Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Follow-Up and Response Assessment
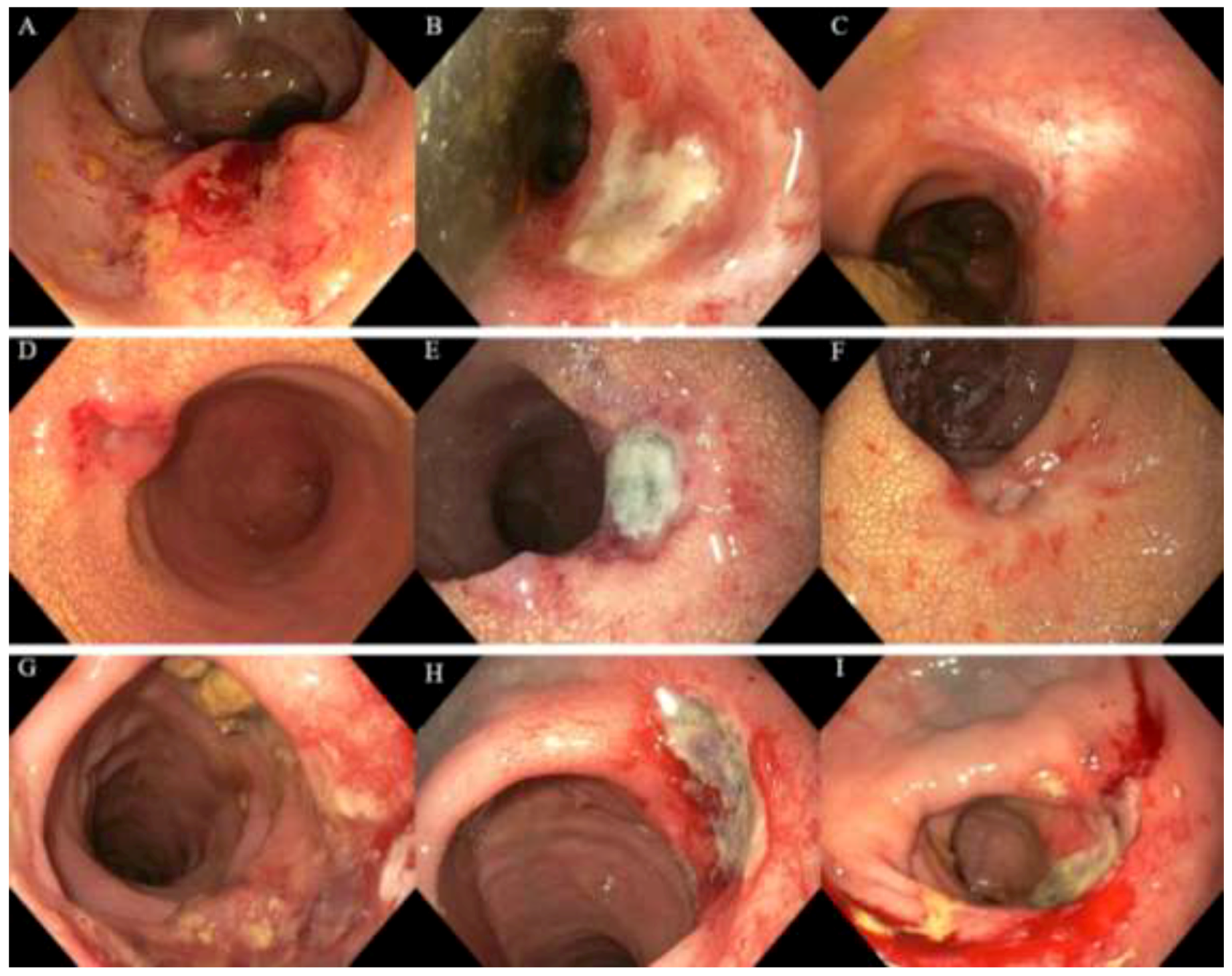
2.3. Endoscopic Toxicity
2.4. Assessment of Functional Outcomes and Quality of Life
2.5. Patients Experience of Undergoing Contact X-ray Brachytherapy
2.6. Statistical Analyses
3. Results
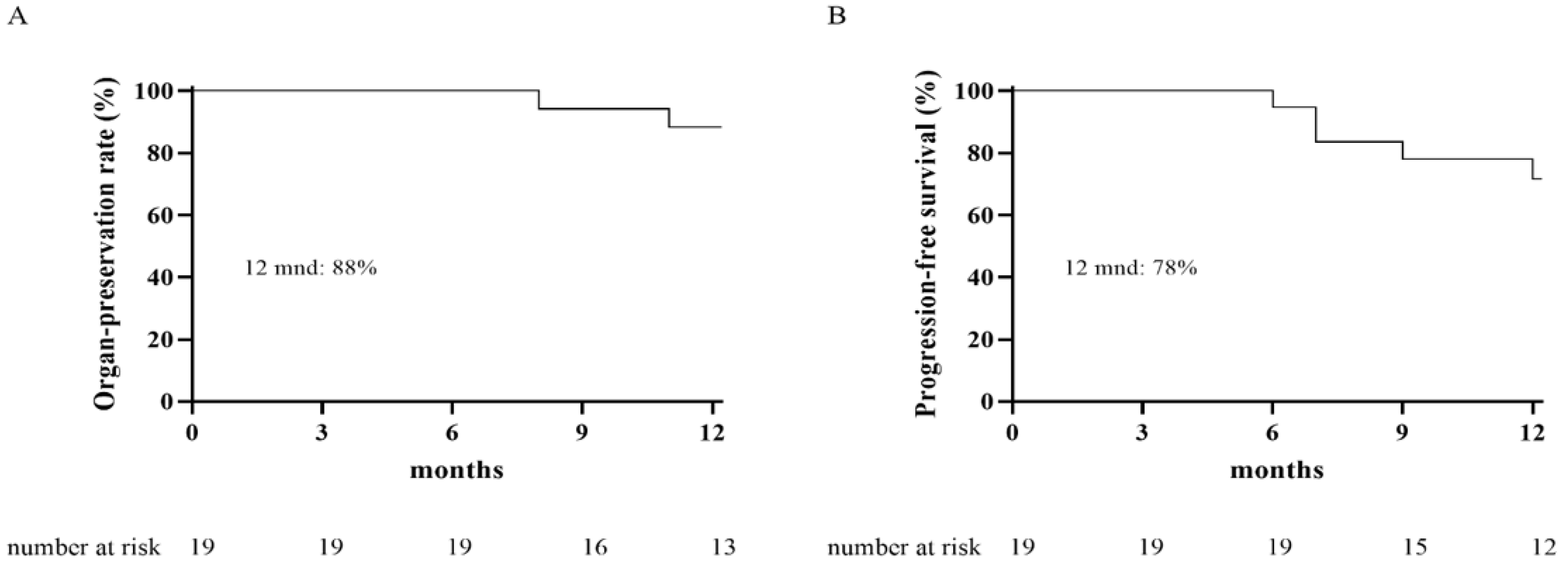
3.1. Oncological Outcome
3.2. Toxicity Scored on Endoscopy
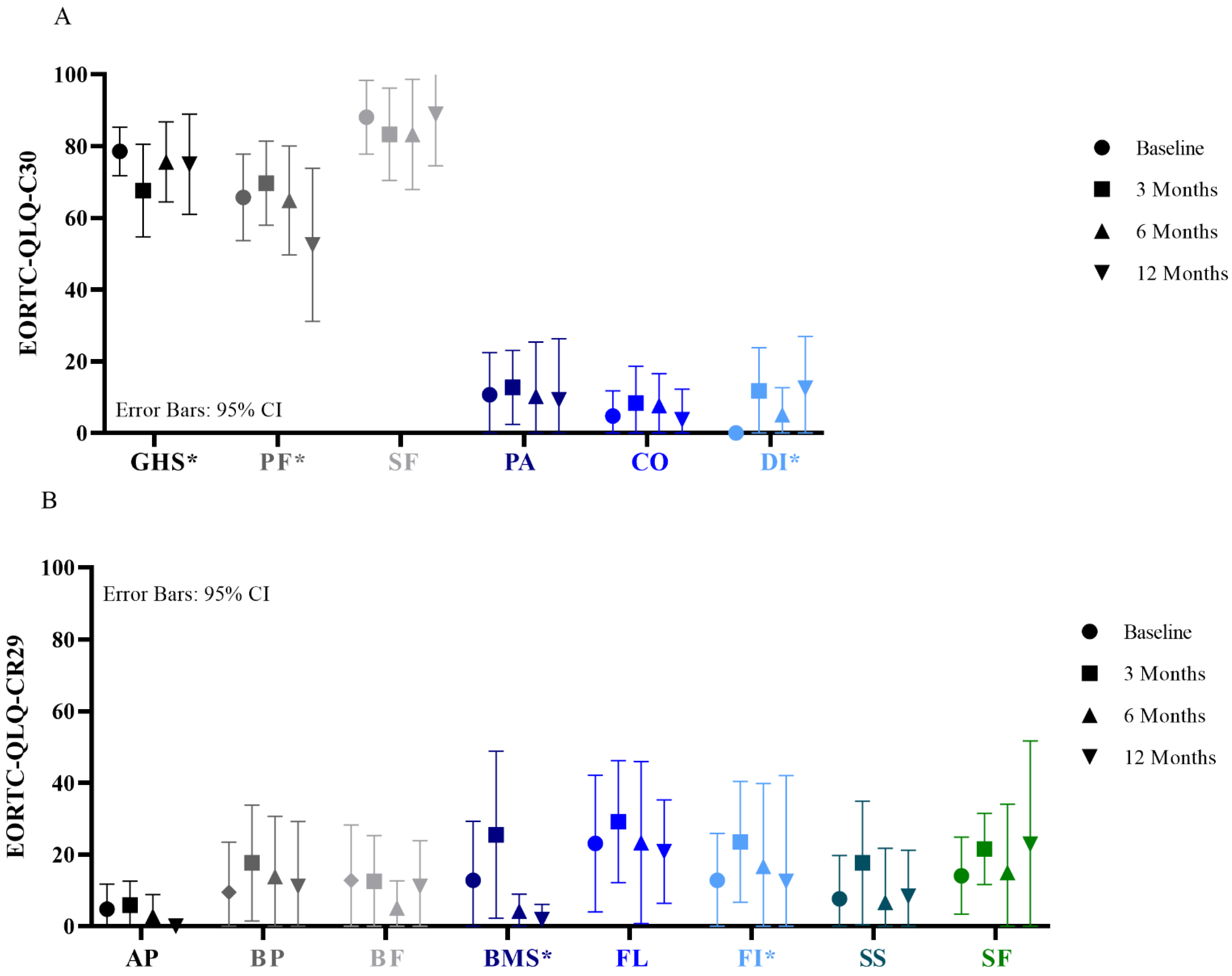
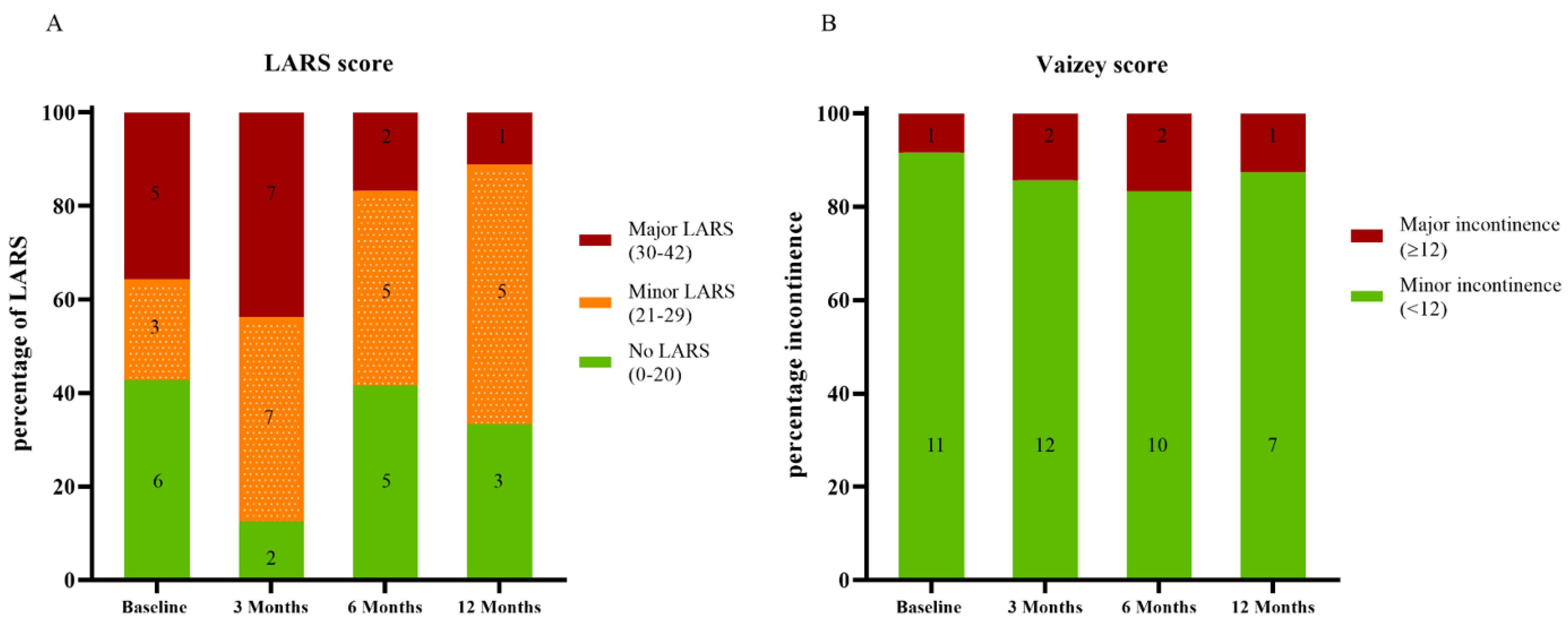
3.3. Functional Outcome and Quality of Life
3.4. In-Depth Interviews
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv263. [Google Scholar] [CrossRef]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S.; Jess, P.; Madsen, M.R.; Nielsen, H.J.; Ovesen, A.U.; Salomon, S.; Nielsen, K.T.; Vilandt, J. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. J. Br. Surg. 2013, 100, 1377–1387. [Google Scholar] [CrossRef]
- Chen, T.Y.-T.; Wiltink, L.M.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomized trial. Clin. Colorectal Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Ketelaers, S.H.J.; Orsini, R.G.; Burger, J.W.A.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T. Significant improvement in postoperative and 1-year mortality after colorectal cancer surgery in recent years. Eur. J. Surg. Oncol. 2019, 45, 2052–2058. [Google Scholar] [CrossRef]
- Breugom, A.J.; Bastiaannet, E.; Dekker, J.W.T.; Wouters, M.W.J.M.; van de Velde, C.J.H.; Liefers, G.J. Decrease in 30-day and one-year mortality over time in patients aged ≥75 years with stage I–III colon cancer: A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1889–1893. [Google Scholar] [CrossRef]
- Fagard, K.; Leonard, S.; Deschodt, M.; Devriendt, E.; Wolthuis, A.; Prenen, H.; Flamaing, J.; Milisen, K.; Wildiers, H.; Kenis, C. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: A systematic review. J. Geriatr. Oncol. 2016, 7, 479–491. [Google Scholar] [CrossRef]
- Lin, H.-S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef] [Green Version]
- Couwenberg, A.M.; de Beer, F.S.A.; Intven, M.P.W.; Burbach, J.P.M.; Smits, A.B.; Consten, E.C.J.; Schiphorst, A.H.W.; Wijffels, N.A.T.; de Roos, M.A.J.; Hamaker, M.E.; et al. The impact of postoperative complications on health-related quality of life in older patients with rectal cancer; a prospective cohort study. J. Geriatr. Oncol. 2018, 9, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujko, K.; Glynne-Jones, R.; Papamichael, D.; Rutten, H.J.T. Optimal management of localized rectal cancer in older patients. J. Geriatr. Oncol. 2018, 9, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Al-Sukhni, E.; Attwood, K.; Mattson, D.M.; Gabriel, E.; Nurkin, S.J. Predictors of Pathologic Complete Response following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann. Surg. Oncol. 2016, 23, 1177–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Malcomson, L.; Emsley, R.; Gollins, S.; Maw, A.; Myint, A.S.; Rooney, P.S.; Susnerwala, S.; Blower, A.; Saunders, M.P.; et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol. 2016, 17, 174–183. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Kranenbarg, E.M.-K.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Appelt, A.L.; Ploen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Dhadda, A.S.; Martin, A.; Killeen, S.; Hunter, I.A. Organ Preservation Using Contact Radiotherapy for Early Rectal Cancer: Outcomes of Patients Treated at a Single Centre in the UK. Clin. Oncol. 2017, 29, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.S.; Smith, F.M.; Gollins, S.W.; Wong, H.; Rao, C.; Whitmarsh, K.; Sripadam, R.; Rooney, P.; Hershman, M.J.; Fekete, Z.; et al. Dose escalation using contact X-ray brachytherapy (Papillon) for rectal cancer: Does it improve the chance of organ preservation? Br. J. Radiol. 2017, 90, 20170175. [Google Scholar] [CrossRef] [PubMed]
- Gérard, J.-P.; Barbet, N.; Gal, J.; Dejean, C.; Evesque, L.; Doyen, J.; Coquard, R.; Gugenheim, J.; Benizri, E.; Schiappa, R.; et al. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur. J. Cancer 2019, 108, 1–16. [Google Scholar] [CrossRef]
- Rijkmans, E.C.; Cats, A.; Nout, R.A.; van den Bongard, D.H.J.G.; Ketelaars, M.; Buijsen, J.; Rozema, T.; Franssen, J.H.; Velema, L.A.; van Triest, B.; et al. Endorectal Brachytherapy Boost After External Beam Radiation Therapy in Elderly or Medically Inoperable Patients with Rectal Cancer: Primary Outcomes of the Phase 1 HERBERT Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 908–917. [Google Scholar] [CrossRef]
- Garant, A.; Magnan, S.; Devic, S.; Martin, A.G.; Boutros, M.; Vasilevsky, C.A.; Ferland, S.; Bujold, A.; DesGroseilliers, S.; Sebajang, H.; et al. Image Guided Adaptive Endorectal Brachytherapy in the Nonoperative Management of Patients with Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1005–1011. [Google Scholar] [CrossRef]
- Rijkmans, E.C.; van Triest, B.; Nout, R.A.; Kerkhof, E.M.; Buijsen, J.; Rozema, T.; Franssen, J.H.; Velema, L.A.; Laman, M.S.; Cats, A.; et al. Evaluation of clinical and endoscopic toxicity after external beam radiotherapy and endorectal brachytherapy in elderly patients with rectal cancer treated in the HERBERT study. Radiother. Oncol. 2018, 126, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Birk, J.W.; Anderson, J.C.; Georgsson, M.; Park, T.L.; Smith, C.J.; Comer, G.M. A prospective randomized placebo-controlled double-blinded pilot study of misoprostol rectal suppositories in the prevention of acute and chronic radiation proctitis symptoms in prostate cancer patients. Am. J. Gastroenterol. 2000, 95, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Gujral, S.; Conroy, T.; Fleissner, C.; Sezer, O.; King, P.M.; Avery, K.N.; Sylvester, P.; Koller, M.; Sprangers, M.A.; Blazeby, J.M.; et al. Assessing quality of life in patients with colorectal cancer: An update of the EORTC quality of life questionnaire. Eur. J. Cancer 2007, 43, 1564–1573. [Google Scholar] [CrossRef]
- King, M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual. Life Res. 1996, 5, 555–567. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S. Low anterior resection syndrome score: Development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef]
- Vaizey, C.J.; Carapeti, E.; Cahill, J.A.; Kamm, M.A. Prospective comparison of faecal incontinence grading systems. Gut 1999, 44, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Ortholan, C.; Romestaing, P.; Chapet, O.; Gerard, J.P. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e165–e171. [Google Scholar] [CrossRef]
- Cameron, M.G.; Kersten, C.; Vistad, I.; van Helvoirt, R.; Weyde, K.; Undseth, C.; Mjaaland, I.; Skovlund, E.; Fossa, S.D.; Guren, M.G. Palliative pelvic radiotherapy for symptomatic rectal cancer—a prospective multicenter study. Acta Oncol. 2016, 55, 1400–1407. [Google Scholar] [CrossRef] [Green Version]
- Minsky, B.D.; Cohen, A.M.; Enker, W.E.; Sigurdson, E. Phase I/II trial of pre-operative radiation therapy and coloanal anastomosis in distal invasive resectable rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 387–392. [Google Scholar] [CrossRef]
- Sandberg, S.; Asplund, D.; Bisgaard, T.; Bock, D.; Gonzalez, E.; Karlsson, L.; Matthiessen, P.; Ohlsson, B.; Park, J.; Rosenberg, J.; et al. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: A longitudinal follow-up within the QoLiRECT study. Colorectal Dis. 2020, 22, 1367–1378. [Google Scholar] [CrossRef]
- Appelt, A.L.; Ploen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jorgensen, J.C.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Popescu, T.; Karlsson, U.; Vinh-Hung, V.; Trigo, L.; Thariat, J.; Vuong, T.; Baumert, B.G.; Motta, M.; Zamagni, A.; Bonet, M.; et al. Challenges Facing Radiation Oncologists in The Management of Older Cancer Patients: Consensus of The International Geriatric Radiotherapy Group. Cancers 2019, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Dale, R.G. The radiobiology of Papillon-type treatments. Clin. Oncol. 2007, 19, 649–654. [Google Scholar] [CrossRef]

| Total | n = 19 | 100% |
|---|---|---|
| Age, median (range), years | 80 | (72–91) |
| Gender | ||
| Male | 13 | 68 |
| Female | 6 | 32 |
| Charlson Comorbidity Index | ||
| 2 | 3 | 16 |
| 3 | 3 | 16 |
| ≥4 | 13 | 68 |
| Clinical T stage | ||
| cT1 | 2 | 11 |
| cT2 | 6 | 32 |
| cT3 | 11 | 58 |
| Clinical N stage | ||
| cN0 | 13 | 68 |
| cN1 | 5 | 26 |
| cN2 | 1 | 5 |
| Distance from anal verge | ||
| 0–5 cm | 9 | 47 |
| 5–10 cm | 9 | 47 |
| 10–15 cm | 1 | 5 |
| Differentiation | ||
| Well | 12 | 63 |
| Moderate | 1 | 5 |
| Poor | 0 | 0 |
| Not known | 6 | 32 |
| Treatment prior to CXB | ||
| Chemoradiotherapy 1 | 6 | 32 |
| 5 × 5 Gy | 6 | 32 |
| 13 × 3 Gy | 2 | 11 |
| Local excision 2 | 4 | 21 |
| HDR 3 | 1 | 5 |
| Tumor size prior to CXB | ||
| ≤3 cm | 16 | 84 |
| >3 cm | 3 | 16 |
| Treatment intent | ||
| Clinical complete response | 8 | 42 |
| Local control of the residual tumor | 11 | 58 |
| Dose of CXB | ||
| 90 Gy | 17 | 89 |
| 60 Gy | 2 | 11 |
| Follow-up, median (range), months | 13 | (6–32) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custers, P.A.; Geubels, B.M.; Huibregtse, I.L.; Peters, F.P.; Engelhardt, E.G.; Beets, G.L.; Marijnen, C.A.M.; van Leerdam, M.E.; van Triest, B. Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up. Cancers 2021, 13, 6333. https://doi.org/10.3390/cancers13246333
Custers PA, Geubels BM, Huibregtse IL, Peters FP, Engelhardt EG, Beets GL, Marijnen CAM, van Leerdam ME, van Triest B. Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up. Cancers. 2021; 13(24):6333. https://doi.org/10.3390/cancers13246333
Chicago/Turabian StyleCusters, Petra A., Barbara M. Geubels, Inge L. Huibregtse, Femke P. Peters, Ellen G. Engelhardt, Geerard L. Beets, Corrie A. M. Marijnen, Monique E. van Leerdam, and Baukelien van Triest. 2021. "Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up" Cancers 13, no. 24: 6333. https://doi.org/10.3390/cancers13246333
APA StyleCusters, P. A., Geubels, B. M., Huibregtse, I. L., Peters, F. P., Engelhardt, E. G., Beets, G. L., Marijnen, C. A. M., van Leerdam, M. E., & van Triest, B. (2021). Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up. Cancers, 13(24), 6333. https://doi.org/10.3390/cancers13246333






