The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinicopathological Characteristics
2.2. Tissue Microarray Construction
2.3. Immunohistochemistry
2.4. Tissue Microarray Evaluation
2.5. Statistical Analysis
3. Results
3.1. TIRC7+ Immune Cells Are Present in the Epithelial and Stromal Compartment of Cholangiocarcinoma
3.2. Correlation of TIRC7 Quantity with Clinicopathological Information
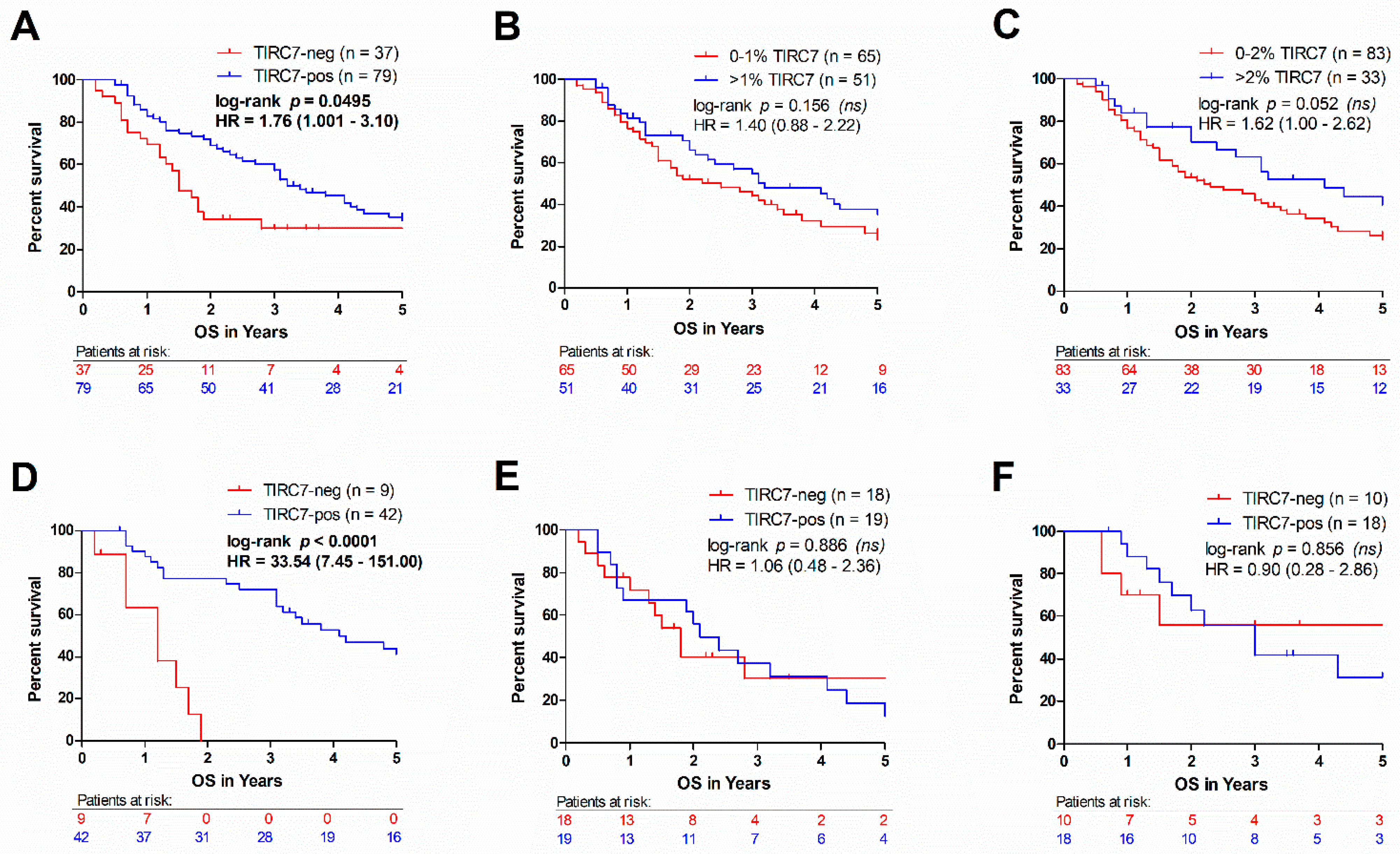
3.3. High Intraepithelial TIRC7+ Immune Cell Density Is Associated with Favorable Outcome in Intrahepatic Cholangiocarcinoma
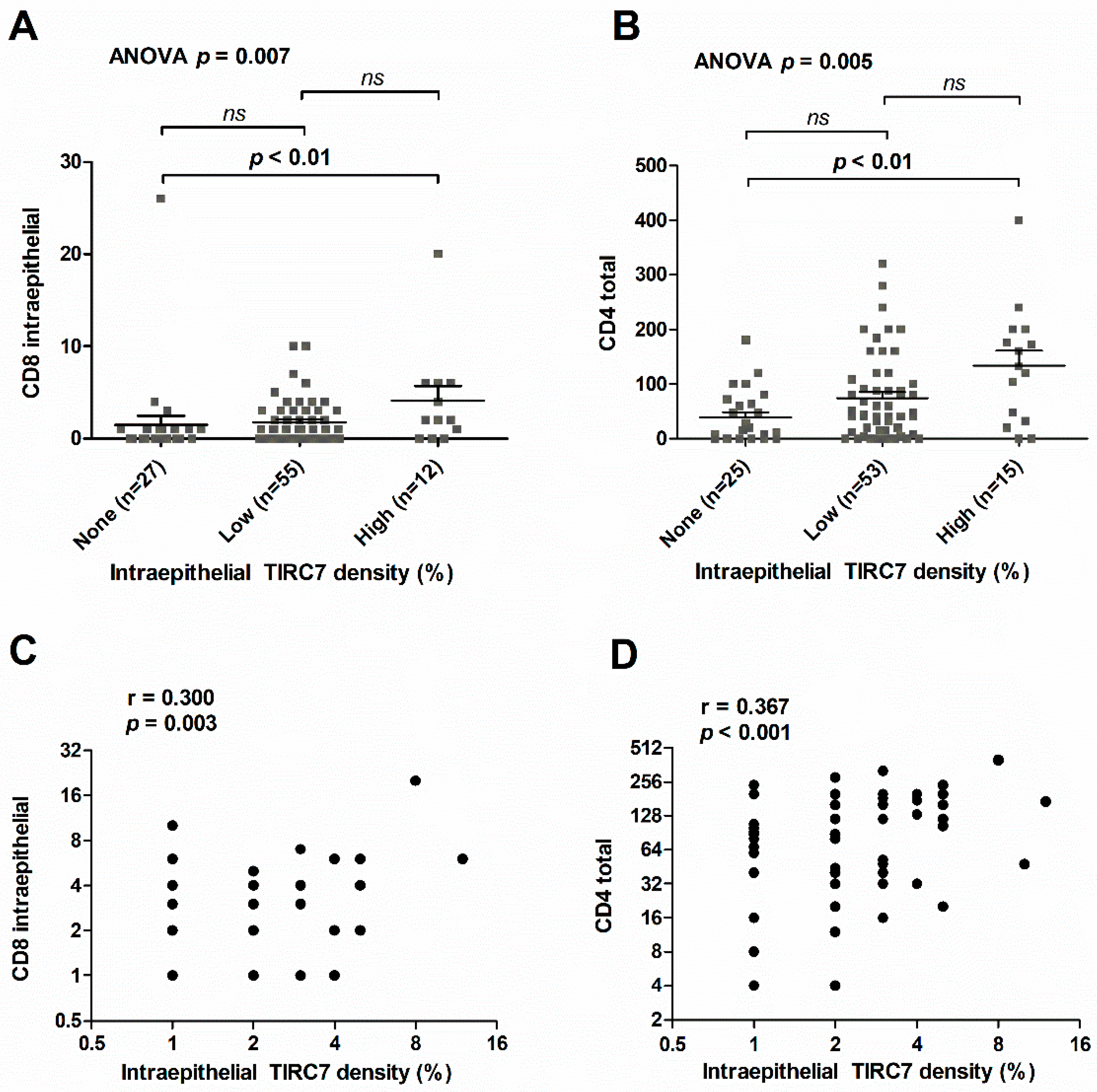
3.4. Intraepithelial TIRC7+ Immune Cell Density in Cholangiocarcinoma Is Associated with the Number of Intraepithelial CD8+ and Total CD4+ Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996, 224, 463–473. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.R.; Campbell, P.J. Evolution of the cancer genome. Nat. Rev. Genet. 2012, 13, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73, 75–85. [Google Scholar] [CrossRef] [PubMed]
- O’Day, S.J.; Maio, M.; Chiarion-Sileni, V.; Gajewski, T.F.; Pehamberger, H.; Bondarenko, I.N.; Queirolo, P.; Lundgren, L.; Mikhailov, S.; Roman, L.; et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann. Oncol. 2010, 21, 1712–1717. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Goeppert, B.; Frauenschuh, L.; Zucknick, M.; Stenzinger, A.; Andrulis, M.; Klauschen, F.; Joehrens, K.; Warth, A.; Renner, M.; Mehrabi, A.; et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br. J. Cancer 2013, 109, 2665–2674. [Google Scholar] [CrossRef]
- Heinemann, T.; Bulwin, G.C.; Randall, J.; Schnieders, B.; Sandhoff, K.; Volk, H.D.; Milford, E.; Gullans, S.R.; Utku, N. Genomic organization of the gene coding for TIRC7, a novel membrane protein essential for T cell activation. Genomics 1999, 57, 398–406. [Google Scholar] [CrossRef]
- Bulwin, G.C.; Walter, S.; Schlawinsky, M.; Heinemann, T.; Schulze, A.; Hohne, W.; Krause, G.; Kalka-Moll, W.; Fraser, P.; Volk, H.D.; et al. HLA-DR alpha 2 mediates negative signalling via binding to Tirc7 leading to anti-inflammatory and apoptotic effects in lymphocytes in vitro and in vivo. PLoS ONE 2008, 3, e1576. [Google Scholar] [CrossRef] [PubMed]
- Utku, N.; Boerner, A.; Tomschegg, A.; Bennai-Sanfourche, F.; Bulwin, G.C.; Heinemann, T.; Loehler, J.; Blumberg, R.S.; Volk, H.D. TIRC7 deficiency causes in vitro and in vivo augmentation of T and B cell activation and cytokine response. J. Immunol. 2004, 173, 2342–2352. [Google Scholar] [CrossRef]
- Zhu, F.; Qiu, T.; Zhu, S.; Zhao, K.; Chen, C.; Qiao, J.; Pan, B.; Yan, Z.; Chen, W.; Liu, Q.; et al. TIRC7 inhibits Th1 cells by upregulating the expression of CTLA4 and STAT3 in mice with acute graftversushost disease. Oncol. Rep. 2020, 44, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Utku, N.; Heinemann, T.; Winter, M.; Bulwin, C.G.; Schlawinsky, M.; Fraser, P.; Nieuwenhuis, E.E.; Volk, H.D.; Blumberg, R.S. Antibody targeting of TIRC7 results in significant therapeutic effects on collagen-induced arthritis in mice. Clin. Exp. Immunol. 2006, 144, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, Y.; Tamura, A.; Volk, H.D.; Reinke, P.; Lohler, J.; Tullius, S.G.; Utku, N. TIRC7 is induced in rejected human kidneys and anti-TIRC7 mAb with FK506 prolongs survival of kidney allografts in rats. Transpl. Immunol. 2006, 16, 238–244. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Tomschegg, A.; Bennai-Sanfourche, F.; Boerner, A.; Kaser, A.; Schmidt-Knosalla, I.; Heinemann, T.; Schlawinsky, M.; Blumberg, R.S.; Volk, H.D.; et al. Monoclonal antibody specific for TIRC7 induces donor-specific anergy and prevents rejection of cardiac allografts in mice. Am. J. Transplant. 2004, 4, 505–514. [Google Scholar] [CrossRef]
- Utku, N.; Heinemann, T.; Tullius, S.G.; Bulwin, G.C.; Beinke, S.; Blumberg, R.S.; Beato, F.; Randall, J.; Kojima, R.; Busconi, L.; et al. Prevention of acute allograft rejection by antibody targeting of TIRC7, a novel T cell membrane protein. Immunity 1998, 9, 509–518. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Goeppert, B.; Frauenschuh, L.; Zucknick, M.; Roessler, S.; Mehrabi, A.; Hafezi, M.; Stenzinger, A.; Warth, A.; Pathil, A.; Renner, M.; et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer 2015, 113, 1343–1349. [Google Scholar] [CrossRef]
- Meng, T.; Huang, R.; Zeng, Z.; Huang, Z.; Yin, H.; Jiao, C.; Yan, P.; Hu, P.; Zhu, X.; Li, Z.; et al. Identification of Prognostic and Metastatic Alternative Splicing Signatures in Kidney Renal Clear Cell Carcinoma. Front. Bioeng. Biotechnol. 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Lei, L.; Hu, J.; Wang, G.; Liu, J.; Ou, S. T cell immune regulator 1 is a prognostic marker associated with immune infiltration in glioblastoma multiforme. Oncol. Lett. 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Andersen, J.B.; Fouassier, L. Intrahepatic cholangiocarcinoma: A single-cell resolution unraveling the complexity of the tumor microenvironment. J. Hepatol. 2020, 73, 1007–1009. [Google Scholar] [CrossRef]
- Albrecht, T.; Brinkmann, F.; Albrecht, M.; Lonsdorf, A.S.; Mehrabi, A.; Hoffmann, K.; Kulu, Y.; Charbel, A.; Vogel, M.N.; Rupp, C.; et al. Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer. Cancers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Goeppert, B.; Roessler, S.; Renner, M.; Loeffler, M.; Singer, S.; Rausch, M.; Albrecht, T.; Mehrabi, A.; Vogel, M.N.; Pathil, A.; et al. Low frequency of mismatch repair deficiency in gallbladder cancer. Diagn. Pathol. 2019, 14, 36. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2019, 29, 3766. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Nam, K.H.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Kim, S.H.; Chang, H.; Lee, J.O.; Kim, Y.J.; Lee, H.S.; et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016, 19, 42–52. [Google Scholar] [CrossRef]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Gadiot, J.; Hooijkaas, A.I.; Kaiser, A.D.M.; van Tinteren, H.; van Boven, H.; Blank, C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011, 117, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Soon, Y.Y.; Lum, J.H.Y.; Tan, C.L.; Tey, J.C.S. Frequency of discordance in programmed death-ligand 1 (PD-L1) expression between primary tumors and paired distant metastases in advanced cancers: A systematic review and meta-analysis. Acta Oncol. 2020, 59, 696–704. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandala, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bulwin, G.C.; Heinemann, T.; Bugge, V.; Winter, M.; Lohan, A.; Schlawinsky, M.; Schulze, A.; Walter, S.; Sabat, R.; Schulein, R.; et al. TIRC7 inhibits T cell proliferation by modulation of CTLA-4 expression. J. Immunol. 2006, 177, 6833–6841. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, Y.; Fan, X.; Zhang, F.; Wang, D.; Qiao, J.; Zhu, S.; Zhao, K.; Pan, B.; Chen, C.; et al. Role of T cell immune response cDNA 7 on the pathology of acute graft-versus-host disease. Oncol. Lett. 2020, 20, 300. [Google Scholar] [CrossRef]



| Clinicopathological Variable | Total 135 (100.0) | TIRC7 Negative 43 (31.9) | TIRC7 Positive 92 (68.1) | p-Value 1 | |
|---|---|---|---|---|---|
| Age | Median | 63.0 | 64.10 | 63.5 | 0.692 |
| Mean | 61.7 | 61.7 | 62.3 | ||
| Interquartile Range | 55.0–69.0 | 56.0–69.4 | 55.2–70.2 | ||
| Sex | Male | 89 (100.0) | 32 (36.0) | 57 (64.0) | 0.177 |
| Female | 46 (100.0) | 11 (23.9) | 35 (76.1) | ||
| Subtype | iCCA | 57 (100.0) | 10 (17.5) | 47 (82.5) | 0.004 |
| pCCA | 43 (100.0) | 21 (48.9) | 22 (51.1) | ||
| dCCA | 35 (100.0) | 12 (34.3) | 23 (65.7) | ||
| Histology 2 | Ductal | 89 (100.0) | 30 (33.7) | 59 (66.3) | 0.520 3 |
| Papillary | 9 (100.0) | 3 (33.3) | 6 (66.7) | ||
| Mucinous | 5 (100.0) | 3 (60.0) | 2 (40.0) | ||
| Solid | 13 (100.0) | 3 (23.1) | 10 (76.9) | ||
| Diffuse/signet ring | 7 (100.0) | 0 (0.0) | 7 (100.0) | ||
| Intestinal | 2 (100.0) | 2 (100.) | 0 (0.0) | ||
| Adenosquamous | 2 (100.0) | 0 (0.0) | 2 (100.0) | ||
| Clear cell | 8 (100.0) | 2 (25.0) | 6 (75.0) | ||
| UICC 4 | UICC 1 | 6 (100.0) | 3 (50.0) | 3 (50.0) | 0.915 |
| UICC 2 | 51 (100.0) | 18 (35.3) | 33 (64.7) | ||
| UICC 3 | 35 (100.0) | 13 (37.1) | 22 (62.9) | ||
| UICC 4 | 5 (100.0) | 2 (40.0) | 3 (60.0) | ||
| NA | 38 (100.0) | 7 (18.4) | 31 (81.6) | ||
| pT | T1 | 10 (100.0) | 3 (30.0) | 7 (70.0) | 0.955 |
| T2 | 77 (100.0) | 26 (33.8) | 51 (66.2) | ||
| T3 | 37 (100.0) | 11 (29.7) | 26 (70.3) | ||
| T4 | 11 (100.0) | 3 (27.3) | 8 (72.7) | ||
| pN | N0 | 46 (100.0) | 16 (34.8) | 30 (65.2) | 0.706 |
| N1 | 52 (100.0) | 20 (38.5) | 32 (61.5) | ||
| NA | 37 (100.0) | 7 (18.9) | 30 (81.1) | ||
| M | M0 | 131 (100.0) | 41 (31.3) | 90 (68.7) | 0.592 |
| M1 | 4 (100.0) | 2 (50.0) | 2 (50.0) | ||
| G | G1 | 8 (100.0) | 3 (37.5) | 5 (62.5) | 0.875 |
| G2 | 99 (100.0) | 32 (32.3) | 67 (67.7) | ||
| G3 | 28 (100.0) | 8 (28.6) | 20 (71.4) | ||
| R | R0 | 65 (100.0) | 20 (30.8) | 45 (69.2) | 0.958 |
| R1 | 44 (100.0) | 14 (31.8) | 30 (68.2) | ||
| R2 | 11 (100.0) | 3 (27.3) | 8 (72.7) | ||
| NA | 15 (100.0) | 6 (40.0) | 9 (60.0) | ||
| L/V | L/V0 | 34 (100.0) | 11 (32.4) | 23 (67.6) | 1.000 |
| L/V1 | 101 (100.0) | 32 (31.7) | 69 (68.3) | ||
| Pn | Pn0 | 65 (100.0 | 16 (24.6) | 49 (75.4) | 0.098 |
| Pn1 | 70 (100.0) | 27 (38.6) | 43 (61.4) | ||
| Hepatobiliary | HBV | 11 (100.0) | 4 (36.4) | 7 (63.6) | 0.345 |
| Disease 5 | HCV | 2 (100.0) | 0 (0.0) | 2 (100.0) | |
| Cholecystitis/-lithiasis | 43 (100.0) | 10 (23.3) | 33 (76.7) | ||
| High-stage fibrosis/cirrhosis | 31 (100.0) | 11 (35.5) | 20 (64.5) | ||
| Fatty liver disease | 13 (100.0) | 3 (23.1) | 10 (76.9) | ||
| PSC | 2 (100.0) | 2 (100.0) | 0 (0.0) | ||
| Chronic pancreatitis | 3 (100.0) | 1 (33.3) | 2 (66.7) | ||
| Hemochromatosis | 2 (100.0) | 1 (50.0) | 1 (50.0) | ||
| Siderosis | 5 (100.0) | 0 (0.0) | 5 (100.0) | ||
| None identified | 50 (100.0) | 15 (30.0) | 35 (70.0) | ||
| Overall survival | Median survival in years (n) | 3.0 (116) | 1.5 (37) | 3.2 (79) | |
| Immune Cell Metric | Intraepithelial TIRC7 Density | ||
|---|---|---|---|
| r Value | p-Value 1 | adj. p-Value 2 | |
| CD4 intraepithelial | 0.164 | 0.116 | 1.000 |
| CD4 total | 0.367 | <0.001 | 0.004 |
| CD8 intraepithelial | 0.300 | 0.003 | 0.043 |
| CD8 total | 0.083 | 0.427 | 1.000 |
| CD20 intraepithelial | NA | NA | NA |
| CD20 total | 0.296 | 0.004 | 0.051 |
| CD68 intraepithelial | 0.013 | 0.902 | 1.000 |
| CD68 total | −0.033 | 0.748 | 1.000 |
| CD25 intraepithelial | 0.037 | 0.721 | 1.000 |
| CD25 total | 0.190 | 0.066 | 0.861 |
| FoxP3 intraepithelial | 0.184 | 0.080 | 1.000 |
| FoxP3 total | 0.150 | 0.156 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, T.; Goeppert, B.; Brinkmann, F.; Charbel, A.; Zhang, Q.; Schreck, J.; Wilhelm, N.; Singer, S.; Köhler, B.C.; Springfeld, C.; et al. The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma. Cancers 2021, 13, 6272. https://doi.org/10.3390/cancers13246272
Albrecht T, Goeppert B, Brinkmann F, Charbel A, Zhang Q, Schreck J, Wilhelm N, Singer S, Köhler BC, Springfeld C, et al. The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma. Cancers. 2021; 13(24):6272. https://doi.org/10.3390/cancers13246272
Chicago/Turabian StyleAlbrecht, Thomas, Benjamin Goeppert, Fritz Brinkmann, Alphonse Charbel, Qiangnu Zhang, Johannes Schreck, Nina Wilhelm, Stephan Singer, Bruno C. Köhler, Christoph Springfeld, and et al. 2021. "The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma" Cancers 13, no. 24: 6272. https://doi.org/10.3390/cancers13246272
APA StyleAlbrecht, T., Goeppert, B., Brinkmann, F., Charbel, A., Zhang, Q., Schreck, J., Wilhelm, N., Singer, S., Köhler, B. C., Springfeld, C., Mehrabi, A., Schirmacher, P., Kühl, A. A., Vogel, M. N., Jansen, H., Utku, N., & Roessler, S. (2021). The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma. Cancers, 13(24), 6272. https://doi.org/10.3390/cancers13246272






