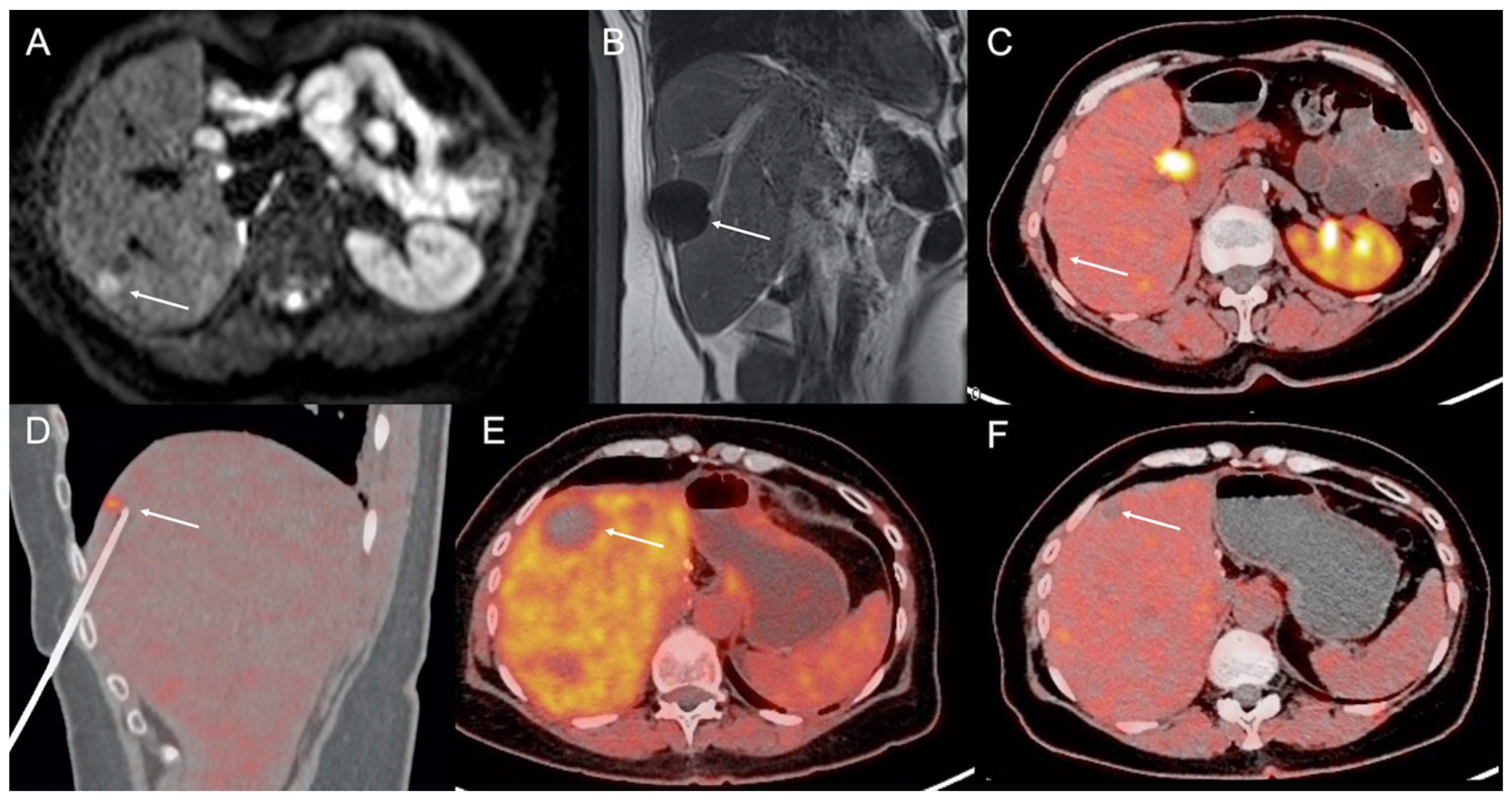
3. Current Imaging for Management of NEN Liver Metastases
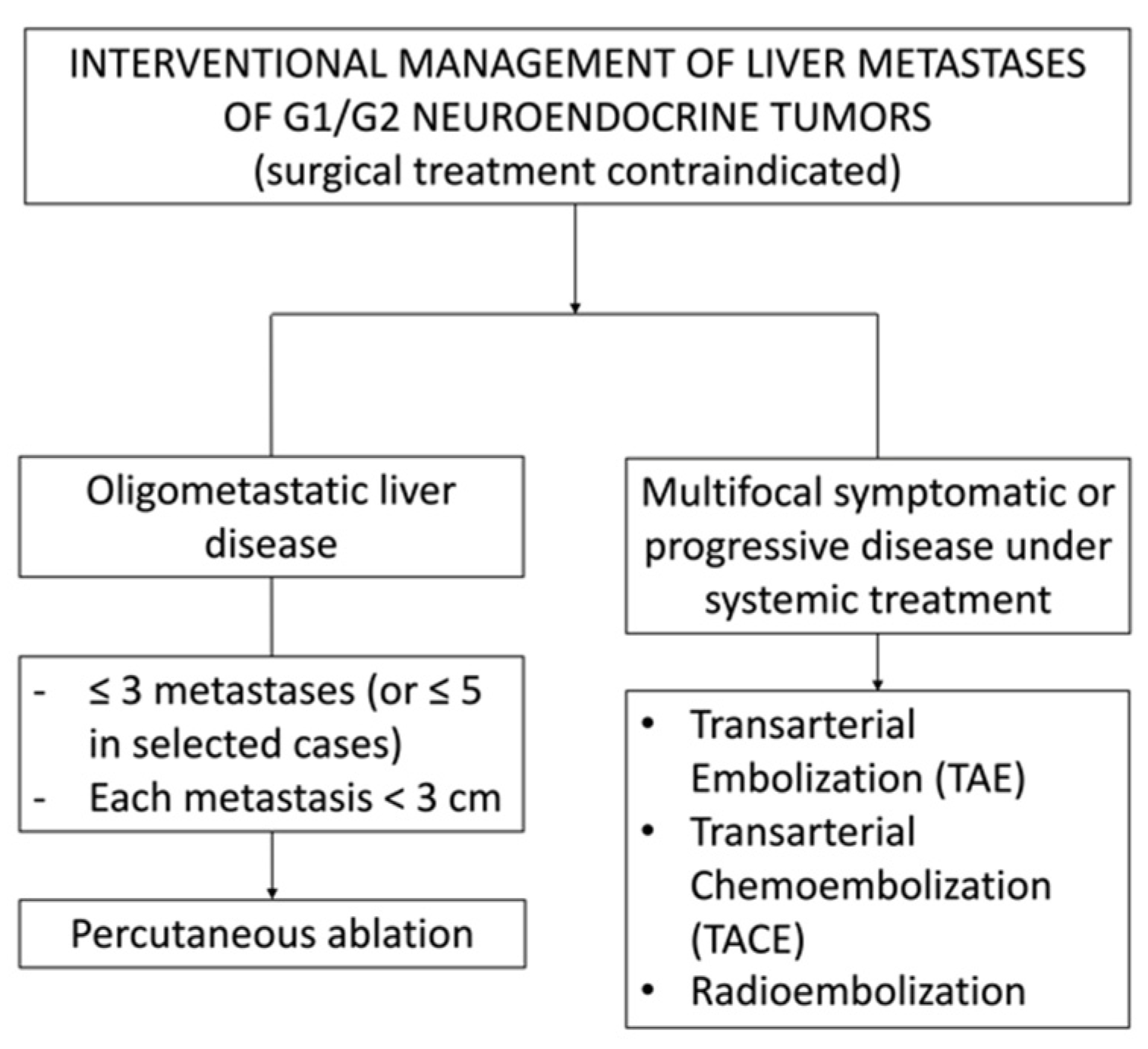
4. Imaging Guidance for Percutaneous Interventional Procedures
5. Percutaneous Ablations
5.1. Laser Ablation (LA)
5.2. Radiofrequency Ablation (RFA)
5.3. Microwave Ablation (MWA)
5.4. Irreversible Electroporation (IE)
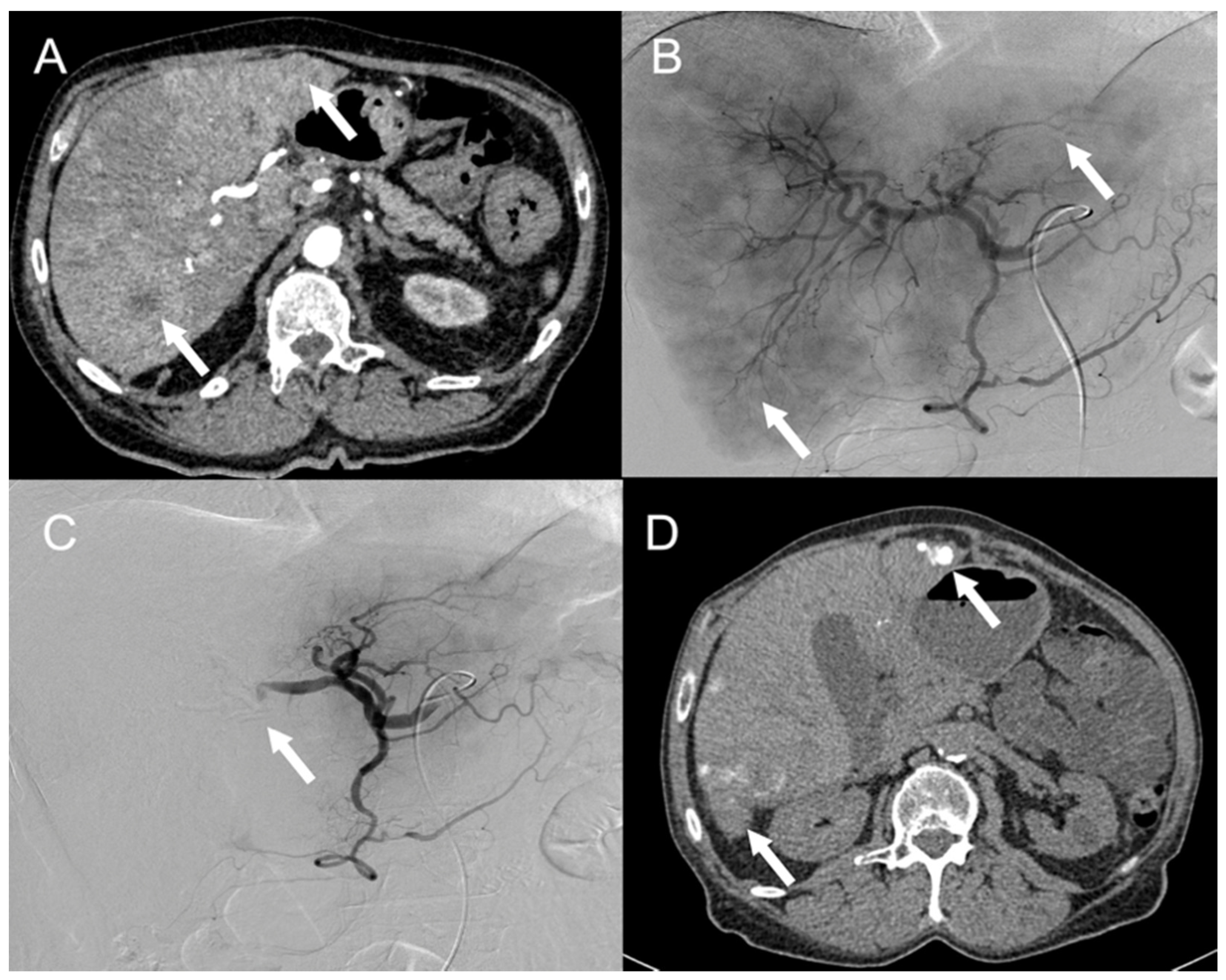
6. Endovascular Treatments
6.1. Transarterial Embolization (TAE) and Transarterial Chemoembolization (TACE)
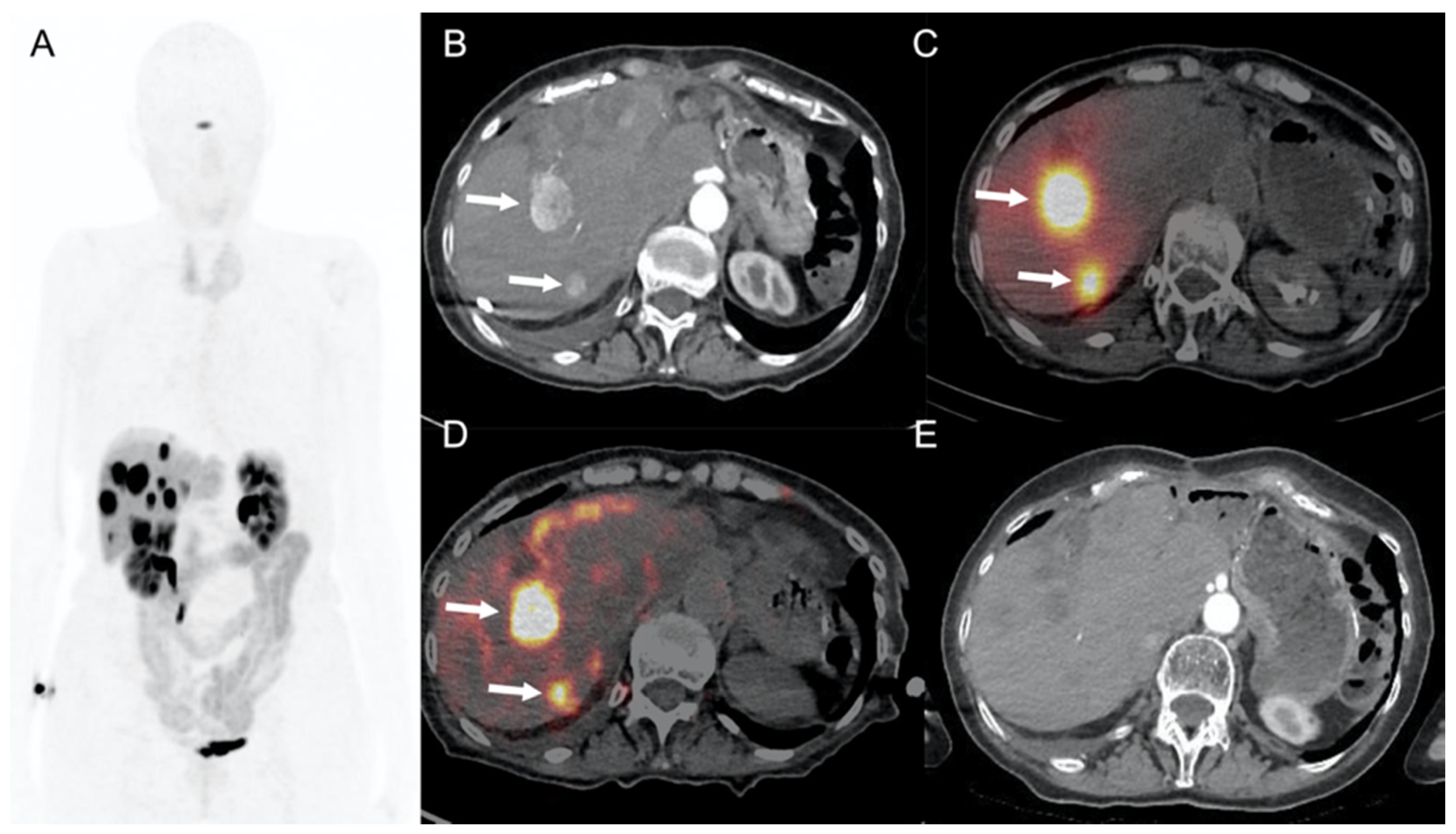
6.2. 90Y-Selective Internal Radiation Therapy (SIRT)
6.3. Targeted Radionuclide Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, M.; Kim, M.; Faggiano, A.; Valk, G. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 691–703. [Google Scholar] [CrossRef]
- Addeo, P.; Bertin, J.-B.; Imperiale, A.; Averous, G.; Terrone, A.; Goichot, B.; Bachellier, P. Outcomes of Simultaneous Resection of Small Bowel Neuroendocrine Tumors with Synchronous Liver Metastases. World J. Surg. 2020, 44, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Falconi, M.; Grana, C.; Botteri, E.; Chiappa, A.; Misitano, P.; Spada, F.; Ravizza, D.; Bazolli, B.; Fazio, N. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. Int. J. Surg. 2015, 20, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; Debraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. 2017, 43, 380–387. [Google Scholar] [CrossRef]
- Plöckinger, U.; Rindi, G.; Arnold, R.; Eriksson, B.; Krenning, E.P.; De Herder, W.W.; Goede, A.; Caplin, M.; Öberg, K.; Reubi, J.C.; et al. Guidelines for the Diagnosis and Treatment of Neuroendocrine Gastrointestinal Tumours. Neuroendocrinology 2004, 80, 394–424. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors: Epidemiology of Metastases. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.; Caplin, M.E.; Yao, J.C. The NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Well-Differentiated Neuroendocrine Tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef] [Green Version]
- Le Treut, Y.P.; Grégoire, E.; Klempnauer, J.; Belghiti, J.; Jouve, E.; Lerut, J.; Castaing, D.; Soubrane, O.; Boillot, O.; Mantion, G.; et al. Liver Transplantation for Neuroendocrine Tumors in Europe—Results and Trends in Patient Selection: A 213-Case European Liver Transplant Registry Study. Ann. Surg. 2013, 257, 807–815. [Google Scholar] [CrossRef]
- Moris, D.; Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Beal, E.; Felekouras, E.; Vernadakis, S.; Fung, J.; Pawlik, T.M. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017, 162, 525–536. [Google Scholar] [CrossRef] [Green Version]
- De Mestier, L.; Brixi, H.; O’Toole, D.; Ruszniewski, P.; Cadiot, G.; Kianmanesh, R. Updating the Surgical Management of Peritoneal Carcinomatosis in Patients with Neuroendocrine Tumors. Neuroendocrinology 2015, 101, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Sposito, C.; Coppa, J.; Miceli, R.; Bhoori, S.; Bongini, M.; Camerini, T.; Milione, M.; Regalia, E.; Spreafico, C.; et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases from Neuroendocrine Tumors. Arab. Archaeol. Epigr. 2016, 16, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.; Duvillard, P.; Goéré, D.; Dromain, C.; Dumont, F.; Baudin, E. Hepatic Metastases from Neuroendocrine Tumors With a “Thin Slice” Pathological Examination: They Are Many More Than You Think. Ann. Surg. 2010, 251, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Elsayes, K.M.; Menias, C.O.; Bowerson, M.; Osman, O.M.; Alkharouby, A.M.; Hillen, T.J. Imaging of Carcinoid Tumors: Spectrum of Findings with Pathologic and Clinical Correlation. J. Comput. Assist. Tomogr. 2011, 35, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; De Baere, T.; Baudin, E.; Galline, J.; Ducreux, M.; Boige, V.; Duvillard, P.; Laplanche, A.; Caillet, H.; Lasser, P.; et al. MR Imaging of Hepatic Metastases Caused by Neuroendocrine Tumors: Comparing Four Techniques. Am. J. Roentgenol. 2003, 180, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Peterson, M.S.; Federle, M.P.; Baron, R.L.; Haradome, H.; Kawamori, Y.; Nawano, S.; Araki, T. Islet Cell Tumor of the Pancreas: Biphasic CT versus MR Imaging in Tumor Detection. Radiology 2000, 216, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Brown, G.; Riddell, A.M.; Scurr, E.; Collins, D.; Allen, S.D.; Chau, I.; Cunningham, D.; Desouza, N.M.; Leach, M.; et al. Detection of colorectal hepatic metastases using MnDPDP MR imaging and diffusion-weighted imaging (DWI) alone and in combination. Eur. Radiol. 2008, 18, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Bali, M.A.; Ba-Ssalamah, A.; Boraschi, P.; Brancatelli, G.; Caseiro-Alves, F.; Grazioli, L.; Helmberger, T.; Lee, J.M.; Manfredi, R.; et al. ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur. Radiol. 2016, 26, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouvrard, E.; Chevalier, E.; Addeo, P.; Sahakian, N.; Detour, J.; Goichot, B.; Bachellier, P.; Karcher, G.; Taïeb, D.; Imperiale, A. Intraindividual comparison of 18 F-FDOPA and 68 Ga-DOTATOC PET/CT detection rate for metastatic assessment in patients with ileal neuroendocrine tumours. Clin. Endocrinol. 2021, 94, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Fiz, F.; Bottoni, G.; Ugolini, M.; Noordzij, W.; Trimboli, P. Head-to-head comparison between 18 F-DOPA PET/CT and 68 Ga-DOTA peptides PET/CT in detecting intestinal neuroendocrine tumours: A systematic review and meta-analysis. Clin. Endocrinol. 2021, 95, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Deroose, C.; Hindié, E.; Kebebew, E.; Goichot, B.; Pacak, K.; Taïeb, D.; Imperiale, A. Molecular Imaging of Gastroenteropancreatic Neuroendocrine Tumors: Current Status and Future Directions. J. Nucl. Med. 2016, 57, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abgral, R.; Leboulleux, S.; Déandreis, D.; Aupérin, A.; Lumbroso, J.; Dromain, C.; Duvillard, P.; Elias, D.; de Baere, T.; Guigay, J.; et al. Performance of 18Fluorodeoxyglucose-Positron Emission Tomography and Somatostatin Receptor Scintigraphy for High Ki67 (≥10%) Well-Differentiated Endocrine Carcinoma Staging. J. Clin. Endocrinol. Metab. 2011, 96, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.-L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High Prognostic Value of 18F-FDG PET for Metastatic Gastroenteropancreatic Neuroendocrine Tumors: A Long-Term Evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef] [Green Version]
- Alevroudis, E.; Spei, M.-E.; Chatziioannou, S.; Tsoli, M.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Clinical Utility of 18F-FDG PET in Neuroendocrine Tumors Prior to Peptide Receptor Radionuclide Therapy: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef] [PubMed]
- Denys, A.; Lachenal, Y.; Duran, R.; Chollet-Rivier, M.; Bize, P. Use of High-Frequency Jet Ventilation for Percutaneous Tumor Ablation. Cardiovasc. Interv. Radiol. 2014, 37, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Cazzato, R.L.; Garnon, J.; Shaygi, B.; Tsoumakidou, G.; Caudrelier, J.; Koch, G.; Gangi, A. How to Perform a Routine Cryoablation under MRI Guidance. Top. Magn. Reson. Imaging 2018, 27, 33–38. [Google Scholar] [CrossRef]
- Cerci, J.J.; Neto, C.C.P.; Krauzer, C.; Sakamoto, D.G.; Vitola, J.V. The impact of coaxial core biopsy guided by FDG PET/CT in oncological patients. Eur. J. Nucl. Med. Mol. Imaging 2012, 40, 98–103. [Google Scholar] [CrossRef]
- Shyn, P.B. Interventional Positron Emission Tomography/Computed Tomography: State-of-the-Art. Tech. Vasc. Interv. Radiol. 2013, 16, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, A.; Garnon, J.; Bachellier, P.; Gangi, A.; Namer, I.J. Simultaneous 18F-FDOPA PET/CT-Guided Biopsy and Radiofrequency Ablation of Recurrent Neuroendocrine Hepatic Metastasis: Further Step Toward a Theranostic Approach. Clin. Nucl. Med. 2015, 40, e334–e335. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Tsoumakidou, G.; Imperiale, A.; Namer, I.J.; Bachellier, P.; Caudrelier, J.; Rao, P.; Koch, G.; et al. 18F-FDOPA PET/CT-Guided Radiofrequency Ablation of Liver Metastases from Neuroendocrine Tumours: Technical Note on a Preliminary Experience. Cardiovasc. Interv. Radiol. 2016, 39, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Garnon, J.; Shaygi, B.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Addeo, P.; Bachellier, P.; Namer, I.J.; Gangi, A. PET/CT-guided interventions: Indications, advantages, disadvantages and the state of the art. Minim. Invasive Ther. Allied Technol. 2017, 27, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Auloge, P.; Cazzato, R.L.; Koch, G.; Caudrelier, J.; De Marini, P.; Garnon, J.; Gangi, A. Destruction tumorale percutanée. Presse Méd. 2019, 48, 1146–1155. [Google Scholar] [CrossRef]
- Spiliotis, A.E.; Gäbelein, G.; Holländer, S.; Scherber, P.-R.; Glanemann, M.; Patel, B. Microwave ablation compared with radiofrequency ablation for the treatment of liver cancer: A systematic review and meta-analysis. Radiol. Oncol. 2021, 55, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-Y.; Li, G.-L.; Chen, J.; Chen, Z.-W.; Chen, Y.-P.; Lin, S.-Z. Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J. Cancer Res. Ther. 2016, 12, 153. [Google Scholar] [CrossRef]
- Mohan, H.; Nicholson, P.; Winter, D.C.; O’Shea, D.; O’Toole, D.; Geoghegan, J.; Maguire, D.; Hoti, E.; Traynor, O.; Cantwell, C.P. Radiofrequency Ablation for Neuroendocrine Liver Metastases: A Systematic Review. J. Vasc. Interv. Radiol. 2015, 26, 935–942.e1. [Google Scholar] [CrossRef]
- Cazzato, R.L.; DE Marini, P.; Leclerc, L.; Dalili, D.; Koch, G.; Rao, P.; Auloge, P.; Garnon, J.; Gangi, A. Large nearly spherical ablation zones are achieved with simultaneous multi-antenna microwave ablation applied to treat liver tumours. Eur. Radiol. 2019, 30, 971–975. [Google Scholar] [CrossRef]
- Garnon, J.; Delmas, L.; De Marini, P.; Dalili, D.; Koch, G.; Auloge, P.; Cazzato, R.L.; Gangi, A. Triple-Antenna Microwave Ablation with Repositioning for the Creation of a Reliable 6-cm Ablation Zone in the Liver. Cardiovasc. Interv. Radiol. 2021, 44, 1291–1295. [Google Scholar] [CrossRef]
- Vogl, T.J.; Nour-Eldin, N.-E.A.; Hammerstingl, R.M.; Panahi, B.; Naguib, N.N.N. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms—Review Article. Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2017, 189, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Pickens, R.C.; Sulzer, J.K.; Passeri, M.J.; Murphy, K.; Vrochides, D.; Martinie, J.B.; Baker, E.H.; Ocuin, L.M.; McKillop, I.H.; Iannitti, D.A. Operative Microwave Ablation for the Multimodal Treatment of Neuroendocrine Liver Metastases. J. Laparoendosc. Adv. Surg. Tech. 2021, 31, 917–925. [Google Scholar] [CrossRef]
- Cannon, R.; Ellis, S.; Hayes, D.; Narayanan, G.; Martin, R.C. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 2013, 107, 544–549. [Google Scholar] [CrossRef]
- Weiss, J.; Garnon, J.; Dalili, D.; Cazzato, R.L.; Koch, G.; Auloge, P.; Gangi, A. The Feasibility of Combined Microwave Ablation and Irreversible Electroporation for Central Liver Metastase. Cardiovasc. Interv. Radiol. 2021, 44, 999–1001. [Google Scholar] [CrossRef]
- Garnon, J.; Auloge, P.; Dalili, D.; Cazzato, R.L.; Gangi, A. Percutaneous irreversible electroporation of porta hepatis lymph node metastasis. Diagn. Interv. Imaging 2020, 102, 53–54. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.H.; Martin, R.C.G. Cardiac synchronization and arrhythmia during irreversible electroporation. J. Surg. Oncol. 2020, 122, 407–411. [Google Scholar] [CrossRef]
- Niessen, C.; Beyer, L.P.; Pregler, B.; Dollinger, M.; Trabold, B.; Schlitt, H.J.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Percutaneous Ablation of Hepatic Tumors Using Irreversible Electroporation: A Prospective Safety and Midterm Efficacy Study in 34 Patients. J. Vasc. Interv. Radiol. 2016, 27, 480–486. [Google Scholar] [CrossRef]
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB 2015, 17, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Pachter, H.L.; Sarpel, U. Hepatic Arterial Embolization for the Treatment of Metastatic Neuroendocrine Tumors. Int. J. Hepatol. 2012, 2012, 471203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barat, M.; Cottereau, A.-S.; Kedra, A.; Dermine, S.; Palmieri, L.-J.; Coriat, R.; Dautry, R.; Tselikas, L.; Soyer, P.; Dohan, A. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J. Clin. Med. 2020, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.; Kennedy, S.; Farrell, A.; Jaberi, A.; Kachura, J.; Beecroft, R. Comparison of Transarterial Bland and Chemoembolization for Neuroendocrine Tumours: A Systematic Review and Meta-Analysis. Curr. Oncol. 2020, 27, 537–546. [Google Scholar] [CrossRef]
- Minh, D.D.; Chapiro, J.; Gorodetski, B.; Huang, Q.; Julius, C.; Smolka, S.; Savic, L.J.; Wainstejn, D.; Lin, M.; Schlachter, T.; et al. Intra-arterial therapy of neuroendocrine tumour liver metastases: Comparing conventional TACE, drug-eluting beads TACE and yttrium-90 radioembolisation as treatment options using a propensity score analysis model. Eur. Radiol. 2017, 27, 4995–5005. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Mazzaferro, V.M.; Sangro, B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: Biological lessons, current challenges, and clinical perspectives. Hepatology 2013, 58, 2188–2197. [Google Scholar] [CrossRef]
- Tong, A.K.T.; Kao, Y.H.; Too, C.W.; Chin, K.F.W.; Ng, D.C.E.; Chow, P.K.H. Yttrium-90 hepatic radioembolization: Clinical review and current techniques in interventional radiology and personalized dosimetry. Br. J. Radiol. 2016, 89, 20150943. [Google Scholar] [CrossRef]
- Giammarile, F.; Therapy, O.A.D.C.T.; Bodei, L.; Chiesa, C.; Flux, G.; Forrer, F.; Kraeber-Bodéré, F.; Brans, B.; Lambert, B.; Konijnenberg, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1393–1406. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur. J. Radiol. 2018, 100, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.Y.; Wild, A.T.; Halappa, V.G.; Kumar, R.; Ellsworth, S.; Ziegler, M.; Garg, T.; Rosati, L.M.; Su, Z.; Hacker-Prietz, A.; et al. Neuroendocrine tumor liver metastases treated with yttrium-90 radioembolization. Contemp. Clin. Trials 2016, 50, 143–149. [Google Scholar] [CrossRef]
- Walker, L.A. Radioactive Yttrium 90: A review of its properties, biological behavior, and clinical uses. Acta Radiol. Ther. Phys. Biol. 1964, 2, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, M.; Kennedy, A.S.; Cao, Z.J.; Sackett, G.D.; Yu, C.X.; Lodge, M.A.; Murthy, R.; Line, B.R.; Van Echo, D.A. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med. Phys. 2003, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Braat, A.J.; Alsafi, A.; Wasan, H.S.; Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.J.A.T.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with 90Y Resin Microspheres of Neuroendocrine Liver Metastases After Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Interv. Radiol. 2020, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, A.; Chua, T.C.; Bester, L.; Kokandi, A.; Morris, D.L. Factors Predicting Response and Survival After Yttrium-90 Radioembolization of Unresectable Neuroendocrine Tumor Liver Metastases. Ann. Surg. 2010, 251, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Capala, J. ‘Image and treat’: An individualized approach to urological tumors. Curr. Opin. Oncol. 2010, 22, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated with 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; López-Benítez, R.; Mier, W.; Haufe, S.; Isermann, B.; Kauczor, H.-U.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocr. Relat. Cancer 2011, 18, 595–602. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Main Indications | Contraindications | Advantages | Disadvantages |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, R.L.; Hubelé, F.; De Marini, P.; Ouvrard, E.; Salvadori, J.; Addeo, P.; Garnon, J.; Kurtz, J.-E.; Greget, M.; Mertz, L.; et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers 2021, 13, 6368. https://doi.org/10.3390/cancers13246368
Cazzato RL, Hubelé F, De Marini P, Ouvrard E, Salvadori J, Addeo P, Garnon J, Kurtz J-E, Greget M, Mertz L, et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers. 2021; 13(24):6368. https://doi.org/10.3390/cancers13246368
Chicago/Turabian StyleCazzato, Roberto Luigi, Fabrice Hubelé, Pierre De Marini, Eric Ouvrard, Julien Salvadori, Pietro Addeo, Julien Garnon, Jean-Emmanuel Kurtz, Michel Greget, Luc Mertz, and et al. 2021. "Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures" Cancers 13, no. 24: 6368. https://doi.org/10.3390/cancers13246368
APA StyleCazzato, R. L., Hubelé, F., De Marini, P., Ouvrard, E., Salvadori, J., Addeo, P., Garnon, J., Kurtz, J.-E., Greget, M., Mertz, L., Goichot, B., Gangi, A., & Imperiale, A. (2021). Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers, 13(24), 6368. https://doi.org/10.3390/cancers13246368







