Acute and Long-Term Outcomes of ST-Elevation Myocardial Infarction in Cancer Patients, a ‘Real World’ Analysis with 175,000 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
Statistical Methods
3. Results
3.1. In-Hospital Treatments and Outcomes of STEMI Patients with Malignancies
3.2. Overall Survival
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dégano, I.R.; Salomaa, V.; Veronesi, G.; Ferriéres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.-B.; Ferrario, M.M.; Meisinger, C.; et al. Acute Myocardial Infarction Trends in Europe (AMITIE) Study Investigators. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar] [CrossRef]
- gbe-bund.de Das Informationssystem der Gesundheitsberichterstattung des Bundes. Available online: https://www.gbe-bund.de (accessed on 1 October 2020).
- OECD iLibrary. Available online: https://www.oecd-ilibrary.org/ (accessed on 1 May 2021).
- Velders, M.A.; Hagström, E.; James, S.K. Temporal trends in the prevalence of cancer and its impact on outcome in patients with first myocardial infarction: A nationwide study. J. Am. Heart Assoc. 2020, 9, e014383. [Google Scholar] [CrossRef]
- European Union. ECIS—European Cancer Information System. 2020. Available online: https://ecis.jrc.ec.europa.eu (accessed on 18 October 2020).
- Rassaf, T.; Totzeck, M.; Backs, J.; Bokemeyer, C.; Hallek, M.; Hilfiker-Kleiner, D.; Hochhaus, A.; Lüftner, D.; Müller, O.J.; Neudorf, U.; et al. Committee for Clinical Cardiovascular Medicine of the German Cardiac Society. Onco-Cardiology: Consensus Paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin. Res. Cardiol. 2020, 109, 1197–1222. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Styczkiewicz, K.; Styczkiewicz, M.; Myćka, M.; Mędrek, S.; Kondraciuk, T.; Czerkies-Bieleń, A.; Wiśniewski, A.; Szmit, S.; Jankowski, P. Clinical Presentation and Treatment of Acute Coronary Syndrome as Well as 1-year Survival of Patients Hospitalized Due to Cancer: A 7-year Experience of a Nonacademic Center. Medicine 2020, 99, e18972. [Google Scholar] [CrossRef]
- Pocock, S.; Bueno, H.; Licour, M.; Medina, J.; Zhang, L.; Annemans, L.; Danchin, N.; Huo, Y.; Van de Werf, F. Predictors of one-year mortality at hospital discharge after acute coronary syndromes: A new risk score from the EPICOR (long-tErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients) study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 509–517. [Google Scholar] [CrossRef]
- Reinöhl, J.; Kaier, K.; Reinecke, H.; Schmoor, C.; Frankenstein, L.; Vach, W.; Cribier, A.; Beyersdorf, F.; Bode, C.; Zehender, M. Effect of Availability of Transcatheter Aortic-Valve Replacement on Clinical Practice. N. Engl. J. Med. 2015, 373, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Scholz, K.H.; Maier, S.K.G.; Maier, L.S.; Lengenfelder, B.; Jacobshagen, C.; Jung, J.; Fleischmann, C.; Werner, G.S.; Olbrich, H.G.; Ott, R.; et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: Results from the German prospective, multicentre FITT-STEMI trial. Eur. Heart J. 2018, 39, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Morales, D.R.; Guthrie, B. Exclusion rates in randomized controlled trials of treatments for physical conditions: A systematic review. Trials 2020, 21, 228. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Goto, S.; Bhatt, D.L.; Steg, P.G.; Storey, R.F.; Cohen, M.; Goodrich, E.; Mauri, L.; Ophuis, T.O.; Ruda, M.; et al. Prevention of Stroke with Ticagrelor in Patients with Prior Myocardial Infarction: Insights from PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54). Circulation 2016, 134, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of low-dose intracoronary alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with acute myocardial infarction: A randomized clinical trial. JAMA 2019, 321, 56–68. [Google Scholar] [CrossRef]
- Grundmann, N.; Meisinger, C.; Trepel, M.; Müller-Nordhorn, J.; Schenkirsch, G.; Linseisen, J. Trends in cancer incidence and survival in the Augsburg study region-results from the Augsburg cancer registry. BMJ Open 2020, 10, e036176. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Potts, J.; Mohamed, M.O.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef]
- Pothineni, N.V.; Shah, N.N.; Rochlani, Y.; Saad, M.; Kovelamudi, S.; Marmagkiolis, K.; Bhatti, S.; Cilingiroglu, M.; Aronow, W.S.; Hakeem, A. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann. Transl. Med. 2017, 5, 482. [Google Scholar] [CrossRef] [Green Version]
- Robert Koch-Institut, Zentrum fuer Krebsregisterdaten und Gesellschaft. Der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg.). Krebs in Deutschland fuer 2015/16. 12. Ausgabe. Berlin. 2019. Available online: https://edoc.rki.de/handle/176904/6012 (accessed on 18 November 2020).
- Elbadawi, A.; Elgendy, I.Y.; Ha, L.D.; Mahmoud, K.; Lenka, J.; Olorunfemi, O.; Reyes, A.; Ogunbayo, G.O.; Saad, M.; Abbott, J.D. National Trends and Outcomes of Percutaneous Coronary Intervention in Patients ≥ 70 Years of Age with Acute Coronary Syndrome (from the National Inpatient Sample Database). Am. J. Cardiol. 2019, 123, 25–32. [Google Scholar] [CrossRef]
- Guddati, A.K.; Joy, P.S.; Kumar, G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J. Cancer Res. Clin. Oncol. 2016, 142, 471–479. [Google Scholar] [CrossRef]
- Angelini, D.E.; Radivoyevitch, T.; McCrae, K.R.; Khorana, A.A. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am. J. Hematol. 2019, 94, 780–785. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, X.; Yu, C.; Hong, J.; Fang, J.; Chen, H. Increased risk of hemorrhage in metastatic colorectal cancer patients treated with bevacizumab: An updated meta-analysis of 12 randomized controlled trials. Medicine 2016, 95, e4232. [Google Scholar] [CrossRef]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [Green Version]
- Donnellan, E.; Khorana, A.A. Cancer and Venous Thromboembolic Disease: A Review. Oncologist 2017, 22, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Sanon, S.; Lenihan, D.J.; Mouhayar, E. Peripheral arterial ischemic events in cancer patients. Vasc. Med. 2011, 16, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Fisher, R.I.; Kuderer, N.M.; Lyman, G.H. Thromboembolism in hospitalized neutropenic cancer patients. J. Clin. Oncol. 2006, 24, 484–490. [Google Scholar] [CrossRef]
- Javid, M.; Magee, T.R.; Galland, R.B. Arterial thrombosis associated with malignant disease. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Lüders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.H.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease. The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Ekman, I.; Ekman, T.; Odén, A.; Rosengren, A. Population impact of heart failure and the most common forms of cancer: A study of 1,162,309 hospital cases in Sweden (1988 to 2004). Circ. Cardiovasc Qual. Outcomes 2010, 3, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Brøndum-Jacobsen, P.; Nordestgaard, B.G.; Nielsen, S.F.; Benn, M. Skin cancer as a marker of sun exposure associates with myocardial infarction, hip fracture and death from any cause. Int. J. Epidemiol. 2013, 42, 1486–1496. [Google Scholar] [CrossRef] [Green Version]
- Wehner, M.R.; Cidre Serrano, W.; Nosrati, A.; Schoen, P.M.; Chren, M.M.; Boscardin, J.; Linos, E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. Am. Acad. Dermatol. 2018, 78, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [Green Version]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
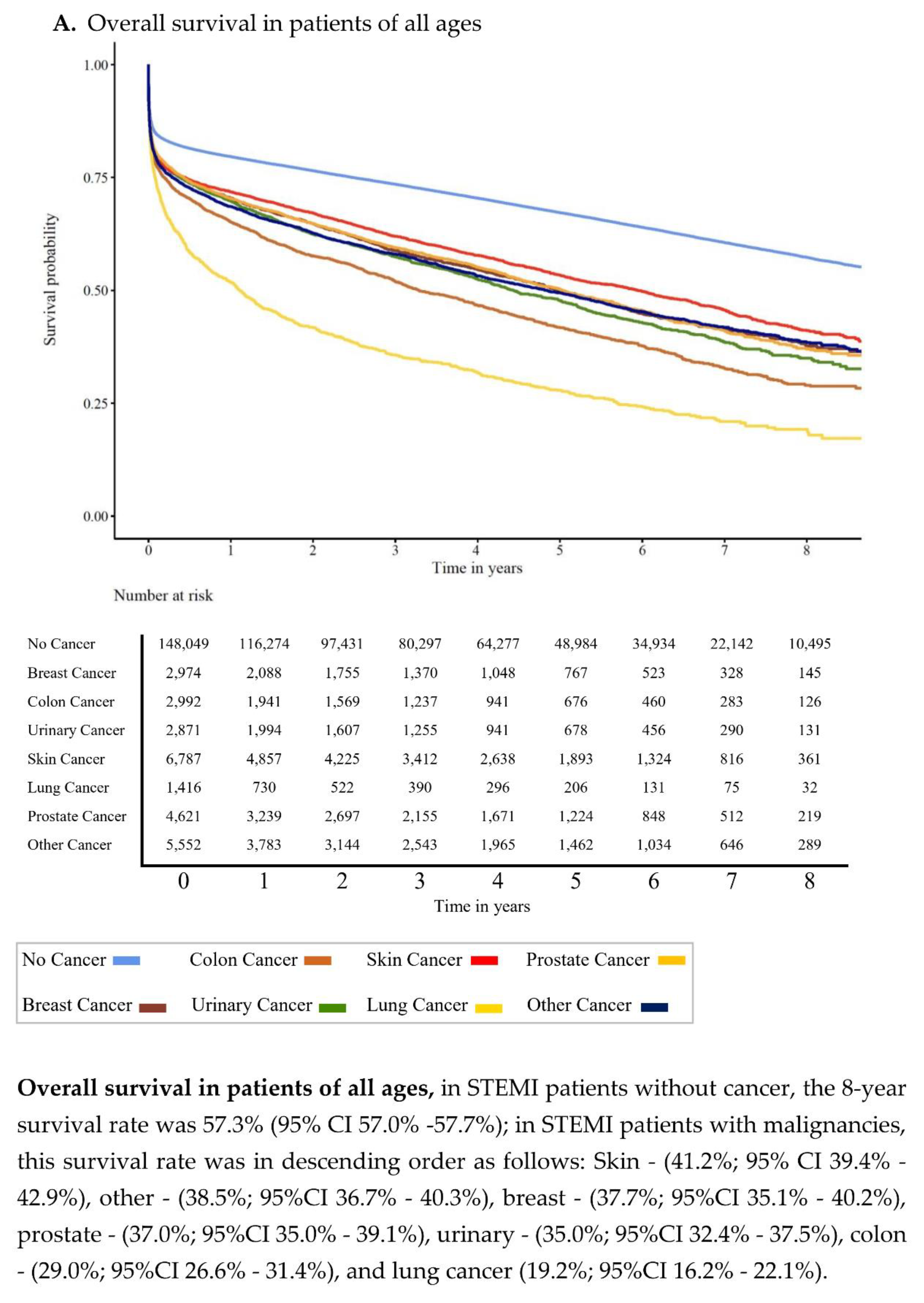

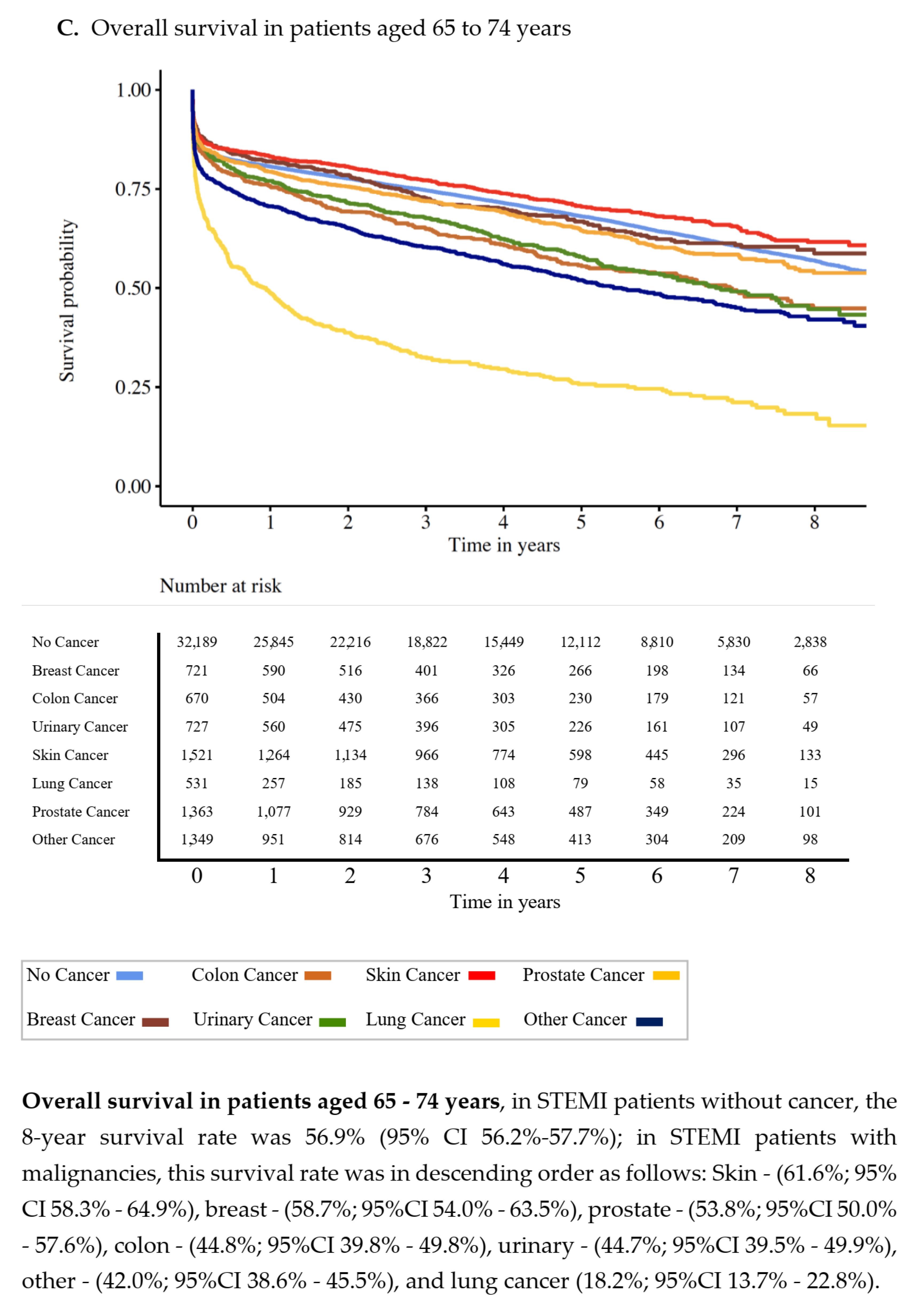
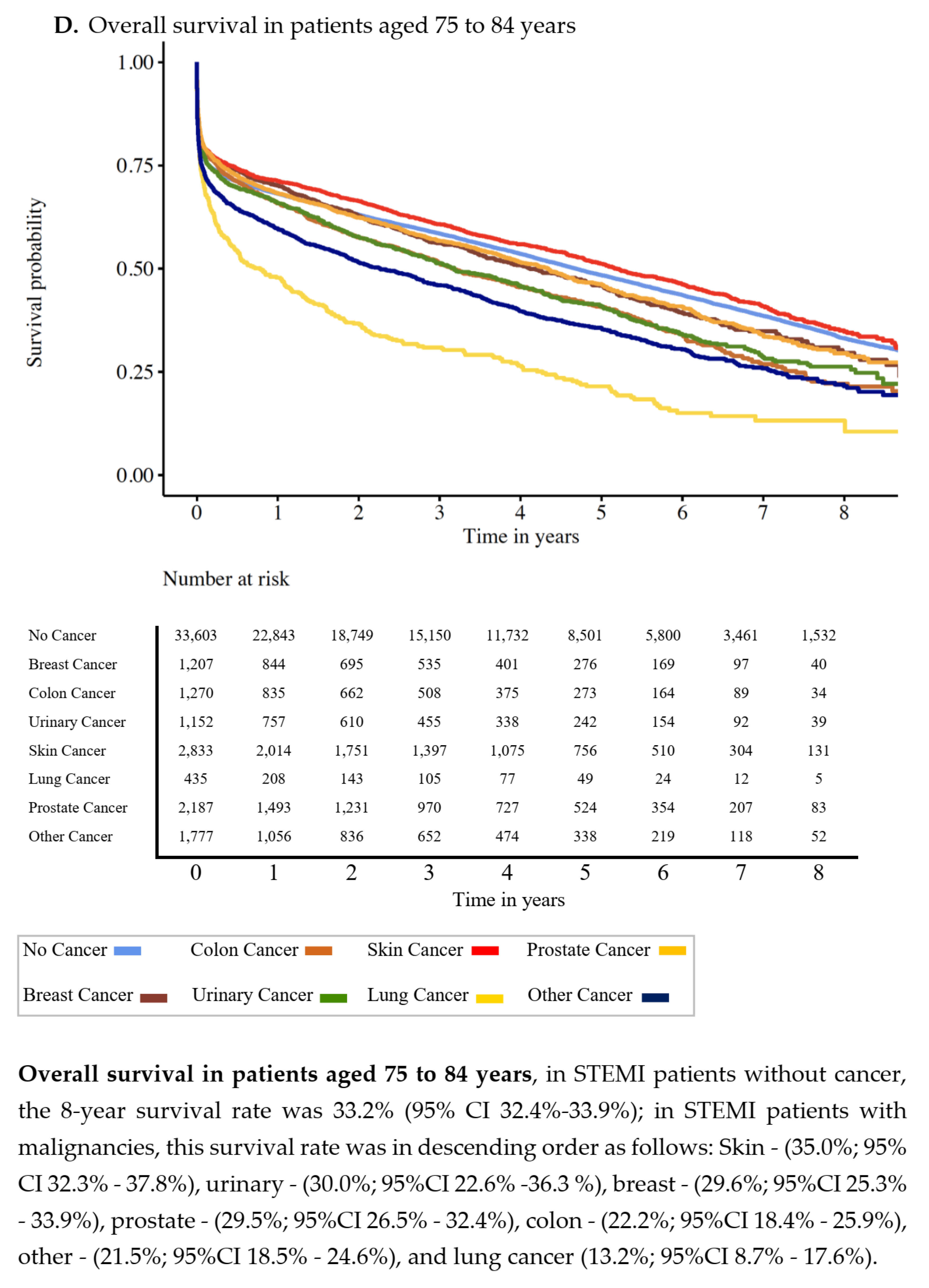
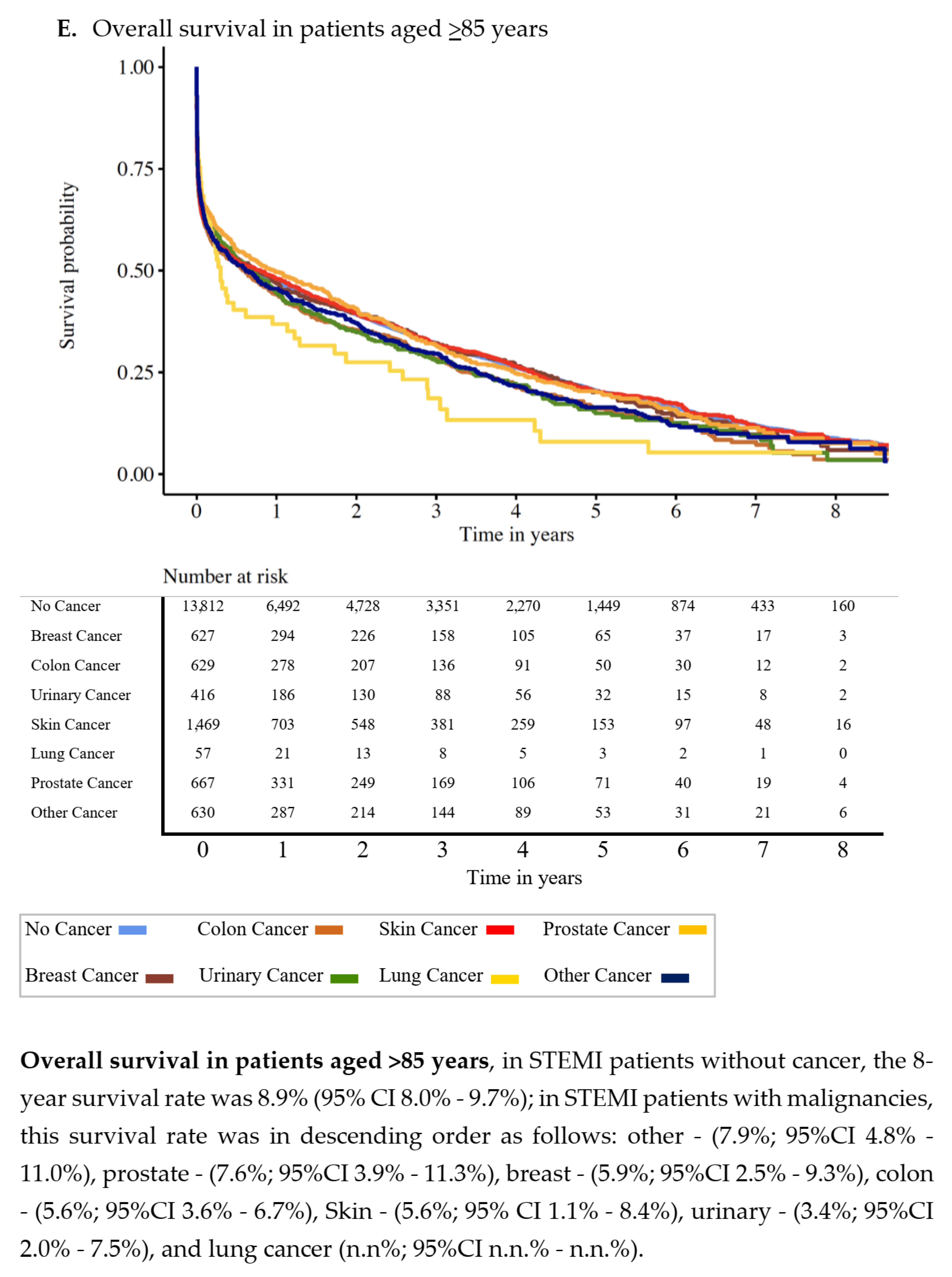
| No Cancer | Prostate | Breast | Lung | Colon | Urinary | Skin | Other Cancers | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Total, Nº | 148,049 | 4621 | 2974 | 1416 | 2992 | 2871 | 6787 | 5552 | - |
| Male, Nº | 96,351 | 4620 | 39 | 1074 | 1866 | 2106 | 4168 | 3083 | <0.001 |
| % | 65.08 | 100 | 1.31 | 75.85 | 62.37 | 73.35 | 61.41 | 55.53 | |
| Female, Nº | 51,698 | 0 | 2935 | 342 | 1126 | 765 | 2619 | 2469 | <0.001 |
| % | 34.92 | - | 98.69 | 24.15 | 37.63 | 26.65 | 38.59 | 44.47 | |
| Median age, years | 66.88 | 77.09 | 77.93 | 71.41 | 77.90 | 76.10 | 78.08 | 72.85 | <0.001 |
| Age IQR, years | 21.83 | 9.98 | 13.43 | 13.54 | 12.40 | 14.47 | 12.73 | 18.57 | |
| No of diseased CV: 3, Nº | 29,849 | 1342 | 649 | 357 | 811 | 742 | 2261 | 1442 | <0.001 |
| % | 42.94 | 51.44 | 38.54 | 41.75 | 46.00 | 45.19 | 45.99 | 42.46 | |
| No of diseased CV: 2, Nº | 17,643 | 630 | 383 | 208 | 415 | 411 | 1216 | 813 | - |
| % | 25.38 | 24.15 | 22.74 | 24.33 | 23.54 | 25.03 | 24.74 | 23.94 | - |
| No of diseased CV: 1, Nº | 15,278 | 381 | 370 | 170 | 291 | 286 | 811 | 670 | - |
| % | 21.98 | 14.60 | 21.97 | 19.88 | 16.51 | 17.42 | 16.50 | 19.73 | - |
| Hypertension, Nº | 125,398 | 4280 | 2788 | 1233 | 2793 | 2682 | 6313 | 4978 | <0.001 |
| % | 84.70 | 92.62 | 93.75 | 87.08 | 93.35 | 93.42 | 93.02 | 89.66 | |
| Diabetes, Nº | 55,549 | 2000 | 1364 | 574 | 1426 | 1371 | 2950 | 2438 | <0.001 |
| % | 37.52 | 43.28 | 45.86 | 40.54 | 47.66 | 47.75 | 43.47 | 43.91 | |
| Dyslipidemia, Nº | 107,820 | 3526 | 2215 | 1021 | 2207 | 2144 | 5213 | 4083 | <0.001 |
| % | 72.83 | 76.30 | 74.48 | 72.10 | 73.76 | 74.68 | 76.81 | 73.54 | |
| Obesity, Nº | 37,555 | 1064 | 865 | 331 | 786 | 800 | 1587 | 1465 | <0.001 |
| % | 25.37 | 23.03 | 29.09 | 23.38 | 26.27 | 27.86 | 23.38 | 26.39 | |
| History of smoking, Nº | 38,682 | 615 | 356 | 604 | 399 | 658 | 916 | 1276 | <0.001 |
| % | 26.13 | 13.31 | 11.97 | 42.66 | 13.34 | 22.92 | 13.50 | 22.98 | |
| CKD, Nº | 35,588 | 1838 | 1096 | 489 | 1223 | 1486 | 2468 | 1871 | <0.001 |
| % | 24.04 | 39.77 | 36.85 | 34.53 | 40.88 | 51.76 | 36.36 | 33.70 | |
| Previous MI, Nº | 43,699 | 1676 | 986 | 525 | 1078 | 1019 | 2351 | 1931 | <0.001 |
| % | 29.52 | 36.27 | 33.15 | 37.08 | 36.03 | 35.49 | 34.64 | 34.78 | |
| Previous PCI, Nº | 5584 | 273 | 107 | 101 | 175 | 178 | 327 | 290 | <0.001 |
| % | 3.77 | 5.91 | 3.60 | 7.13 | 5.85 | 6.20 | 4.82 | 5.22 | |
| Previous CABG, Nº | 5430 | 331 | 81 | 88 | 165 | 208 | 390 | 229 | <0.001 |
| % | 3.67 | 7.16 | 2.72 | 6.21 | 5.51 | 7.24 | 5.75 | 4.12 | |
| Previous valve replacement, Nº | 670 | 42 | 22 | 14 | 27 | 27 | 52 | 40 | <0.001 |
| % | 0.45 | 0.91 | 0.74 | 0.99 | 0.90 | 0.94 | 0.77 | 0.72 | |
| Chronic heart failure, Nº | 69,503 | 2591 | 1726 | 784 | 1759 | 1647 | 3788 | 2895 | <0.001 |
| % | 46.95 | 56.07 | 58.04 | 55.37 | 58.79 | 57.37 | 55.81 | 52.14 | |
| Previous stroke, Nº | 12,994 | 607 | 377 | 210 | 428 | 407 | 903 | 658 | <0.001 |
| % | 8.78 | 13.14 | 12.68 | 14.83 | 14.31 | 14.18 | 13.31 | 11.85 | |
| Atrial flutter/fibrillation, Nº | 28,114 | 1357 | 862 | 373 | 885 | 831 | 1967 | 1343 | <0.001 |
| % | 18.99 | 29.37 | 28.98 | 26.34 | 29.58 | 28.94 | 28.98 | 24.19 | |
| Cerebrovascular disease, Nº | 12,293 | 627 | 326 | 215 | 374 | 393 | 934 | 760 | <0.001 |
| % | 8.30 | 13.57 | 10.96 | 15.18 | 12.50 | 13.69 | 13.76 | 13.69 | |
| PAD RF stage 1–3, Nº | 8623 | 416 | 175 | 203 | 210 | 297 | 501 | 483 | <0.001 |
| % | 5.82 | 9.00 | 5.88 | 14.34 | 7.02 | 10.34 | 7.38 | 8.70 | |
| PAD RF stage 4–6, Nº | 4295 | 132 | 68 | 93 | 140 | 141 | 222 | 233 | <0.001 |
| % | 2.90 | 2.86 | 2.29 | 6.57 | 4.68 | 4.91 | 3.27 | 4.20 | |
| Metastasis, Nº | 0 | 473 | 402 | 568 | 419 | 202 | 135 | 743 | - |
| % | 0 | 10.24 | 13.52 | 40.11 | 14.00 | 7.04 | 1.99 | 13.38 | - |
| No Cancer | Prostate | Breast | Lung | Colon | Urinary | Skin | Other Cancers | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| In-hospital PCI, Nº | 124,058 | 3769 | 2291 | 1069 | 2284 | 2266 | 5345 | 4405 | <0.001 |
| % | 83.80 | 81.56 | 77.03 | 75.49 | 76.34 | 78.93 | 78.75 | 79.34 | |
| With DES, Nº | 88,571 | 2395 | 1477 | 587 | 1381 | 1455 | 3497 | 2804 | <0.001 |
| % | 59.83 | 51.83 | 49.66 | 41.46 | 46.16 | 50.68 | 51.53 | 50.50 | |
| Only with BMS, Nº | 27,649 | 1072 | 625 | 386 | 710 | 638 | 1449 | 1275 | <0.001 |
| % | 18.68 | 23.20 | 21.02 | 27.26 | 23.73 | 22.22 | 21.35 | 22.97 | |
| In-hospital CABG, Nº | 11,797 | 516 | 175 | 99 | 241 | 277 | 566 | 397 | <0.001 |
| % | 7.97 | 11.17 | 5.88 | 6.99 | 8.06 | 9.65 | 8.34 | 7.15 | |
| Shock, Nº | 20,638 | 678 | 437 | 214 | 443 | 444 | 932 | 852 | 0.006 |
| % | 13.94 | 14.67 | 14.69 | 15.13 | 14.81 | 15.47 | 13.73 | 15.35 | |
| Shock/Resuscitation/ LV-support, Nº | 29,371 | 964 | 605 | 292 | 646 | 619 | 1338 | 1184 | 0.004 |
| % | 19.84 | 20.86 | 20.34 | 20.62 | 21.59 | 21.56 | 19.71 | 21.33 | |
| Death (discharge status index case), Nº | 19,831 | 764 | 520 | 297 | 560 | 498 | 1218 | 972 | <0.001 |
| % | 13.40 | 16.53 | 17.49 | 20.98 | 18.72 | 17.35 | 17.95 | 17.51 | |
| Death within case chain, Nº | 22,048 | 877 | 577 | 346 | 635 | 549 | 1326 | 1101 | <0.001 |
| % | 14.89 | 18.98 | 19.40 | 24.44 | 21.22 | 19.12 | 19.54 | 19.83 | |
| Ischemic stroke, Nº | 1660 | 75 | 49 | 25 | 47 | 40 | 152 | 81 | 0.05 |
| % | 2.39 | 2.87 | 2.91 | 2.92 | 2.67 | 2.44 | 3.09 | 2.39 | |
| Hemorrhagic stroke, Nº | 283 | <10 | <10 | <10 | <10 | <10 | 24 | 10 | 0.676 |
| % | 0.41 | - | - | - | - | - | 0.49 | 0.29 | |
| Impella, Nº | 578 | 18 | <10 | <10 | <10 | <10 | 25 | 18 | 0.829 |
| % | 0.39 | 0.39 | - | - | - | - | 0.37 | 0.32 | |
| IABP, Nº | 5042 | 147 | 73 | 40 | 89 | 98 | 181 | 168 | 0.002 |
| % | 3.41 | 3.18 | 2.46 | 2.83 | 2.98 | 3.41 | 2.67 | 3.03 | |
| ECMO, Nº | 1738 | 44 | 24 | <10 | 23 | 23 | 55 | 55 | 0.001 |
| % | 1.17 | 0.95 | 0.81 | - | 0.77 | 0.80 | 0.81 | 0.99 | |
| GpIIb/IIIa inhibitor, Nº | 36,618 | 1097 | 598 | 244 | 574 | 581 | 1447 | 1176 | <0.001 |
| % | 24.73 | 23.74 | 20.11 | 17.23 | 19.19 | 20.24 | 21.32 | 21.18 | |
| Bleeding, Nº | 12,434 | 399 | 307 | 115 | 344 | 285 | 628 | 517 | <0.001 |
| % | 8.40 | 8.64 | 10.32 | 8.12 | 11.50 | 9.93 | 9.25 | 9.31 | |
| Blood transfusion, Nº | 7237 | 356 | 226 | 125 | 269 | 246 | 593 | 538 | <0.001 |
| % | 10.41 | 13.65 | 13.42 | 14.62 | 15.26 | 14.98 | 12.06 | 15.84 | |
| Bleeding or transfusion, Nº | 11,036 | 497 | 319 | 170 | 372 | 337 | 852 | 733 | <0.001 |
| % | 15.88 | 19.05 | 18.94 | 19.88 | 21.10 | 20.52 | 17.33 | 21.58 | |
| ARF, Nº | 10,713 | 433 | 264 | 147 | 293 | 318 | 542 | 488 | <0.001 |
| % | 7.24 | 9.37 | 8.88 | 10.38 | 9.79 | 11.08 | 7.99 | 8.79 | |
| ARF and/or renal replacement therapy, Nº | 12,347 | 514 | 289 | 161 | 341 | 411 | 640 | 571 | <0.001 |
| % | 8.34 | 11.12 | 9.72 | 11.37 | 11.40 | 14.32 | 9.43 | 10.29 | |
| Renal replacement therapy, Nº | 5459 | 219 | 112 | 58 | 133 | 207 | 244 | 244 | <0.001 |
| % | 3.69 | 4.74 | 3.77 | 4.10 | 4.45 | 7.21 | 3.60 | 4.40 |
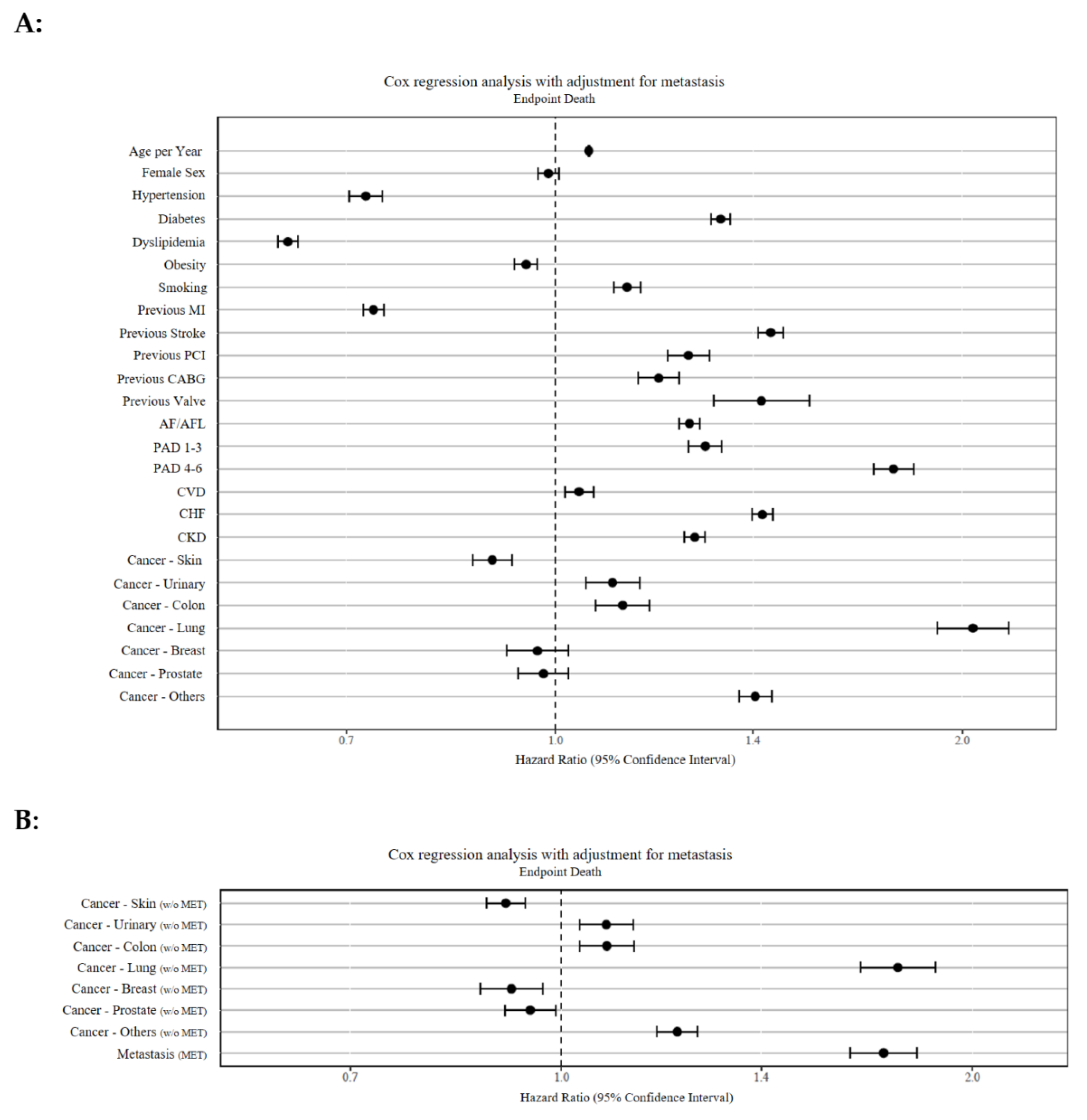
| Co-Morbidities | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Lung cancer | 2.04 | 1.92–2.17 | p < 0.001 |
| PAD (RF stage 4–6) | 1.78 | 1.72–1.84 | p < 0.001 |
| Lung cancer (w/o MET) | 1.76 | 1.65– 1.88 | p < 0.001 |
| Metastasis | 1.72 | 1.63–1.82 | p < 0.001 |
| Previous stroke | 1.44 | 1.31–1.54 | p < 0.001 |
| Chronic heart failure | 1.42 | 1.40–1.45 | p < 0.001 |
| Previous valve surgery | 1.42 | 1.31–1.54 | p < 0.001 |
| Other cancers | 1.41 | 1.37–1.45 | p < 0.001 |
| Diabetes mellitus | 1.33 | 1.30–1.35 | p < 0.001 |
| PAD (RF stage 1–3) | 1.29 | 1.26–1.33 | p < 0.001 |
| Chronic kidney disease | 1.27 | 1.25–1.29 | p < 0.001 |
| AF or AFL | 1.26 | 1.23–1.28 | p < 0.001 |
| Previous PCI | 1.25 | 1.21–1.30 | p < 0.001 |
| Other cancers (w/o MET) | 1.21 | 1.17–1.26 | p < 0.001 |
| Previous CABG | 1.19 | 1.15–1.25 | p < 0.001 |
| Cancer of the urinary tract | 1.10 | 1.05–1.15 | p < 0.001 |
| Smoking | 1.13 | 1.10–1.16 | p < 0.001 |
| Colon cancer | 1.12 | 1.07–1.17 | p < 0.001 |
| Colon cancer (w/o MET) | 1.08 | 1.03–1.13 | p < 0.001 |
| Cancer of the urinary tract (w/o MET) | 1.08 | 1.03–1.13 | p < 0.001 |
| Age | 1.06 | 1.057–1.059 | p < 0.001 |
| Cerebrovascular disease | 1.04 | 1.02–1.07 | p < 0.001 |
| Sex | 0.99 | 0.97–1.01 | p = 0.212 |
| Cancer—Breast | 0.97 | 0.92–1.02 | p = 0.248 |
| Cancer—Prostate | 0.98 | 0.94–1.02 | p = 0.345 |
| Cancer—Prostate (w/o MET) | 0.95 | 0.91–0.99 | p = 0.05 |
| Obesity | 0.95 | 0.93–0.97 | p < 0.001 |
| Cancer—Breast (w/o MET) | 0.92 | 0.87–0.97 | p < 0.01 |
| Cancer of the skin | 0.91 | 0.88–0.94 | p < 0.001 |
| Previous MI | 0.73 | 0.72–0.75 | p < 0.001 |
| Hypertension | 0.72 | 0.70–0.74 | p < 0.001 |
| Dyslipidemia | 0.63 | 0.62–0.65 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, S.A.; Feld, J.; Kühnemund, L.; Köppe, J.; Makowski, L.; Engelbertz, C.M.; Gerß, J.; Dröge, P.; Ruhnke, T.; Günster, C.; et al. Acute and Long-Term Outcomes of ST-Elevation Myocardial Infarction in Cancer Patients, a ‘Real World’ Analysis with 175,000 Patients. Cancers 2021, 13, 6203. https://doi.org/10.3390/cancers13246203
Lange SA, Feld J, Kühnemund L, Köppe J, Makowski L, Engelbertz CM, Gerß J, Dröge P, Ruhnke T, Günster C, et al. Acute and Long-Term Outcomes of ST-Elevation Myocardial Infarction in Cancer Patients, a ‘Real World’ Analysis with 175,000 Patients. Cancers. 2021; 13(24):6203. https://doi.org/10.3390/cancers13246203
Chicago/Turabian StyleLange, Stefan A., Jannik Feld, Leonie Kühnemund, Jeanette Köppe, Lena Makowski, Christiane M. Engelbertz, Joachim Gerß, Patrik Dröge, Thomas Ruhnke, Christian Günster, and et al. 2021. "Acute and Long-Term Outcomes of ST-Elevation Myocardial Infarction in Cancer Patients, a ‘Real World’ Analysis with 175,000 Patients" Cancers 13, no. 24: 6203. https://doi.org/10.3390/cancers13246203
APA StyleLange, S. A., Feld, J., Kühnemund, L., Köppe, J., Makowski, L., Engelbertz, C. M., Gerß, J., Dröge, P., Ruhnke, T., Günster, C., Freisinger, E., & Reinecke, H. (2021). Acute and Long-Term Outcomes of ST-Elevation Myocardial Infarction in Cancer Patients, a ‘Real World’ Analysis with 175,000 Patients. Cancers, 13(24), 6203. https://doi.org/10.3390/cancers13246203






