Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer
Abstract
Simple Summary
Abstract
1. Gastric Cancer: Incidence and Subtypes
2. Gastric Cancer: Sporadic and Hereditary Forms
Helicobacter pylori and Gastric Cancer
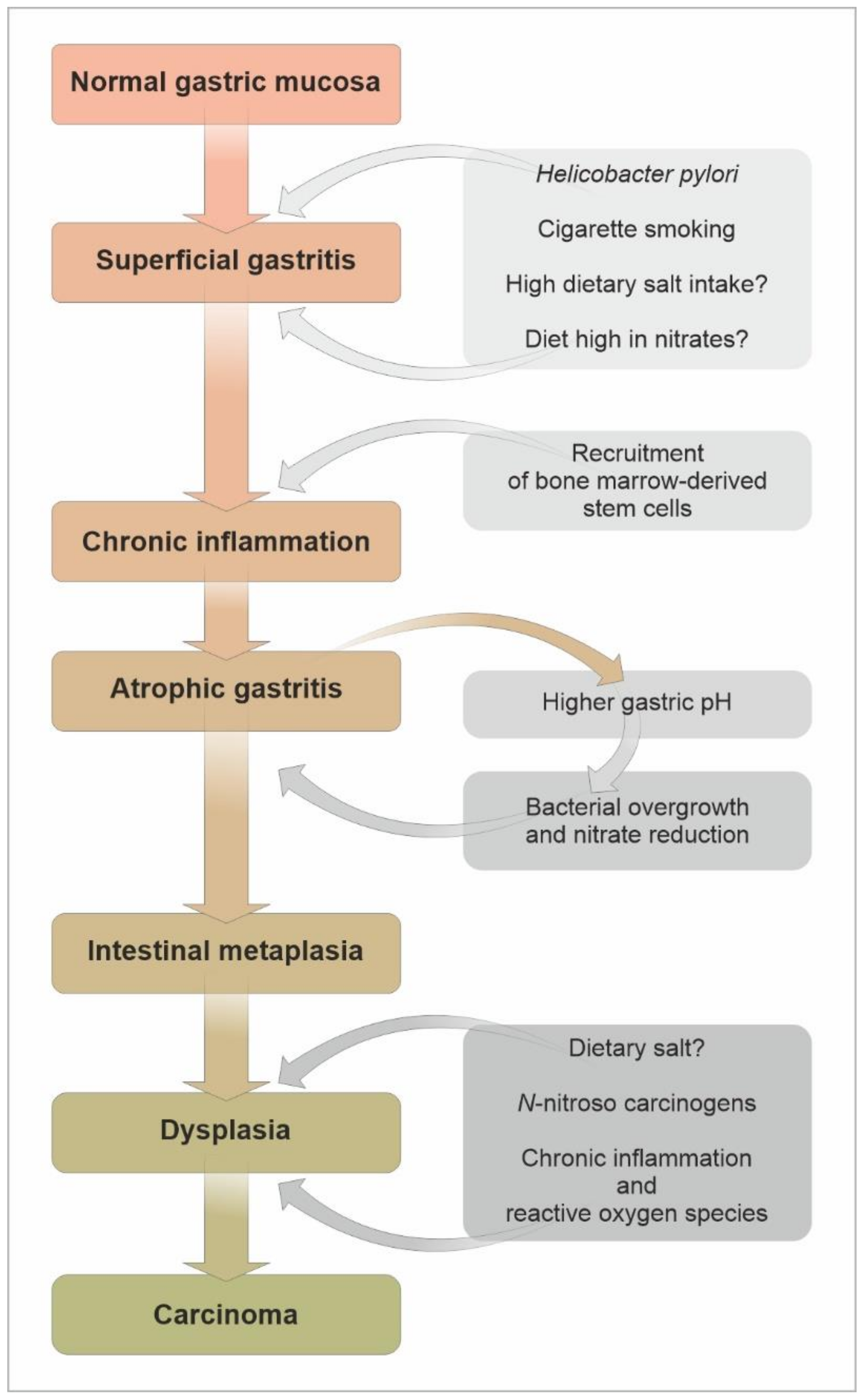
3. Gastric Atrophy and Intestinal Metaplasia as Precursors of Gastric Cancer
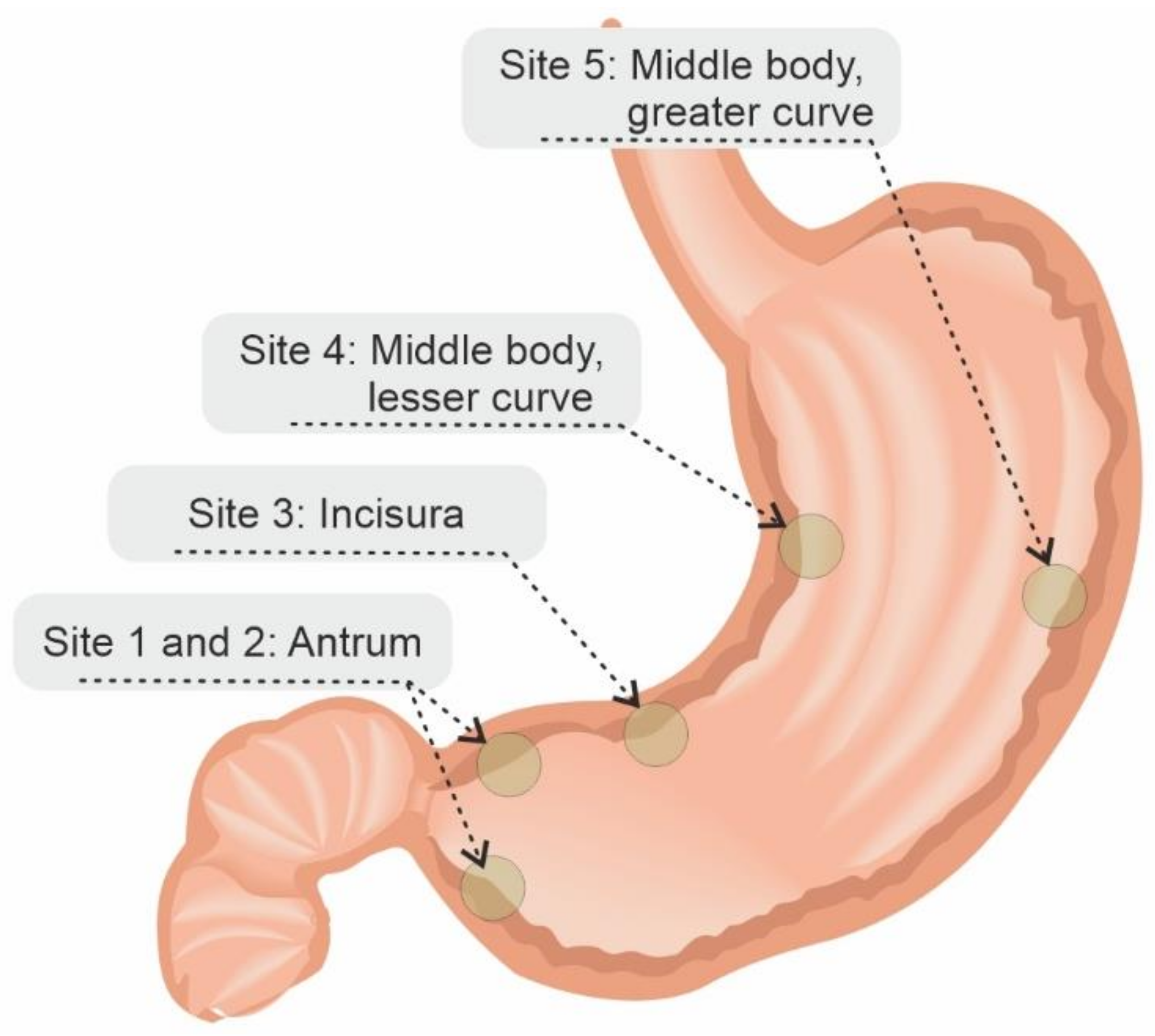
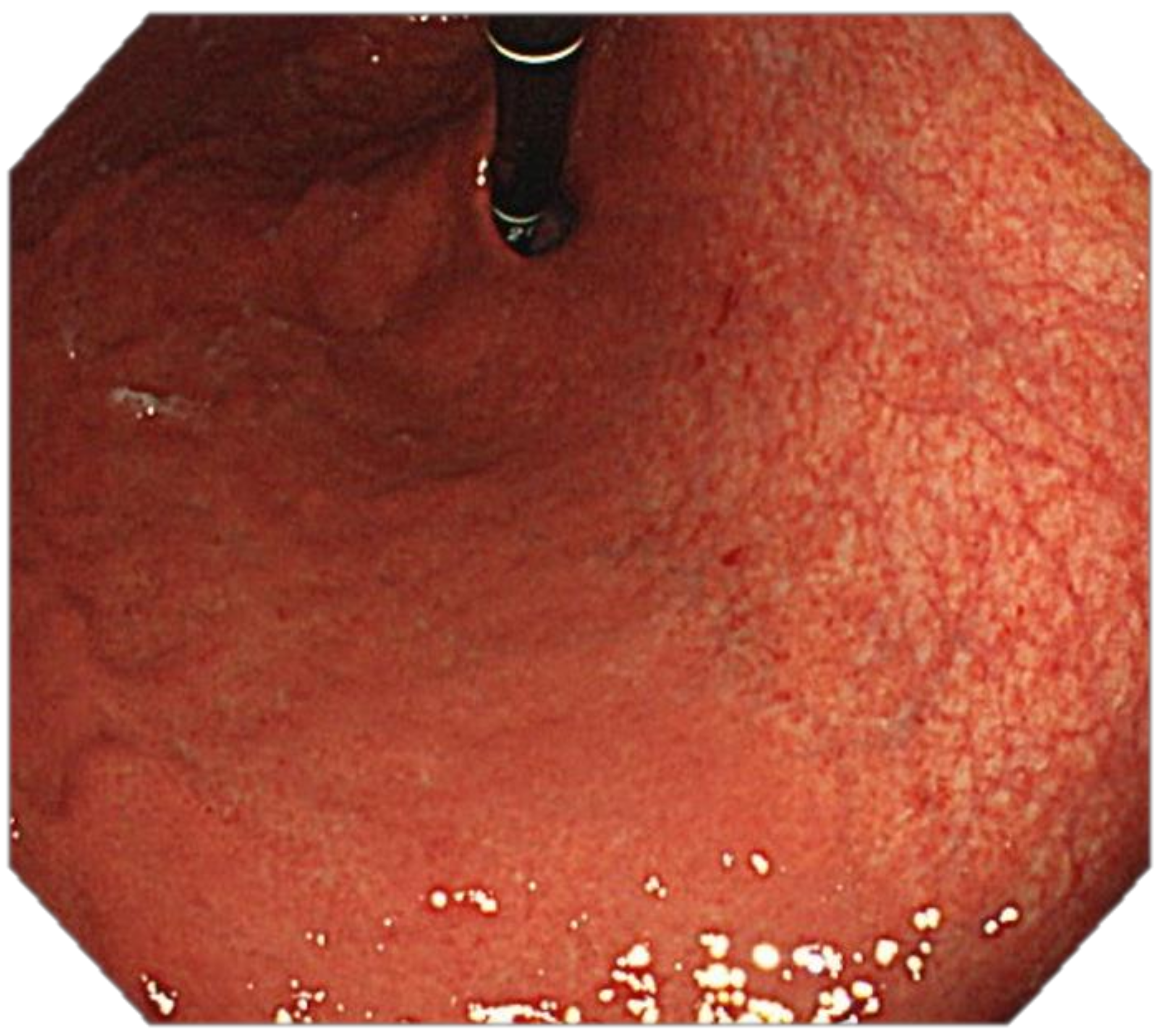
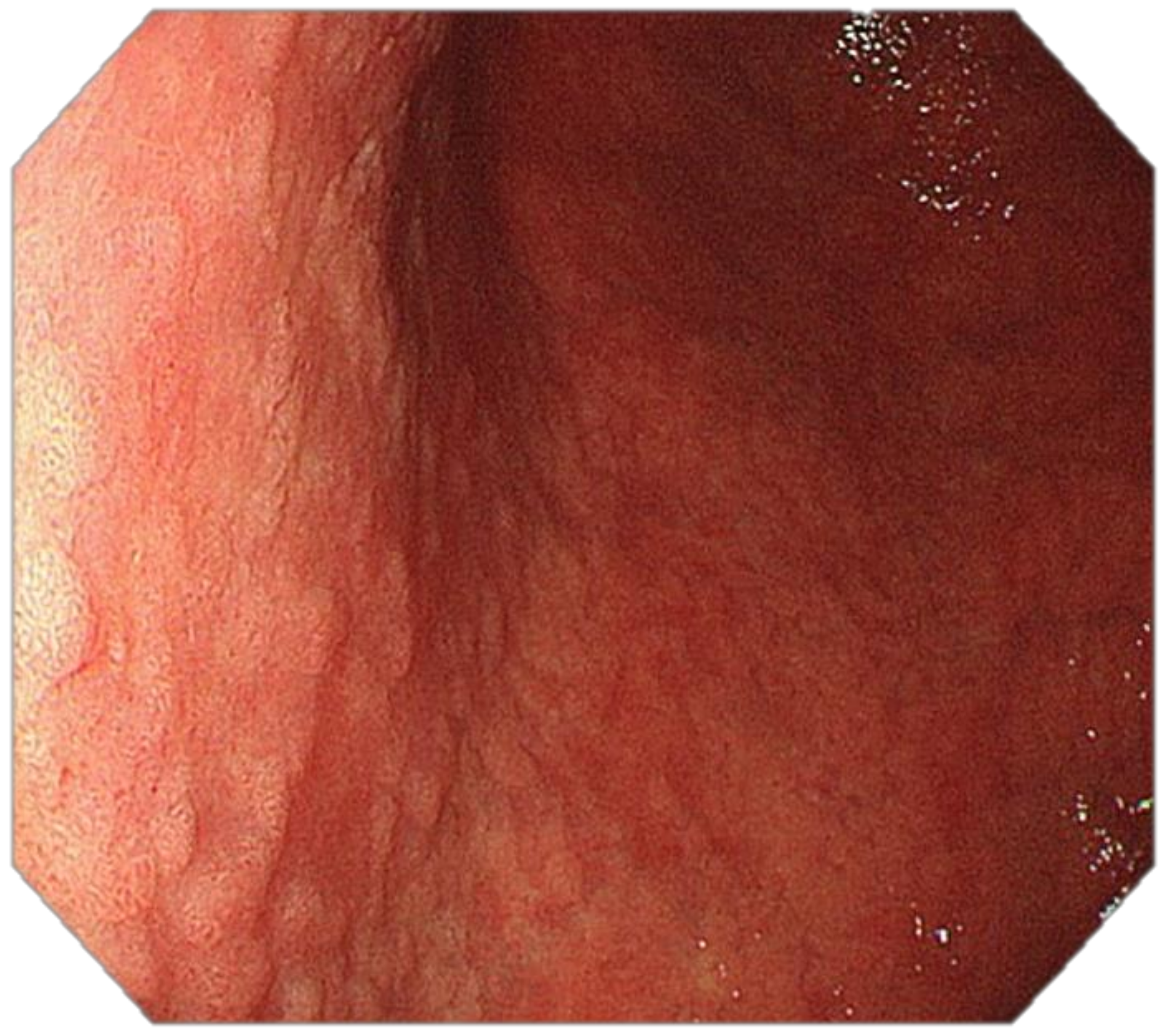
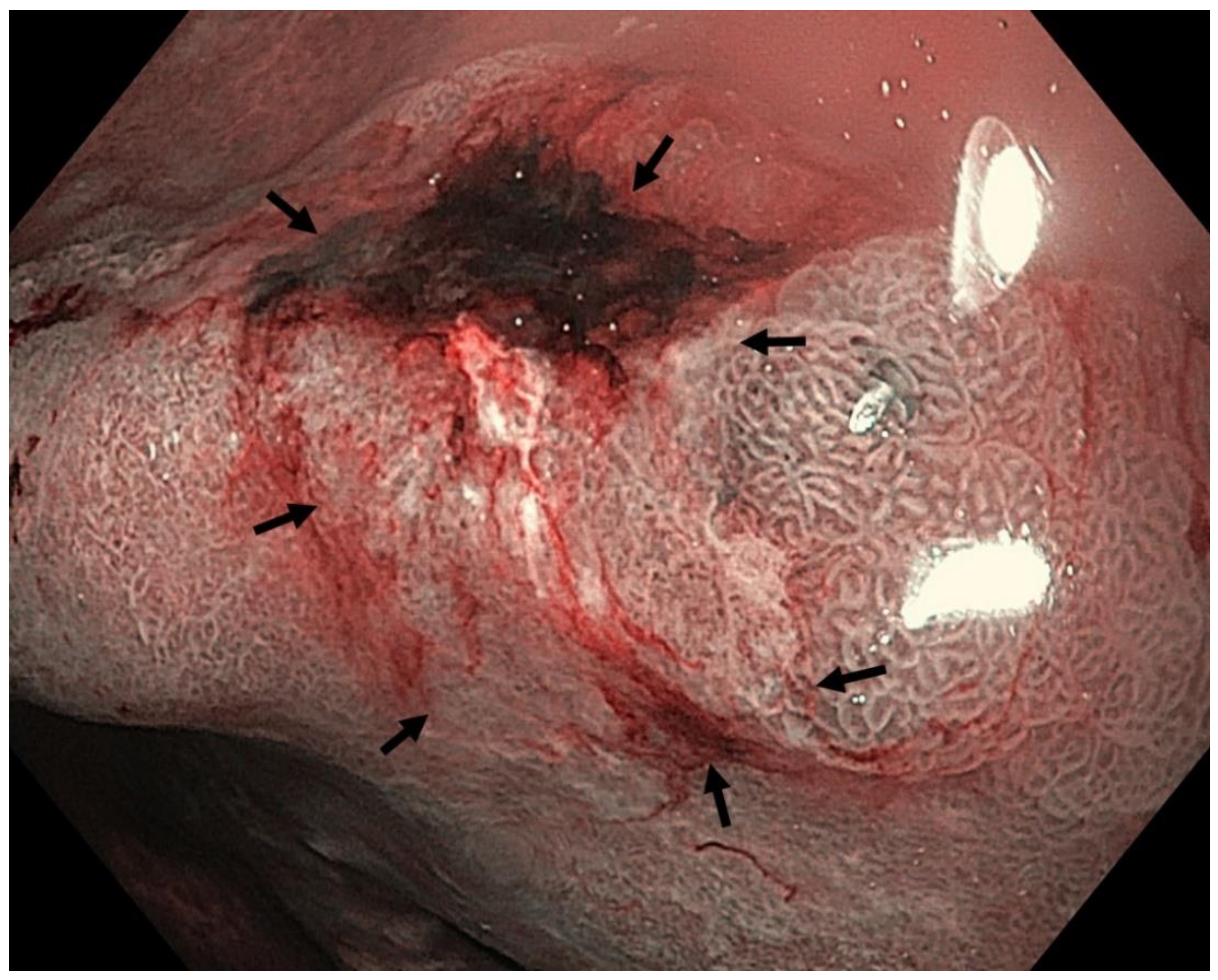
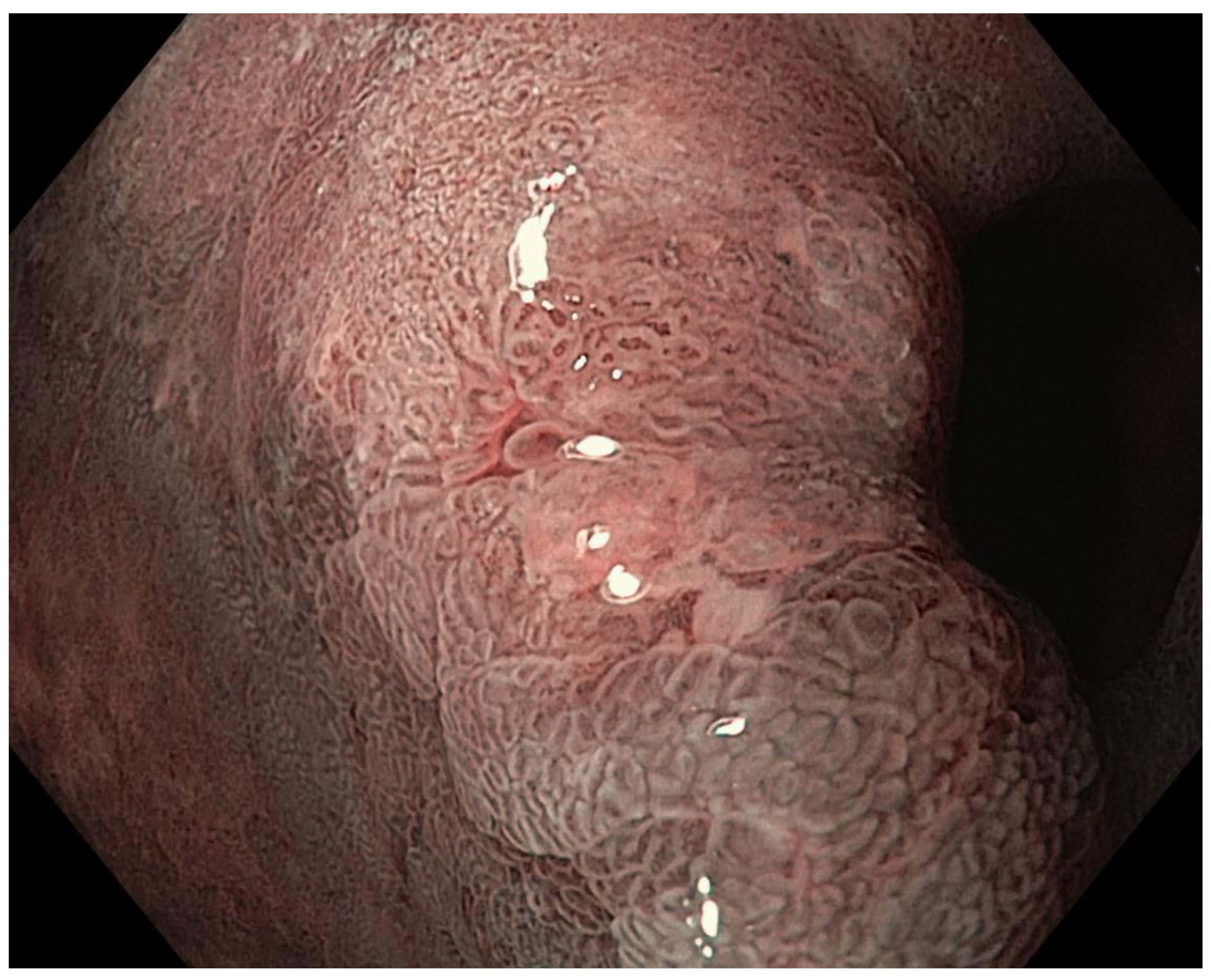
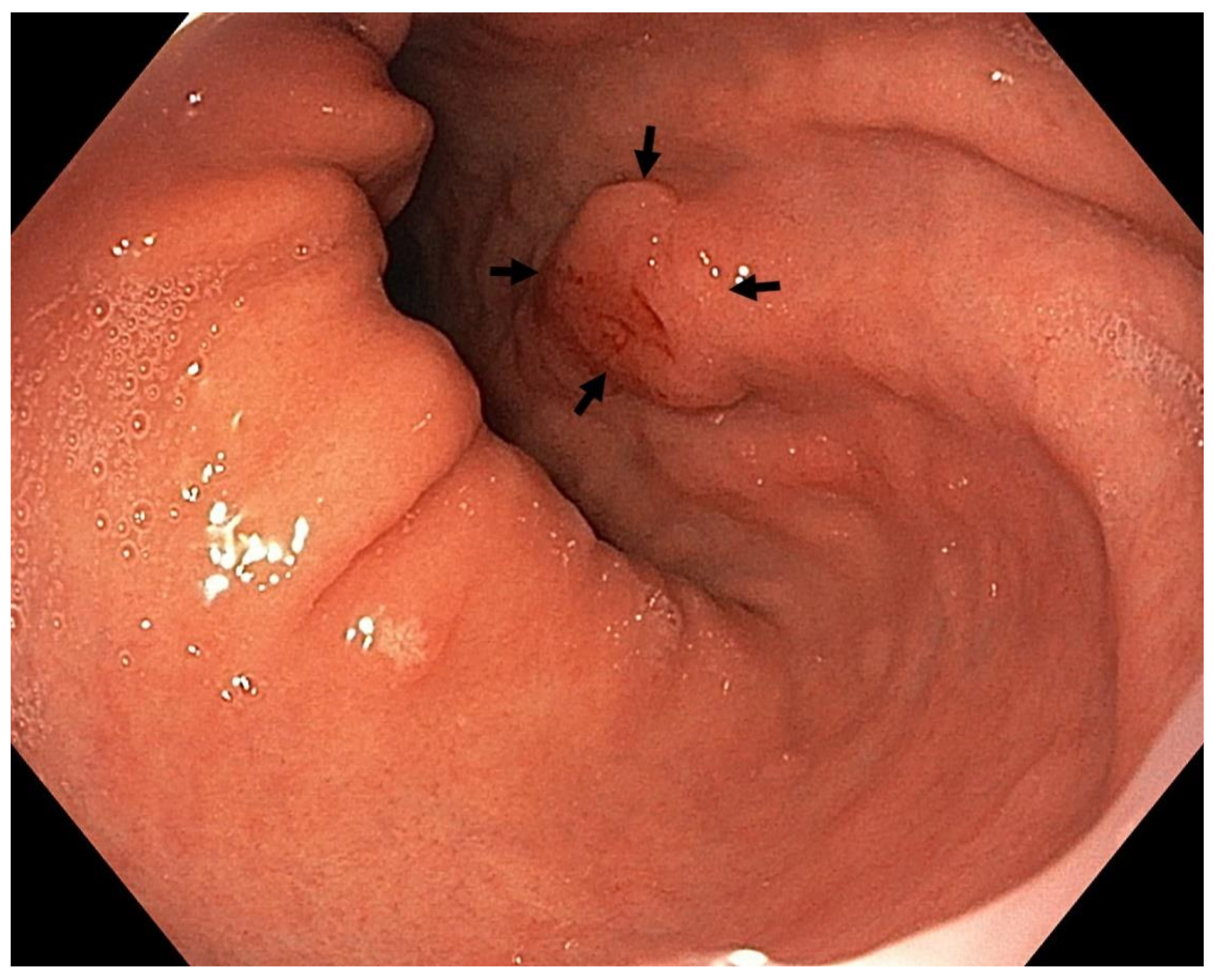
4. Features Indicative of Gastric Atrophy and Intestinal Metaplasia on White-Light Endoscopy
5. Image-Enhanced Endoscopy and Magnification
6. Optimal Endoscopy Setting for Detection of an Early Gastric Neoplasia
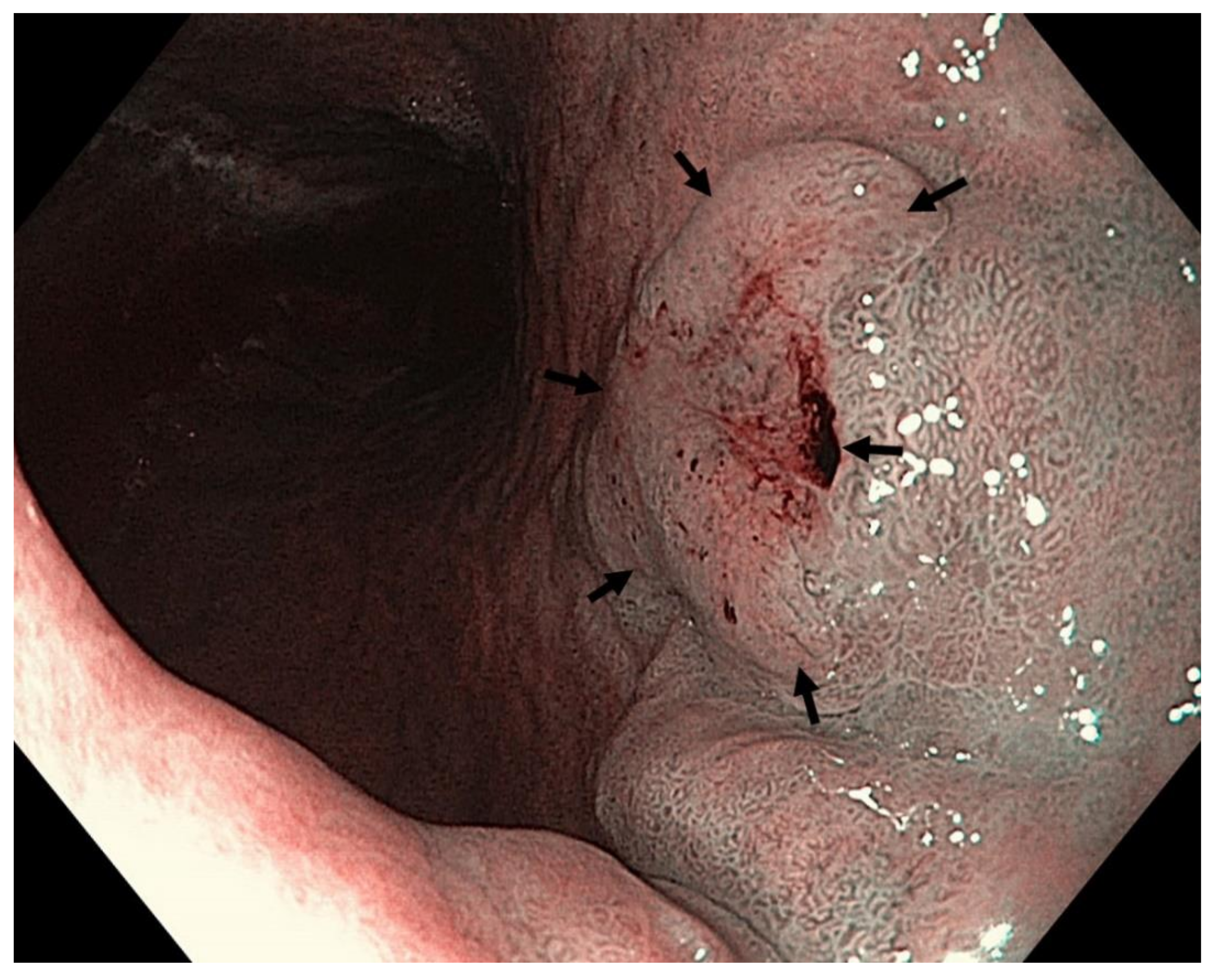
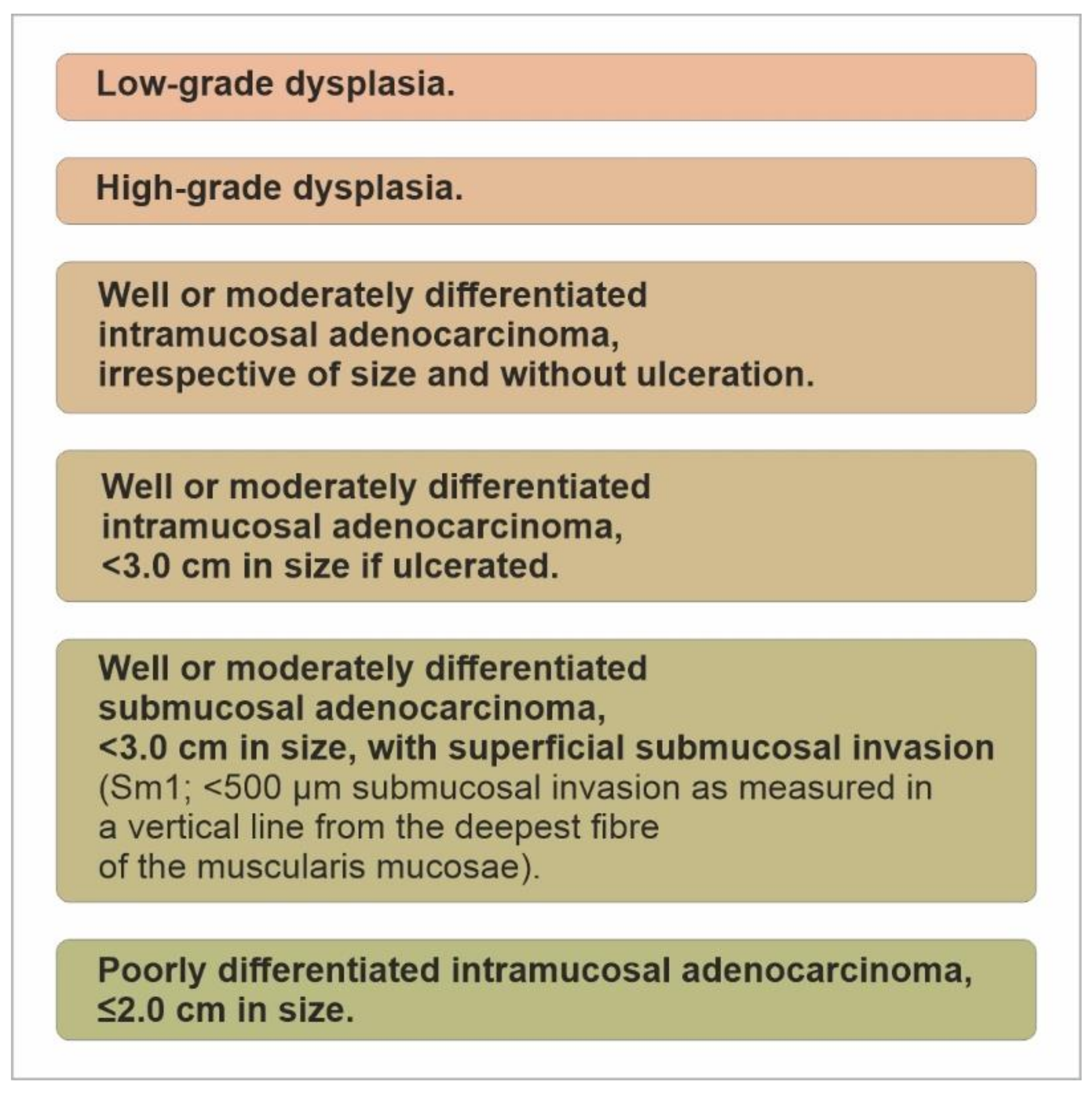
7. Endoscopic Therapy for Early Gastric Neoplasia
- Low-grade dysplasia;
- High-grade dysplasia;
- 3.
- Well- or moderately differentiated intramucosal adenocarcinoma, <3.0 cm in size if ulcerated;
- 4.
- Well- or moderately differentiated submucosal adenocarcinoma, <3.0 cm in size, with superficial submucosal invasion (Sm1; <500 μm submucosal invasion as measured in a vertical line from the deepest fibre of the muscularis mucosae);
- 5.
- Poorly differentiated intramucosal adenocarcinoma, ≤2.0 cm in size. (Figure 14).
8. Endoscopic Evaluation and Surveillance of Individuals with Inherited Predisposition to Gastric Adenocarcinoma
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; Samuel, D.G.; Thorne, K.; Akbari, A.; Williams, J.G. Review article: The prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment. Pharmacol. Ther. 2016, 43, 334–345. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100 Pt B, 1–441. [Google Scholar]
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing Trends in Stomach Cancer Throughout the World. Curr. Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Abdi, E.; Latifi-Navid, S.; Zahri, S.; Yazdanbod, A.; Pourfarzi, F. Risk factors predisposing to cardia gastric adenocarcinoma: Insights and new perspectives. Cancer Med. 2019, 8, 6114–6126. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The Two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Thrift, A.P.; Nguyen, T.H. Gastric Cancer Epidemiology. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 425–439. [Google Scholar] [CrossRef]
- Rustgi, S.D.; Ching, C.K.; Kastrinos, F. Inherited Predisposition to Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 467–487. [Google Scholar] [CrossRef] [PubMed]
- van der Post, R.S.; Oliveira, C.; Guilford, P.; Carneiro, F. Hereditary gastric cancer: What’s new? Update 2013–2018. Fam. Cancer 2019, 18, 363–367. [Google Scholar] [CrossRef]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Phillips, K.D.; Wayte, N.; Schrader, K.A.; Healey, S.; Kaurah, P.; Shulkes, A.; Grimpen, F.; Clouston, A.; Moore, D.; et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut 2012, 61, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Repak, R.; Kohoutova, D.; Podhola, M.; Rejchrt, S.; Minarik, M.; Benesova, L.; Lesko, M.; Bures, J. The first European family with gastric adenocarcinoma and proximal polyposis of the stomach: Case report and review of the literature. Gastrointest. Endosc. 2016, 84, 718–725. [Google Scholar] [CrossRef]
- Li, J.; Woods, S.L.; Healey, S.; Beesley, J.; Chen, X.; Lee, J.S.; Sivakumaran, H.; Wayte, N.; Nones, K.; Waterfall, J.J.; et al. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am. J. Hum. Genet. 2016, 98, 830–842. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.; Liang, W.Y.; Alfaro, E.; Kelly, P.; Chung, D.C.; Odze, R.D.; Lauwers, G.Y. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology 2014, 65, 353–362. [Google Scholar] [CrossRef]
- Bianchi, L.K.; Burke, C.A.; Bennett, A.E.; Lopez, R.; Hasson, H.; Church, J.M. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2008, 6, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Mankaney, G.; Leone, P.; Cruise, M.; LaGuardia, L.; O’Malley, M.; Bhatt, A.; Church, J.; Burke, C.A. Gastric cancer in FAP: A concerning rise in incidence. Fam. Cancer 2017, 16, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Kopacova, M.; Tacheci, I.; Rejchrt, S.; Bures, J. Peutz-Jeghers syndrome: Diagnostic and therapeutic approach. World J. Gastroenterol. 2009, 15, 5397–5408. [Google Scholar] [CrossRef]
- Blatter, R.H.; Plasilova, M.; Wenzel, F.; Gokaslan, S.T.; Terracciano, L.; Ashfaq, R.; Heinimann, K. Somatic alterations in juvenile polyps from BMPR1A and SMAD4 mutation carriers. Genes Chromosomes Cancer 2015, 54, 575–582. [Google Scholar] [CrossRef]
- Katona, B.W.; Powers, J.; McKenna, D.B.; Long, J.M.; Le, A.N.; Hausler, R.; Zelley, K.; Jennings, S.; Domchek, S.M.; Nathanson, K.L.; et al. Upper Gastrointestinal Cancer Risk and Surveillance Outcomes in Li-Fraumeni Syndrome. Am. J. Gastroenterol. 2020, 115, 2095–2097. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273. [Google Scholar]
- Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- Goodwin, C.S. Helicobacter pylori: 10th anniversary of its culture in April 1982. Gut 1993, 34, 293–294. [Google Scholar] [CrossRef][Green Version]
- Moran, A.P.; Wadström, T. Pathogenesis of Helicobacter pylori. Curr. Opin. Gastroenterol. 1998, 14, S9–S14. [Google Scholar]
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24, e12638. [Google Scholar] [CrossRef] [PubMed]
- Denic, M.; Touati, E.; De Reuse, H. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Jensen, P.J.; Howden, C.W. Gastritis and gastropathy. In Sleisenger and Fordtrans Gastrointestinal and Liver Disease, 11th ed.; Feldman, M., Friedman, L.S., Chung, T., Rubin, D.T., Wilcox, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 781–805. [Google Scholar]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factor Cytotoxin-Associated Gene A (CagA)-Mediated Gastric Pathogenicity. Int. J. Mol. Sci. 2020, 21, 7430. [Google Scholar] [CrossRef] [PubMed]
- Chiba, N. Ulcer disease and Helicobacter pylori infection: Current treatment. In Evidece-Based Gastroenterology and Hepatology, 4th ed.; McDonald, J.W.D., Feagan, B.G., Jalan, R., Kahrilas, P.J., Eds.; Wiley Blackwell: Oxford, UK, 2019; pp. 68–85. [Google Scholar]
- Kontizas, E.; Tastsoglou, S.; Karamitros, T.; Karayiannis, Y.; Kollia, P.; Hatzigeorgiou, A.G.; Sgouras, D.N. Impact of Helicobacter pylori Infection and Its Major Virulence Factor CagA on DNA Damage Repair. Microorganisms 2020, 8, 2007. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Quante, M.; Bornschein, J. Adenocarcinoma of the stomach and other gastric tumors. Gastritis and gastropathy. In Sleisenger and Fordtrans Gastrointestinal and Liver Disease, 11th ed.; Feldman, M., Friedman, L.S., Chung, T., Rubin, D.T., Wilcox, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 820–841. [Google Scholar]
- Forman, D.; Newell, D.G.; Fullerton, F.; Yarnell, J.W.; Stacey, A.R.; Wald, N.; Sitas, F. Association between infection with Helicobacter pylori and risk of gastric cancer: Evidence from a prospective investigation. Br. Med. J. 1991, 302, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Sierra, R.; Muñoz, N.; Peña, A.S.; Biemond, I.; van Duijn, W.; Lamers, C.B.; Teuchmann, S.; Hernandez, S.; Correa, P. Antibodies to Helicobacter pylori and pepsinogen levels in children from Costa Rica: Comparison of two areas with different risks for stomach cancer. Cancer Epidemiol. Biomarkers Prev. 1992, 1, 449–454. [Google Scholar] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Stemmermann, G.N.; Chyou, P.H.; Kato, I.; Perez-Perez, G.I.; Blaser, M.J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 1991, 325, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Zinsmeister, A.R.; Weaver, A.; DiMagno, E.P.; Carpenter, H.A.; Perez-Perez, G.I.; Blaser, M.J. Gastric adenocarcinoma and Helicobacter pylori infection. J. Natl. Cancer Inst. 1991, 83, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241.
- Watanabe, T.; Tada, M.; Nagai, H.; Sasaki, S.; Nakao, M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology 1998, 115, 642–648. [Google Scholar] [CrossRef]
- Petersen, C.P.; Mills, J.C.; Goldenring, J.R. Murine Models of Gastric Corpus Preneoplasia. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Fox, J.G.; Wang, T.C. The Origins of Gastric Cancer From Gastric Stem Cells: Lessons From Mouse Models. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process: First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef]
- Kadeerhan, G.; Gerhard, M.; Gao, J.J.; Mejías-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; Liu, W.D.; et al. Microbiota alteration at different stages in gastric lesion progression: A population-based study in Linqu, China. Am. J. Cancer Res. 2021, 11, 561–575. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef]
- Seta, T.; Takahashi, Y.; Noguchi, Y.; Shikata, S.; Sakai, T.; Sakai, K.; Yamashita, Y.; Nakayama, T. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: A systematic review and meta-analysis comparing risk ratio with risk difference. PLoS ONE 2017, 12, e0183321. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection: The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar]
- Ford, A.C.; Yuan, Y.; Forman, D.; Hunt, R.; Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 2020, 7, CD005583. [Google Scholar]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori. World Gastroenterology Organisation Global Guidelines. World Gastroenterology Organisation. April 2021. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/helicobacter-pylori-english-2021.pdf (accessed on 27 August 2021).
- Inoue, M. Public Health Interventions for Gastric Cancer Control. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, T.C. Helicobacter pylori and Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 451–465. [Google Scholar] [CrossRef]
- Huang, R.J.; Hwang, J.H. Improving the Early Diagnosis of Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 503–517. [Google Scholar] [CrossRef]
- Fuccio, L.; Zagari, R.M.; Eusebi, L.H.; Laterza, L.; Cennamo, V.; Ceroni, L.; Grilli, D.; Bazzoli, F. Meta-analysis: Can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann. Intern. Med. 2009, 151, 121–128. [Google Scholar] [CrossRef]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. Br. Med. J. 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Mukai, T.; Okamoto, S.; Yamaguchi, S.; Mashiba, H.; Taniyama, K.; Sasaki, N.; Haruma, K.; Sumii, K.; Kajiyama, G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 639–642. [Google Scholar] [CrossRef]
- Forbes, G.M.; Threlfall, T.J. Treatment of Helicobacter pylori infection to reduce gastric cancer incidence: Uncertain benefits of a community based programme in Australia. J. Gastroenterol. Hepatol. 1998, 13, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.; Lam, S.K.; Wong, W.M.; Chen, J.S.; Zheng, T.T.; Feng, R.E.; Lai, K.C.; Hu, W.H.; Yuen, S.T.; Leung, S.Y.; et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. J. Am. Med. Assoc. 2004, 291, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Pisegna, J.R.; Surti, B.; Scott, D.R. Clinical trial report: Eradication of Helicobacter pylori reduces the risk for subsequent gastric cancer. Curr. Gastroenterol. Rep. 2010, 12, 427–430. [Google Scholar] [CrossRef][Green Version]
- Kosunen, T.U.; Pukkala, E.; Sarna, S.; Seppälä, K.; Aromaa, A.; Knekt, P.; Rautelin, H. Gastric cancers in Finnish patients after cure of Helicobacter pylori infection: A cohort study. Int. J. Cancer 2011, 128, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Heo, J.; Lee, H.S.; Cho, C.M.; Jeon, S.W. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment. Pharmacol. Ther. 2014, 39, 609–618. [Google Scholar] [CrossRef]
- Shiotani, A.; Haruma, K.; Graham, D.Y. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J. Gastroenterol. 2014, 20, 11552–11559. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Iijima, K.; Shimosegawa, T. Gastric cancer development after the successful eradication of Helicobacter pylori. World J. Gastrointest. Oncol. 2016, 8, 271–281. [Google Scholar] [CrossRef]
- Choi, I.J.; Kook, M.C.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Mei, D.; Luo, R.; Lu, H.; Xu, H.; Huang, B. Does Helicobacter pylori Eradication Reduce the Incidence of Metachronous Gastric Cancer After Curative Endoscopic Resection of Early Gastric Cancer: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2020, 54, 235–241. [Google Scholar] [CrossRef] [PubMed]
- den Hoed, C.M.; Holster, I.L.; Capelle, L.G.; de Vries, A.C.; den Hartog, B.; Ter Borg, F.; Biermann, K.; Kuipers, E.J. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy 2013, 45, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.F.; Zhang, L.; Gerhard, M.; Ma, J.L.; Liu, W.D.; Ulm, K.; Wang, J.X.; Zhang, L.; Zhang, Y.; Bajbouj, M.; et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: Baseline results and factors affecting the eradication. Gut 2016, 65, 9–18. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M.; et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Graham, D.Y.; Zou, W.Y. Guilt by association: Intestinal metaplasia does not progress to gastric cancer. Curr. Opin. Gastroenterol. 2018, 34, 458–464. [Google Scholar] [CrossRef]
- Bures, J.; Kopacova, M.; Koupil, I.; Vorisek, V.; Rejchrt, S.; Beranek, M.; Seifert, B.; Pozler, O.; Zivny, P.; Douda, T.; et al. Epidemiology of Helicobacter pylori infection in the Czech Republic. Helicobacter 2006, 11, 56–65. [Google Scholar] [CrossRef]
- Bures, J.; Kopacova, M.; Koupil, I.; Seifert, B.; Skodova Fendrichova, M.; Spirkova, J.; Vorisek, V.; Rejchrt, S.; Douda, T.; Kral, N.; et al. Significant decrease in prevalence of Helicobacter pylori in the Czech Republic. World J. Gastroenterol. 2012, 18, 4412–4418. [Google Scholar] [CrossRef]
- Institute of Health Information and Statistics of the Czech Republic. Available online: www.uzis.cz (accessed on 27 August 2021).
- den Hoed, C.M.; Vila, A.J.; Holster, I.L.; Perez-Perez, G.I.; Blaser, M.J.; de Jongste, J.C.; Kuipers, E.J. Helicobacter pylori and the birth cohort effect: Evidence for stabilized colonization rates in childhood. Helicobacter 2011, 16, 405–409. [Google Scholar] [CrossRef][Green Version]
- Blaser, M.J. Hypothesis: The changing relationships of Helicobacter pylori and humans: Implications for health and disease. J. Infect. Dis. 1999, 179, 1523–1530. [Google Scholar] [CrossRef]
- Blaser, M.J. Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep. 2006, 7, 956–960. [Google Scholar] [CrossRef]
- Blaser, M.J. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev. Res. 2008, 1, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Chyou, P.H.; Nomura, A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995, 55, 562–565. [Google Scholar] [PubMed]
- Hansson, L.E.; Nyrén, O.; Hsing, A.W.; Bergström, R.; Josefsson, S.; Chow, W.H.; Fraumeni, J.F., Jr.; Adami, H.O. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 1996, 335, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: A model. Gastroenterology 1997, 113, 1983–1991. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Hsu, P.I.; Li, C.N.; Tseng, H.H.; Lai, K.H.; Hsu, P.N.; Lo, G.H.; Lo, C.C.; Yeh, J.J.; Ger, L.P.; Hsiao, M.; et al. The interleukin-1 RN polymorphism and Helicobacter pylori infection in the development of duodenal ulcer. Helicobacter 2004, 9, 605–613. [Google Scholar] [CrossRef]
- Tahara, T.; Arisawa, T.; Wang, F.; Shibata, T.; Nakamura, M.; Sakata, M.; Hirata, I.; Nakano, H. Toll-like receptor 2 (TLR) -196 to 174del polymorphism in gastro-duodenal diseases in Japanese population. Dig. Dis. Sci. 2008, 53, 919–924. [Google Scholar] [CrossRef]
- Ubukata, H.; Nagata, H.; Tabuchi, T.; Konishi, S.; Kasuga, T.; Tabuchi, T. Why is the coexistence of gastric cancer and duodenal ulcer rare? Examination of factors related to both gastric cancer and duodenal ulcer. Gastric Cancer 2011, 14, 4–12. [Google Scholar] [CrossRef]
- Datta De, D.; Roychoudhury, S. To be or not to be: The host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J. Gastroenterol. 2015, 21, 2883–2895. [Google Scholar] [CrossRef]
- Henrik Simán, J.; Forsgren, A.; Berglund, G.; Florén, C.H. Helicobacter pylori infection is associated with a decreased risk of developing oesophageal neoplasms. Helicobacter 2001, 6, 310–316. [Google Scholar] [CrossRef]
- Tacheci, I.; Repak, R.; Podhola, M.; Benesova, L.; Cyrany, J.; Bures, J.; Kohoutova, D. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)—A Helicobacter-opposite point. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101728. [Google Scholar] [CrossRef]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef]
- Lundell, L.; Vieth, M.; Gibson, F.; Nagy, P.; Kahrilas, P.J. Systematic review: The effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment. Pharmacol. Ther. 2015, 42, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Leung, W.K. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Ther. Adv. Gastroenterol. 2019, 12, 1756284819834511. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Wahlin, K.; Engstrand, L.; Lagergren, J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open 2017, 7, e017739. [Google Scholar] [CrossRef]
- Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. [Google Scholar] [CrossRef]
- Holcombe, C. Helicobacter pylori: The African enigma. Gut 1992, 33, 429–431. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Chaturvedi, R.; Correa, P. The enigma of Helicobacter pylori infection and gastric cancer. Indian J. Gastroenterol. 2010, 29, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.F. Helicobacter pylori associated Asian enigma: Does diet deserve distinction? World J. Gastrointest. Oncol. 2016, 8, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.K.; Williams, S.M. Helicobacter pylori infection causes both protective and deleterious effects in human health and disease. Genes Immun. 2021, 22, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Wang, T.C.; Nagler-Anderson, C. The African enigma: The parasite’s perspective. Gut 2001, 49, 156–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whary, M.T.; Sundina, N.; Bravo, L.E.; Correa, P.; Quinones, F.; Caro, F.; Fox, J.G. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: Possible implications for gastric carcinogenesis. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1464–1469. [Google Scholar] [CrossRef]
- Hussain, Z.; El-Omar, E.; Lee, Y.Y. Dual infective burden of Helicobacter pylori and intestinal parasites: Good or bad news for the host? Indian J. Gastroenterol. 2020, 39, 111–116. [Google Scholar] [CrossRef]
- Agha, A.; Graham, D.Y. Evidence-based examination of the African enigma in relation to Helicobacter pylori infection. Scand. J. Gastroenterol. 2005, 40, 523–529. [Google Scholar] [CrossRef]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar]
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of gastric intestinal metaplasia: Clinical implications. Am. J. Gastroenterol. 2010, 105, 493–498. [Google Scholar] [CrossRef]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J. Cancer Prev. 2015, 20, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; Romiti, A.; Giusto, M.; Guerriero, C.; Lorenzetti, R.; Campo, S.M.; Tomao, S. Follow-up of intestinal metaplasia in the stomach: When, how and why. World J. Gastrointest. Oncol. 2012, 4, 30–36. [Google Scholar] [CrossRef]
- You, W.C.; Li, J.Y.; Blot, W.J.; Chang, Y.S.; Jin, M.L.; Gail, M.H.; Zhang, L.; Liu, W.D.; Ma, J.L.; Hu, Y.R.; et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int. J. Cancer 1999, 83, 615–619. [Google Scholar] [CrossRef]
- de Vries, A.C.; van Grieken, N.C.; Looman, C.W.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Leung, W.K.; Lin, S.R.; Ching, J.Y.; To, K.F.; Ng, E.K.; Chan, F.K.; Lau, J.Y.; Sung, J.J. Factors predicting progression of gastric intestinal metaplasia: Results of a randomised trial on Helicobacter pylori eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Partida-Rodríguez, O.; Camorlinga-Ponce, M.; Flores-Luna, L.; Lazcano, E.; Gómez, A.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Torres, J. Polymorphisms in HLA-DQ genes, together with age, sex, and Helicobacter pylori infection, as potential biomarkers for the early diagnosis of gastric cancer. Helicobacter 2017, 22, e12326. [Google Scholar] [CrossRef]
- Engel, L.S.; Chow, W.H.; Vaughan, T.L.; Gammon, M.D.; Risch, H.A.; Stanford, J.L.; Schoenberg, J.B.; Mayne, S.T.; Dubrow, R.; Rotterdam, H.; et al. Population attributable risks of esophageal and gastric cancers. J. Natl. Cancer Inst. 2003, 95, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, K.; Lu, Y.; Nilsen, T.I.; Ye, W.; Hveem, K.; Vatten, L.; Lagergren, J. Smoking and alcohol drinking in relation to risk of gastric cancer: A population-based, prospective cohort study. Int. J. Cancer 2007, 120, 128–132. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S.; Heo, N.J.; Lim, J.H.; Yang, S.Y.; Chung, G.E.; Kim, J.S. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1133–1138. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Osborn, J.; Annibale, B. Systematic review: Gastric cancer incidence in pernicious anaemia. Aliment. Pharmacol. Ther. 2013, 37, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.D.; Cardwell, C.R.; McMenamin, Ú.C.; Hicks, B.M.; Johnston, B.T.; Murray, L.J.; Coleman, H.G. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: A systematic review. BMC Gastroenterol. 2017, 17, 157. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Annibale, B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World J. Gastroenterol. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Choi, A.Y.; Strate, L.L.; Fix, M.C.; Schmidt, R.A.; Ende, A.R.; Yeh, M.M.; Inadomi, J.M.; Hwang, J.H. Association of gastric intestinal metaplasia and East Asian ethnicity with the risk of gastric adenocarcinoma in a U.S. population. Gastrointest. Endosc. 2018, 87, 1023–1028. [Google Scholar] [CrossRef]
- González, C.A.; Sanz-Anquela, J.M.; Companioni, O.; Bonet, C.; Berdasco, M.; López, C.; Mendoza, J.; Martín-Arranz, M.D.; Rey, E.; Poves, E.; et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: Results of the Spanish follow-up multicenter study. J. Gastroenterol. Hepatol. 2016, 31, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Lopes, C.; da Costa-Pereira, A.; Guilherme, M.; Barbosa, J.; Lomba-Viana, H.; Silva, R.; Moreira-Dias, L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J. Clin. Pathol. 2004, 57, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- den Hollander, W.J.; Holster, I.L.; den Hoed, C.M.; Capelle, L.G.; Tang, T.J.; Anten, M.P.; Prytz-Berset, I.; Witteman, E.M.; Ter Borg, F.; Hartog, G.D.; et al. Surveillance of premalignant gastric lesions: A multicentre prospective cohort study from low incidence regions. Gut 2019, 68, 585–593. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Leja, M.; Linē, A. Early detection of gastric cancer beyond endoscopy—New methods. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101731. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.; Ma, J.; Xu, J.; Yang, L.; Xu, W.; Que, H.; Chen, M.; Xu, H. Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med. 2019, 8, 1576–1583. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Zhu, X.; Chen, L.; Ma, Y.; Wang, J.; Yang, X.; Liu, Z. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int. J. Clin. Oncol. 2020, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, Z.Y.; Ruan, S.M.; Mo, S.; Qin, J.J.; Cheng, X.D. Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 2020, 19, 96. [Google Scholar] [CrossRef]
- Huang, Z.B.; Zhang, H.T.; Yu, B.; Yu, D.H. Cell-free DNA as a liquid biopsy for early detection of gastric cancer. Oncol. Lett. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pariente, C.; Montori, S.; Llach, J.; Bofill, A.; Albeniz, E.; Moreira, L. Biomarkers for Gastric Cancer Screening and Early Diagnosis. Biomedicines 2021, 9, 1448. [Google Scholar] [CrossRef]
- Calanzani, N.; Druce, P.E.; Snudden, C.; Milley, K.M.; Boscott, R.; Behiyat, D.; Saji, S.; Martinez-Gutierrez, J.; Oberoi, J.; Funston, G.; et al. Identifying Novel Biomarkers Ready for Evaluation in Low-Prevalence Populations for the Early Detection of Upper Gastrointestinal Cancers: A Systematic Review. Adv. Ther. 2021, 38, 793–834. [Google Scholar] [CrossRef]
- Nozaki, K.; Ogawa, M.; Williams, J.A.; Lafleur, B.J.; Ng, V.; Drapkin, R.I.; Mills, J.C.; Konieczny, S.F.; Nomura, S.; Goldenring, J.R. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008, 134, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.G.; Goldenring, J.R. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 2009, 12, 189–197. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010, 138, 2207–2210. [Google Scholar] [CrossRef]
- Leja, M.; Funka, K.; Janciauskas, D.; Putnins, V.; Ruskule, A.; Kikuste, I.; Kojalo, U.; Tolmanis, I.; Misins, J.; Purmalis, K.; et al. Interobserver variation in assessment of gastric premalignant lesions: Higher agreement for intestinal metaplasia than for atrophy. Eur. J. Gastroenterol. Hepatol. 2013, 25, 694–699. [Google Scholar] [CrossRef]
- Rugge, M.; Fassan, M.; Pizzi, M.; Farinati, F.; Sturniolo, G.C.; Plebani, M.; Graham, D.Y. Operative link for gastritis assessment vs. operative link on intestinal metaplasia assessment. World J. Gastroenterol. 2011, 17, 4596–4601. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Kono, S.; Gotoda, T.; Yoshida, S.; Oda, I.; Kondo, H.; Gatta, L.; Naylor, G.; Dixon, M.; Moriyasu, F.; Axon, A. Can endoscopic atrophy predict histological atrophy? Historical study in United Kingdom and Japan. World J. Gastroenterol. 2015, 21, 13113–13123. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pimentel-Nunes, P.; Angeletti, S.; Castro, R.; Libânio, D.; Galli, G.; Lahner, E.; Di Giulio, E.; Annibale, B.; Dinis-Ribeiro, M. Endoscopic grading of gastric intestinal metaplasia (EGGIM): A multicenter validation study. Endoscopy 2019, 51, 515–521. [Google Scholar] [CrossRef]
- Dai, Y.C.; Tang, Z.P.; Zhang, Y.L. How to assess the severity of atrophic gastritis. World J. Gastroenterol. 2011, 17, 1690–1693. [Google Scholar] [CrossRef]
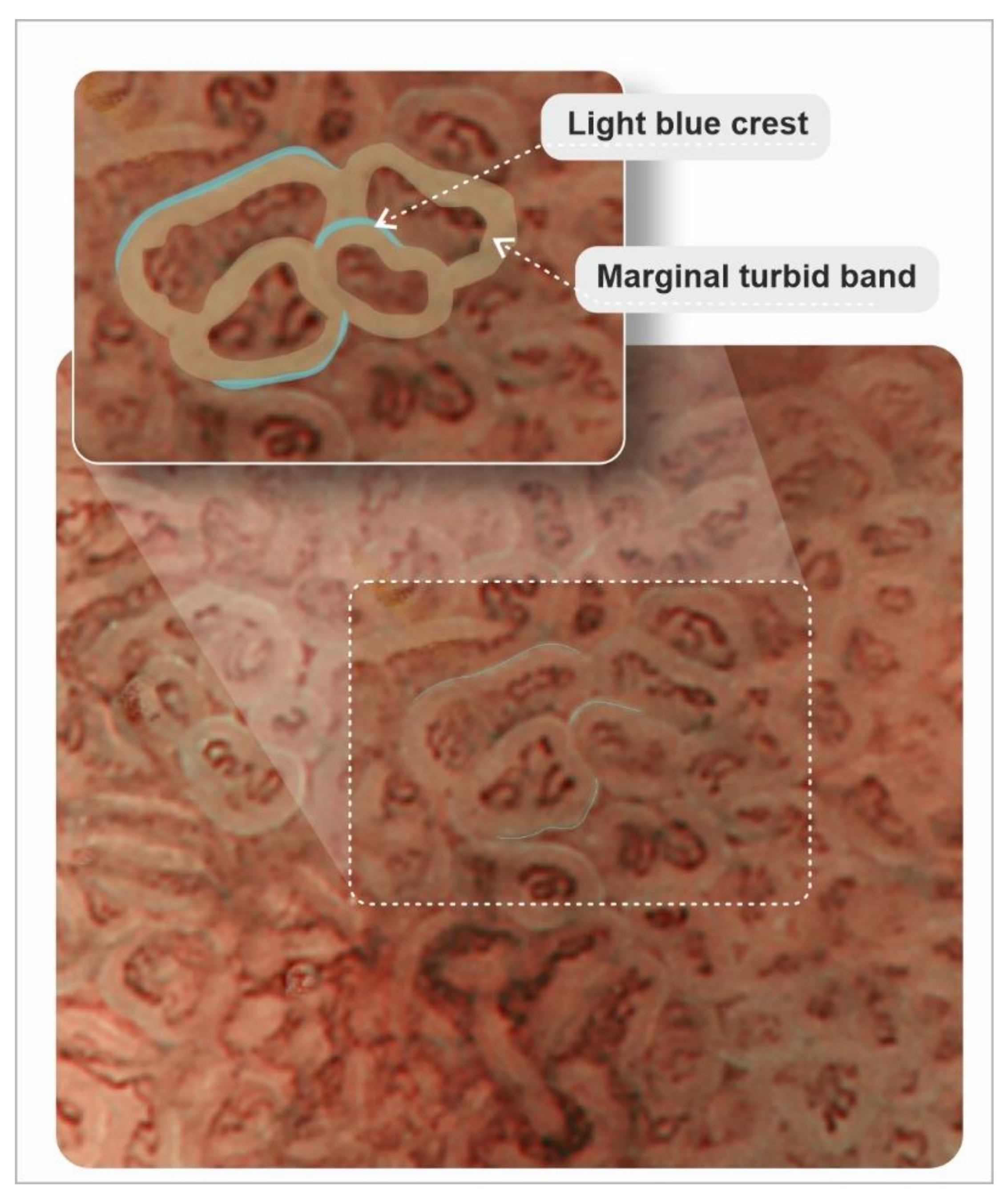
- An, J.K.; Song, G.A.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Lee, B.E.; Woo, H.Y.; Ryu, D.Y.; Kim, D.U.; Heo, J. Marginal turbid band and light blue crest, signs observed in magnifying narrow-band imaging endoscopy, are indicative of gastric intestinal metaplasia. BMC Gastroenterol. 2012, 12, 169. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Evans, J.A.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Fisher, D.A.; Foley, K.; Hwang, J.H.; Jue, T.L.; et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest. Endosc. 2015, 82, 1–8. [Google Scholar]
- Zhu, L.; Qin, J.; Wang, J.; Guo, T.; Wang, Z.; Yang, J. Early Gastric Cancer: Current Advances of Endoscopic Diagnosis and Treatment. Gastroenterol. Res. Pract. 2016, 2016, 9638041. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; Areia, M.; Coron, E.; Dobru, D.; Kaskas, B.; Kuvaev, R.; Pech, O.; Ragunath, K.; Weusten, B.; Familiari, P.; et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United Eur. Gastroenterol. J. 2016, 4, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Ragunath, K.; Wyman, A.; Banks, M.; Trudgill, N.; Pritchard, D.M.; Riley, S.; Anderson, J.; Griffiths, H.; Bhandari, P.; et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017, 66, 1886–1899. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yang, D.H.; Kim, J.W.; Kim, J.H.; Kim, J.H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intest. Res. 2021, 19, 127–157. [Google Scholar] [CrossRef]
- Teh, J.L.; Tan, J.R.; Lau, L.J.; Saxena, N.; Salim, A.; Tay, A.; Shabbir, A.; Chung, S.; Hartman, M.; So, J.B. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin. Gastroenterol. Hepatol. 2015, 13, 480–487.e2. [Google Scholar] [CrossRef]
- Kawamura, T.; Wada, H.; Sakiyama, N.; Ueda, Y.; Shirakawa, A.; Okada, Y.; Sanada, K.; Nakase, K.; Mandai, K.; Suzuki, A.; et al. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig. Endosc. 2017, 29, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Huo, S.M.; Lee, H.H.; Lee, B.I.; Song, H.J.; Choi, M.G. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017, 153, 460–469.e1. [Google Scholar] [CrossRef]
- Yoshimizu, S.; Hirasawa, T.; Horiuchi, Y.; Omae, M.; Ishiyama, A.; Yoshio, T.; Tsuchida, T.; Fujisaki, J. Differences in upper gastrointestinal neoplasm detection rates based on inspection time and esophagogastroduodenoscopy training. Endosc. Int. Open 2018, 6, E1190–E1197. [Google Scholar] [CrossRef]
- Waddingham, W.; Nieuwenburg, S.A.V.; Carlson, S.; Rodriguez-Justo, M.; Spaander, M.; Kuipers, E.J.; Jansen, M.; Graham, D.G.; Banks, M. Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterol. 2020, 12, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Asge Technology Committee; Song, L.M.; Adler, D.G.; Conway, J.D.; Diehl, D.L.; Farraye, F.A.; Kantsevoy, S.V.; Kwon, R.; Mamula, P.; Rodriguez, B.; et al. Narrow band imaging and multiband imaging. Gastrointest. Endosc. 2008, 67, 581–589. [Google Scholar]
- Matsumoto, K.; Ueyama, H.; Yao, T.; Abe, D.; Oki, S.; Suzuki, N.; Ikeda, A.; Yatagai, N.; Akazawa, Y.; Komori, H.; et al. Diagnostic limitations of magnifying endoscopy with narrow-band imaging in early gastric cancer. Endosc. Int. Open. 2020, 8, E1233–E1242. [Google Scholar] [CrossRef]
- Osawa, H.; Yamamoto, H.; Miura, Y.; Yoshizawa, M.; Sunada, K.; Satoh, K.; Sugano, K. Diagnosis of extent of early gastric cancer using flexible spectral imaging color enhancement. World J. Gastrointest. Endosc. 2012, 4, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, J.; Nishikawa, J.; Nakamura, M.; Goto, A.; Hamabe, K.; Hashimoto, S.; Okamoto, T.; Suenaga, M.; Fujita, Y.; Hamamoto, Y.; et al. Efficacy of i-Scan Imaging for the Detection and Diagnosis of Early Gastric Carcinomas. Gastroenterol. Res. Pract. 2014, 2014, 819395. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Oono, Y.; Yano, T.; Ikematsu, H.; Odagaki, T.; Yoda, Y.; Yagishita, A.; Sato, A.; Nomura, S. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc. Int. Open 2014, 2, E212–E219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, W.; Du, J.; Chen, Y.; Yang, J. Diagnostic yield of the light blue crest sign in gastric intestinal metaplasia: A meta-analysis. PLoS ONE 2014, 9, e92874. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M. Chromoendoscopy for early diagnosis of gastric cancer. Eur. J. Gastroenterol. Hepatol. 2006, 18, 831–838. [Google Scholar] [CrossRef]
- Singh, R.; Chiam, K.H.; Leiria, F.; Pu, L.Z.C.T.; Choi, K.C.; Militz, M. Chromoendoscopy: Role in modern endoscopic imaging. Transl. Gastroenterol. Hepatol. 2020, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Persiani, R.; Nista, E.C.; Rausei, S.; Lecca, G.; Migneco, A.; Cananzi, F.C.; Cammarota, G.; D’Ugo, D.; Gasbarrini, G.; et al. A case-control study comparing methylene blue directed biopsies and random biopsies for detecting pre-cancerous lesions in the follow-up of gastric cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 291–296. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; da Costa-Pereira, A.; Lopes, C.; Moreira-Dias, L. Feasibility and cost-effectiveness of using magnification chromoendoscopy and pepsinogen serum levels for the follow-up of patients with atrophic chronic gastritis and intestinal metaplasia. J. Gastroenterol. Hepatol. 2007, 22, 1594–1604. [Google Scholar] [CrossRef]
- Taghavi, S.A.; Membari, M.E.; Eshraghian, A.; Dehghani, S.M.; Hamidpour, L.; Khademalhoseini, F. Comparison of chromoendoscopy and conventional endoscopy in the detection of premalignant gastric lesions. Can. J. Gastroenterol. 2009, 23, 105–108. [Google Scholar] [CrossRef]
- Eleftheriadis, N.; Inoue, H.; Ikeda, H.; Onimaru, M.; Yoshida, A.; Maselli, R.; Santi, G.; Kudo, S.E. Acetic acid spray enhances accuracy of narrow-band imaging magnifying endoscopy for endoscopic tissue characterization of early gastric cancer. Gastrointest. Endosc. 2014, 79, 712. [Google Scholar] [CrossRef] [PubMed]
- Yao, K. The endoscopic diagnosis of early gastric cancer. Ann. Gastroenterol. 2013, 26, 11–22. [Google Scholar]
- An, P.; Yang, D.; Wang, J.; Wu, L.; Zhou, J.; Zeng, Z.; Huang, X.; Xiao, Y.; Hu, S.; Chen, Y.; et al. A deep learning method for delineating early gastric cancer resection margin under chromoendoscopy and white light endoscopy. Gastric Cancer 2020, 23, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Ohnita, K.; Isomoto, H.; Shikuwa, S.; Yamaguchi, N.; Nakayama, T.; Nishiyama, H.; Okamoto, K.; Fukuda, E.; Takeshima, F.; Hayashi, T.; et al. Magnifying chromoendoscopic findings of early gastric cancer and gastric adenoma. Dig. Dis. Sci. 2011, 56, 2715–2722. [Google Scholar] [CrossRef]
- Zheng, X.; Mao, X.; Xu, K.; Lü, L.; Peng, X.; Wang, M.; Xu, G.; Hua, Z.; Wang, J.; Xue, H.; et al. Massive Endoscopic Screening for Esophageal and Gastric Cancers in a High-Risk Area of China. PLoS ONE 2015, 10, e0145097. [Google Scholar] [CrossRef]
- Canakis, A.; Kim, R. Endoscopic Advances for Gastric Neoplasia Detection. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 543–561. [Google Scholar] [CrossRef]
- Kaise, M.; Ohkura, Y.; Iizuka, T.; Kimura, R.; Nomura, K.; Kuribayashi, Y.; Yamada, A.; Yamashita, S.; Furuhata, T.; Kikuchi, D.; et al. Endocytoscopy is a promising modality with high diagnostic accuracy for gastric cancer. Endoscopy 2015, 47, 19–25. [Google Scholar] [CrossRef]
- Abad, M.R.A.; Inoue, H.; Ikeda, H.; Manolakis, A.; Rodriguez de Santiago, E.; Sharma, A.; Fujiyoshi, Y.; Fukuda, H.; Sumi, K.; Onimaru, M.; et al. Utilizing fourth-generation endocytoscopy and the ‘enlarged nuclear sign’ for in vivo diagnosis of early gastric cancer. Endosc. Int. Open 2019, 7, E1002–E1007. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Park, Y.; Kim, H.K.; Jo, J.H.; Park, C.H.; Kim, E.H.; Jung, D.H.; Chung, H.; Shin, S.K.; Lee, S.K.; et al. Probe-based confocal laser endomicroscopy in the margin delineation of early gastric cancer for endoscopic submucosal dissection. J. Gastroenterol. Hepatol. 2017, 32, 1046–1054. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tajiri, H.; Seike, E.; Shitaya, M.; Tounou, S.; Mine, M.; Oba, K. Detection of early gastric cancer by a real-time autofluorescence imaging system. Cancer Lett. 2001, 165, 155–159. [Google Scholar] [CrossRef]
- Tada, K.; Oda, I.; Yokoi, C.; Taniguchi, T.; Sakamoto, T.; Suzuki, H.; Nonaka, S.; Yoshinaga, S.; Saito, Y.; Gotoda, T. Pilot study on clinical effectiveness of autofluorescence imaging for early gastric cancer diagnosis by less experienced endoscopists. Diagn. Ther. Endosc. 2011, 2011, 419136. [Google Scholar] [CrossRef] [PubMed]
- Testoni, P.A.; Mangiavillano, B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J. Gastroenterol. 2008, 14, 6444–6452. [Google Scholar] [CrossRef]
- Kanzaki, H.; Takenaka, R.; Kawahara, Y.; Kawai, D.; Obayashi, Y.; Baba, Y.; Sakae, H.; Gotoda, T.; Kono, Y.; Miura, K.; et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc. Int. Open. 2017, 5, E1005–E1013. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Osawa, H.; Hayashi, Y.; Lefor, A.K.; Yamamoto, H. Linked color imaging for the detection of early gastrointestinal neoplasms. Therap. Adv. Gastroenterol. 2019, 12, 1756284819885246. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Manner, H.; Rey, J.W.; Kiesslich, R. A guide to multimodal endoscopy imaging for gastrointestinal malignancy—an early indicator. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 421–434. [Google Scholar] [CrossRef]
- Ali, H.; Yasmin, M.; Sharif, M.; Rehmani, M.H. Computer assisted gastric abnormalities detection using hybrid texture descriptors for chromoendoscopy images. Comput. Methods Programs Biomed. 2018, 157, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, L.; Ling, T.; Lv, Y.; Ni, M.; Zhan, Q.; Fu, Y.; Zhuang, D.; Guo, H.; Dou, X.; et al. Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: A multicentre retrospective diagnostic study. EBioMedicine 2020, 62, 103146. [Google Scholar] [CrossRef]
- Jiang, K.; Jiang, X.; Pan, J.; Wen, Y.; Huang, Y.; Weng, S.; Lan, S.; Nie, K.; Zheng, Z.; Ji, S.; et al. Current Evidence and Future Perspective of Accuracy of Artificial Intelligence Application for Early Gastric Cancer Diagnosis With Endoscopy: A Systematic and Meta-Analysis. Front. Med. 2021, 8, 629080. [Google Scholar] [CrossRef]
- Hsiao, Y.J.; Wen, Y.C.; Lai, W.Y.; Lin, Y.Y.; Yang, Y.P.; Chien, Y.; Yarmishyn, A.A.; Hwang, D.K.; Lin, T.C.; Chang, Y.C.; et al. Application of artificial intelligence-driven endoscopic screening and diagnosis of gastric cancer. World J. Gastroenterol. 2021, 27, 2979–2993. [Google Scholar] [CrossRef]
- Ueyama, H.; Kato, Y.; Akazawa, Y.; Yatagai, N.; Komori, H.; Takeda, T.; Matsumoto, K.; Ueda, K.; Matsumoto, K.; Hojo, M.; et al. Application of artificial intelligence using a convolutional neural network for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging. J. Gastroenterol. Hepatol. 2021, 36, 482–489. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; Liu, M.; Xie, H.; An, P.; Zhang, J.; Zhang, H.; Ai, Y.; Tong, Q.; Guo, M.; et al. Evaluation of the effects of an artificial intelligence system on endoscopy quality and preliminary testing of its performance in detecting early gastric cancer: A randomized controlled trial. Endoscopy 2021, 53, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Jang, J.S.; Ryu, H.C.; Kim, M.C.; Kim, K.H.; Kim, D.K. Risk factors associated with multiple and missed gastric neoplastic lesions after endoscopic resection: Prospective study at a single institution in south Korea. Hepatogastroenterology 2015, 62, 512–517. [Google Scholar]
- Oka, K.; Iwai, N.; Okuda, T.; Hara, T.; Inada, Y.; Tsuji, T.; Komaki, T.; Sakagami, J.; Naito, Y.; Kagawa, K.; et al. Clinical Features of False-Negative Early Gastric Cancers: A Retrospective Study of Endoscopic Submucosal Dissection Cases. Gastroenterol. Res. Pract. 2021, 2021, 6635704. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef]
- Hirasawa, T.; Gotoda, T.; Miyata, S.; Kato, Y.; Shimoda, T.; Taniguchi, H.; Fujisaki, J.; Sano, T.; Yamaguchi, T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009, 12, 148–152. [Google Scholar] [CrossRef]
- Oda, I.; Gotoda, T.; Hamanaka, H.; Eguchi, T.; Saito, Y.; Matsuda, T.; Bhandari, P.; Emura, F.; Saito, D.; Ono, H. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2005, 17, 54–58. [Google Scholar] [CrossRef]
- Hasuike, N.; Ono, H.; Boku, N.; Mizusawa, J.; Takizawa, K.; Fukuda, H.; Oda, I.; Doyama, H.; Kaneko, K.; Hori, S.; et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): The Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018, 21, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.; Schneider, A.; Schaller, T.; Anthuber, M.; Ebigbo, A.; Messmann, H. Endoscopic submucosal dissection for early gastric cancer: Are expanded resection criteria safe for Western patients? Endoscopy 2017, 49, 855–865. [Google Scholar] [CrossRef]
- Facciorusso, A.; Antonino, M.; Di Maso, M.; Muscatiello, N. Endoscopic submucosal dissection vs. endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J. Gastrointest. Endosc. 2014, 6, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Chen, S.; Zhang, Y.; Qiu, F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest. Endosc. 2012, 76, 763–770. [Google Scholar] [CrossRef]
- Park, Y.M.; Cho, E.; Kang, H.Y.; Kim, J.M. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg. Endosc. 2011, 25, 2666–2677. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; Ichinose, M.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 2016, 28, 3–15. [Google Scholar] [CrossRef]
- The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 2003, 58 (Suppl. 6), S3–S43. [CrossRef]
- Abe, S.; Oda, I.; Shimazu, T.; Kinjo, T.; Tada, K.; Sakamoto, T.; Kusano, C.; Gotoda, T. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer 2011, 14, 35–40. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Aslanian, H.R.; Sethi, A.; Bhutani, M.S.; Goodman, A.J.; Krishnan, K.; Lichtenstein, D.R.; Melson, J.; Navaneethan, U.; Pannala, R.; et al. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE 2019, 4, 343–350. [Google Scholar] [CrossRef] [PubMed]
- van der Post, R.S.; Vogelaar, I.P.; Carneiro, F.; Guilford, P.; Huntsman, D.; Hoogerbrugge, N.; Caldas, C.; Schreiber, K.E.; Hardwick, R.H.; Ausems, M.G.; et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J. Med. Genet. 2015, 52, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Balaguer, F.; Dekker, E.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Ricciardiello, L.; Rupińska, M.; et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohoutova, D.; Banks, M.; Bures, J. Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer. Cancers 2021, 13, 6242. https://doi.org/10.3390/cancers13246242
Kohoutova D, Banks M, Bures J. Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer. Cancers. 2021; 13(24):6242. https://doi.org/10.3390/cancers13246242
Chicago/Turabian StyleKohoutova, Darina, Matthew Banks, and Jan Bures. 2021. "Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer" Cancers 13, no. 24: 6242. https://doi.org/10.3390/cancers13246242
APA StyleKohoutova, D., Banks, M., & Bures, J. (2021). Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer. Cancers, 13(24), 6242. https://doi.org/10.3390/cancers13246242





