Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Targets in Pancreatic Ductal Adenocarcinoma (PDAC)
2.1. Target Characteristics for PDAC Imaging
2.2. Overview of PDAC-Associated Molecular Targets for Imaging Purposes
2.2.1. CA19.9
2.2.2. Cathepsin-E
2.2.3. CDCP-1
2.2.4. CEA/CEACAM-5
2.2.5. EGFR
2.2.6. Endoglin
2.2.7. EpCAM
2.2.8. FAP
2.2.9. Fibronectin
2.2.10. GRP78
2.2.11. Integrins
2.2.12. Mesothelin
2.2.13. MT1-MMP/MMP-14
2.2.14. Mucin-1
2.2.15. NTSR1
2.2.16. PSMA
2.2.17. TF
2.2.18. TfR1
2.2.19. uPA/uPAR System
2.2.20. VEGFR/VEGF-A
2.3. The Effect of (Neo)Adjuvant Treatment on Target Expression
2.4. Summary
| Target | Biological Function (Subtype) | Biological Effect Related to Expression by Tumor-(Associated) Cells | Location, Expression on Pancreatic Cell-Type | Target Expression in PDAC (% of +) | Advantages for PDAC Imaging | Disadvantages for PDAC Imaging | Expression Profile (0/−/+/++) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| CA19.9 | Glycan | Cell-to-cell recognition processes | Cell membrane, (neoplastic) pancreatic cells | 70–90% |
|
| NPT: + Pancreatitis: ++ Precursor lesions: −/+ PDAC: ++ | [53,54,55] | |
| Cathepsin-E | Hydrolytic aspartic protease | Regulation of immune response, protein turnover, induction of apoptosis | Intracellular, (neoplastic) pancreatic cells | ~92% |
|
| NPT: 0 Pancreatitis: − Precursor lesions: +/++ PDAC: ++ | [57,59] | |
| CDCP-1 | Glycosylated receptor protein | Cell proliferation, tumor invasiveness, metastasis | Cell membrane, (neoplastic) pancreatic epithelial cells | ~92% |
|
| NPT: 0 Pancreatitis: N/A Precursor lesions: −/+ PDAC: −/+/++ | [145,146] | |
| CEA | Cell Adhesion Molecule | Oncogenic signaling protein, inhibition of apoptosis | Cell membrane, (neoplastic) pancreatic cells | 70–85% |
|
| NPT: 0 Pancreatitis + Precursor lesions: + PDAC: ++ | [44,65,147] | |
| EGFR | Tyrosine kinase Receptor (TKR) | Cell proliferation, metastasis, tumor angiogenesis | Cell membrane, (neoplastic) pancreatic cells | 69–90% |
|
| NPT: − Pancreatitis: N/A Precursor lesions: + PDAC: ++ | [42,44] | |
| Endoglin | Co-receptor for TGF-β | Tumor angiogenesis, tumor growth, metastasis | Cell membrane, (neoplastic) pancreatic vascular endothelial cells | N/A |
|
| NPT: 0 Pancreatitis: N/A Precursor lesions: −/+ PDAC: −/+/++ | [77,78] | |
| EpCAM | Cell Adhesion Molecule | Cell proliferation, metastasis, oncogenic signaling protein | Cell membrane, (neoplastic) pancreatic epithelial cells | 56–78% |
|
| NPT: −/+ Pancreatitis: −/+ Precursor lesions: −/+ PDAC: +/++ | [44,80,148] | |
| FAP-α | Cell membrane associated enzyme | Fibroblast activation, promoting angiogenesis | Cell membrane, Cancer Associated Fibroblasts (CAFs) in stroma | 73–76% |
|
| NPT: − Pancreatitis + Precursor lesions: N/A PDAC: ++ | [82] | |
| Fibronectin (FN) | Component of ECM | Cell proliferation, metastasis, resistance to chemotherapy | Cell membrane, pancreatic fibroblastic cells and CAFs | ~85% |
|
| NPT: 0 Pancreatitis: N/A Precursor lesions: N/A PDAC: ++ | [86,87] | |
| GRP78 | Neoplastic cells: Co-Receptor for various proteins Normal cells: Chaperone protein localized in ER | Cell-to-cell and cell-to-matrix recognition processes, induction of endoplasmic reticulum stress for cell aging, survival, metastasis | Cell membrane, pancreatic neoplastic cells (in non-tumor cells located in ER) | N/A |
|
| NPT: − Pancreatitis: N/A Precursor lesions: − PDAC: ++ | [90,149] | |
| Integrin αvβ3 | Cell Adhesion Molecule | Tumor angiogenesis, tumor growth, metastasis | Cell membrane, (neoplastic) stromal and endothelial pancreatic cells | ~68% |
|
| NPT: − to −/+ Pancreatitis −/+ Precursor lesions: −/+ PDAC: +/++ | [93,97] | |
| Integrin αvβ6 | Cell Adhesion Molecule | Tumor growth, metastasis | Cell membrane, (neoplastic) epithelial cells | 80–88% |
|
| NPT: −/+ Pancreatitis −/+ Precursor lesions: −/+ PDAC: ++ | [44,96,98] | |
| Mesothelin | GPI-anchored protein (Adhesion molecule) | Cell proliferation, migration, metastasis, inhibition of apoptosis | Cell membrane of pancreatic (neoplastic) mesothelial cells | >90% |
|
| NPT: 0 Pancreatitis: 0 Precursor lesions: 0/− PDAC: ++ | [102,103] | |
| MT1-MMP/MMP-14 | Cell membrane associated enzyme | Tumor growth, invasiveness, resistance to chemotherapy | Cell membrane, (neoplastic) pancreatic stromal cells | ~75% |
|
| NPT: − Pancreatitis: −/+ Precursor lesions: −/+ PDAC: ++ | [108,150] | |
| Mucin-1 | Protective cell coating | Cell proliferation, tumor invasiveness due to upregulated cell motility, metastasis | Cell membrane, (neoplastic) pancreatic epithelial cells | ~90% |
|
| NPT: − Pancreatitis: N/A Precursor lesions: −/+, + PDAC: ++ | [112,151] | |
| NTSR1 | G-protein-coupled Receptor (GPCR) | Cell proliferation, inhibition of apoptosis. | Cell membrane, (neoplastic) pancreatic cells | 79–88% |
|
| NPT: − Pancreatitis: + Precursor lesions: N/A PDAC: ++ | [114,115] | |
| PSMA | Cell membrane associated enzyme | Tumor angiogenesis | Cell membrane, neovascular associated cells and tumor cells | ~68% |
|
| NPT: 0 Pancreatitis: 0 Precursor lesions: N/A PDAC: +/++ | [119,120] | |
| Tissue Factor (TF) | Cytokine-receptor | Initiating blood coagulation cascades, metastasis | Cell membrane, (neoplastic) pancreatic cells | 50–90% |
|
| NPT: − Pancreatitis −/+ Precursor lesions: +/++ PDAC: ++ | [127,128,129] | |
| TfR1 | Ion-channel coupled Receptor | Cell proliferation, regulation of iron uptake/release. | Cell membrane, (neoplastic) pancreatic cells | >90% |
|
| NPT: 0 Pancreatitis: N/A Precursor lesions: N/A PDAC: ++ | [135,136] | |
| uPAR/uPA system | GPI-anchored receptor | Degradation of ECM, tumor angiogenesis, metastasis | Cell membrane, stromal (neoplastic) cells Cell membrane, endothelial (neoplastic) pancreatic cells | ~80% ~67% |
|
| NPT: − Pancreatitis: N/A Precursor lesions: + PDAC: +/++ | [44,138,140] | |
| VEGFR-2/ VEGF-A | Tyrosine kinase Receptor (TKR) Growth factor | Tumor angiogenesis | Cell membrane, pancreatic vascular endothelial cells | >70% |
|
| NPT: − Pancreatitis: − Precursor lesions: N/A PDAC: ++ | [143,152] |
3. Positron Emission Tomography—Computed Tomography (PET/CT)
3.1. Primary Diagnostic Work-Up and Monitoring Response to Neoadjuvant Treatment
3.2. Clinically Available PET-Tracers for PDAC Imaging
3.2.1. 18F-FDG
3.2.2. 18F-Fluorothymidine (FLT)
3.2.3. 18F-Fluoromisonidazole (FMISO) and 18F-Fluoroazomycin Arabinoside (FAZA)
3.3. PDAC-Targeted PET-Tracers in Clinical Early Clinical Trials
3.3.1. CA19.9
3.3.2. Fibroblast Activating Protein (FAP)
3.3.3. Integrin αvβ6
3.3.4. PSMA
3.4. Preclinical Evaluation and Development of PDAC-Targeted PET-Tracers
3.5. Summary
4. NIR-Fluorescence Imaging and Fluorescence-Guided Surgery
4.1. Clinically Tested PDAC Targeted NIRF-Tracers
4.1.1. CEA
4.1.2. EGFR
4.1.3. VEGF-A
4.2. Preclinical Evaluation and Development of PDAC-Targeted NIRF-Tracers
4.3. Summary
5. Future Perspectives: Application of Improved Targeting Strategies, Technological Advances, and a Theranostic Approach
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblast |
| CAM | Cell adhesion molecule |
| Cath-E | Cathepsin-E |
| CA19.9 | Carbohydrate antigen 19.9 |
| CDCP1 CUB | domain-containing protein1 |
| Ce-CT | Contrast-enhanced Computed Tomography |
| CEA | Carcinoembryonic antigen |
| EGFR | Epidermoid growth factor receptor |
| EpCAM | Epithelial cell adhesion molecule |
| ESMO | European Society of Medical Oncology |
| FAP | Fibroblast-activating protein |
| FAPI | Fibroblast-activating protein inhibitor |
| FAZA | Fluoroazomycin arabinoside |
| FDG | Fluorodeoxyglucose |
| FGS | Fluorescence-guided surgery |
| FLT | Fluorothymidine |
| FMISO | Fluoromisonidazole |
| GRP78 | Glucose-regulating protein-78 |
| LAPC | Locally-advanced pancreatic cancer |
| mAb | Monoclonal antibody |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| NCCN | National Comprehensive Cancer Network |
| NET | Neuro endocrine tumor |
| NIR | Near infrared |
| NIRF | Near infrared fluorescence |
| NPT | Normal pancreatic tissue |
| NT | Neoadjuvant therapy |
| NTS | Neurotensin |
| NTSR-1 | Neurotensin receptor-1 |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| PSMA | Prostate membrane antigen |
| PET | Positron emission tomography |
| scFv | Single-chain variable fragment |
| SPECT | Single-photon emission computed tomography |
| SMI | Small molecule inhibitor |
| SUV | Standardized uptake value |
| TfR1 | Transferrin receptor-1 |
| TBR | Tumor-to-background ratio |
| TF | Tissue Factor |
| uPA | Urokinase-type plasminogen activator |
| uPAR | Urokinase-type plasminogen activator Receptor |
| VEGFR | Vascular endothelial growth factor receptor |
| VEGF-A | Vascular endothelial growth factor A |
| WLI | White light inspection |
Appendix A
| Target | Tracer | Type | Modality (Control) | Study Design | Number of Patients | Infusion- Imaging Window | Main Outcome (Time-Point) | Results | Highlights | Main Elimination Route | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA19.9 | 89Zr-DFO-Hu Mab-5B1 | mAb, fully human | PET/CT (ce-CT) | Prospective (Phase II) | 12 patients with local ized PDAC (LAPC) | After 24 h, 3 d, 4 d, 7 d | SUVmax (SD) | PDAC 24h: 3.51 (±2.58) PDAC Day 3: 9.3 (±14.3) PDAC Day 4: 12.6 (±14.5) PDAC Day 7: 16.5 (±17.3) | First in-human clinical study, high target affinity of CA19.9+ (PDAC) tumors. Location of tracer uptake on the imaging studies should allow for differentiation of PDAC and other malignan cies. Limitation: No histopatho logical confirmation of LN, Ideal injection-imaging win dow long (7 days), disad vantage for screening and early detection of PDAC, relatively high radiation ex posure. | Hepatic system | [165] | |
| In vivo Tumor-to-background ratio (SD) | 18.4 (±1) 12/12 patients, at least one additional suspect metastatic lesion was identified. | |||||||||||
| CEA | SGM-101-BM-104 | mAb, chimiric | NIRF- 700 nm (−) | Prospective (Phase II) | 12 Resectable PDAC patients (5, 7.5, 10 mg) | After 48 h | Identified pri mary tumors with NIRF | 11/11 (100%), one surgical procedure abandoned before imaging | Proof-of-concept and safety targeting CEA with SMG101 for NIR-imaging of PDAC, metastatic lymph nodes, and distant metasta sis. | Hepatic system | [68] | |
| In vivo Tumor-to-background ratio (SD) | 1.6 (±0.37) | |||||||||||
| In vivo Metas tasis-to-back ground ratio (SD) | 1.7 (±0.42) | |||||||||||
| EGFR | panitumumab-IRDye800CW | mAb, chimeric | NIRF- 800 nm (−) | Prospective (Phase I) | 11 PDAC patients | After 2–5 days | Tumor-to-background ratio per dosage (SD) | 25 mg: 3.0 (±0.5) 50 mg: 4.0 (±0.6) 75 mg: 3.7 (±0.4) | Proof-of-concept and safety of targeting EGFR with 50 mg panitumumab-IRDye800CW is best suita ble for NIR-imaging of PDAC, metastatic lymph nodes, and distant metasta sis. | Hepatic system | [187] | |
| Sensitivity Specificity (95% CI) | 90.3% (84.5–94.2) 74.5% (65.1–82.1) | |||||||||||
| Ex vivo Differ entiating tumor from normal pancreatic parenchyma | Fluorescence signal delineat ing tumor correlated with his topathology in all cases MFI and the tumor-to-back ground ratio of the +LN were significantly higher than those of -LN (p < 0.001) | |||||||||||
| cetuximab-IRDye800CW, monoclonal antibody | mAb, chimeric | NIRF- 800 nm (−) | Prospective (Phase I/II) | 7 Pancreatic tumors (5 PDAC, 2 NET) | After 2–5 days | NIRF Identifi cation of primary tumor | 4/6 patients (67%) | Proof-of-concept and safety of targeting EGFR with 50 mg cetuximab-IRDye800CW is best suita ble for NIR-imaging of pan creatic tumors, metastatic lymph nodes, and distant metastasis. Potential to dif ferentiate pancreatitis and PDAC. | Hepatic system | [185,186] | ||
| In vivo Tumor-to-background ratio (50 mg) | Primary Tumor: 2.3 (±0.72) Tumor+ LN: 6.3 (±0.82) | |||||||||||
| Ex vivo Tumor-to-back ground ratio (50 mg) | Primary Tumor: 3.4 (±0.4) | |||||||||||
| Sensitivity Specificity (95% CI) | 96.1% (92.2–98.4%) 67.0% (59.7–73.8%) | |||||||||||
| FAP | 68Ga-FAPI-04 | SMI | PET/CT (−) | Prospective, retrospectively analyzed (Phase II) | 51 PDAC patients | After 1 h | SUVmax | PDAC: 6–12 (range) Blood pool: 1.4 Muscle: 1.0 | High FAPI uptake in FAP+ PDAC. Low background healthy tissues, including liver, resulting in moderate/high TBR’s in PDAC. Due to fast tracer uptake and clearance, optimal win dow for imaging: 10 min−1 h after injection, results in re duced radiation doses. | Renal System | [83] | |
| 68Ga-FAPI-04, 68Ga-FAPI-46 | SMI | PET/CT (ce-CT) | Prospective, retrospect-tive analysis (Phase II) | 19 PDAC patients 7 primary; 12 progres-sive or recurrent disease | After 1 h | SUVmax (SD) | Pancreatitis: 7.50 (±3.52) PDAC: 13.37 (±5.45) Metastatic Lymph nodes: 14.13 (±8.50) Distant metastases: 7.34 (±2.48) Blood pool: 2.3 (8.31) Muscle: 2.4 (8.72) | High FAPI uptake in pri mary FAP+ PDAC, lymph nodes, distant metastases. Low background healthy tissues, including liver, re sulting in adequate TBR’s for PDAC. Differentiation with pancreatitis challeng ing. Clinical value should be ad dressed separately in ho mogenous group for pri mary diagnosis and ability of response monitoring. | Renal System | [167] | ||
| Restaging in 10 out of 19 patients compared to ceCT | ||||||||||||
| SUVmax (Tumor-to-background ratio) | Low hepatic background (SUV 1.7, compared to FDG-PET/CT (SUV 2.8) | |||||||||||
| Integrin αvβ6 | 18F-FP-R01-MG-F2 | Peptide, cyclic | PET/CT (ce-CT) | Prospective (Phase I) | 14 PDAC patients | After 1 h | SUVmax (categories) | PDAC: high Pancreas: moderate/high | Proof-of-concept and safety for PET-imaging with high specific affinity for αvβ6+ PDAC and metastatic le sions. | Renal System | [171] * | |
| Detection of known PDAC lesions | 14/14 (100%) | |||||||||||
| 18F-FP-R01-MG-F | Peptide, cyclic | PET/CT (FDG-PET/CT) | Prospective pre-clinical/clinical study (Phase I/II) | 10 Healthy volunteers 1 PDAC patient | After 1 h | Healthy volun teers: SUVmean | Liver: <1 Pancreas: 2 Muscle: 1.5 Stomach: 10 Small Intestines: 9 | Proof-of-concept and safety for αvβ6+ targeted PET/CT-imaging in healthy volunteers and 1 PDAC patient. Compared to FDG-PET/CT lower SUVmean in surrounding/adjacent structures of the pancreas, except the stomach. Clinical evaluation in larger cohort of PDAC patients warranted (Nakamoto et al. above) Primary diagnosis as well as response monitoring could be evaluated. | Renal System | [170] | ||
| After 1 h | PDAC patient SUVmean | αvβ6-PET/CT Liver: <1 PDAC: 6.2 Muscle: 1.8 Stomach: 22 Small Intestines: 9 | FDG/PET/CT Liver: 2.9 PDAC: 4.1 Muscle: <1 Stomach: <1 | |||||||||
| MSLN | 89Zr-MMOT0530A | mAb, humanized | PET/CT (ce-CT) | Prospective (Phase I/II) | 11 patients total 7 PDAC 4 Ovarian | After 2, 4, and 7 days | SUVmax (SD) (Day 4) | PDAC: 11.5 (±5.6) Metastases: 12.1 (±6.0) Muscle: 2.4 (±1.3) | MSLN was able to visualize PDAC, although high varia bility between SUVmax in PDAC patients. Resulting in relatively poor TBR’s. Optimal injection-imaging window 4 days. High up take in liver, possibly inter fering with surgical field of view. Potentially interesting to evaluate/predict response to MSLN targeted therapy | Hepatic system | [214] | |
| Tumor-to-back ground ratio | Day 1: 0.70 Day 4: 1.1 Day 7: 1.28 | |||||||||||
| 2 MSLN+ lung nodules missed on MSLN-PET/CT which were seen on ce-CT | ||||||||||||
| NTSR1 | 68Ga-DOTA-NT-20.3 | Peptide, linear | PET/CT (−) | Prospective (Phase I) | 3 patients localized or metastatic PDAC | After 5–25 min, 25–45 min, 45–65 min, and 65–85 min | Uptake in primary tu mors, meta static disease | Primary tumor: 3/3 patients Metastases: 2/2 patients | Proof-of-concept for safety and tolerability of 68Ga-DOTA-NT-20.3 in patients with proven localized or metastatic PDAC. Uptake of NT-20.3 uptake in all PDAC and in 2/3 patients with liver metastases. | Renal System | [118] | |
| PSMA | 68Ga-PSMA-11 | Peptide, linear | PET/CT (FDG-PET/CT) | Prospective (Phase II) | 19 PDAC 21 Benign pancreatic lesions | After 1 h | SUVmax (IQR) | PSMA- PET/CT: | FDG- PET/CT: | PSMA-PET/CT out-performed FDG-PET/CT in primary diagnosis of PSMA+ PDAC lesions. Lesions with inflammatory origin were not visualized with PSMA-PET/CT, in contrast to FDG-PET/CT (false positive). Promising results for PSMA-PET/CT, further evaluation in a multicenter study will be able to substantiate the diagnostic value of PSMA-PET/CT in the primary diagnosis of PDAC. | Renal System | [124] |
| Benign: 3.9 (3.4) Malignant: 7.6 (10.8) | Benign: 3.5 (1.5) Malignant: 7.4 (4.5) | |||||||||||
| Sensitivity Specificity PPV NPV Accuracy | 94.7% 90.5% 90% 95% 92.5% | 89.5% 57.1% 65.4% 87.5% 72.5% | ||||||||||
| VEGF-A | Bevacizu-mabIRDye 800CW, monoclonal antibody | mAb, chime-ric | NIRF- 800 nm (−) | Prospective (Phase II) | 10 suspected pancreatic tumors (PDAC, NET, Periampul-lary, IPMN) | After 3 days | Detailed results have not yet been published | Bevacizumab-800CW was safe without adverse events related to the study drug although due to residual in different fluorescent signals in non-tumoral tissue after complete tumor resection in the majority of included pa tients, this study was termi nated early. Feasibility for further clini cal translation of VEGF-tar geted FGS of PDAC is based on these results not proven. | Hepatic system | Eudra-CT 2015-004247-39 | ||
| Target | Tracer | Type | Modality | Design | Subjects (Cell Line) | Infusion-Imaging Window | Main Outcome | Results | Highlights | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| CA19.9 | Alexa Fluor 488-anti-CA19.9 | mAb, hu manized | Fluorescence imaging- 500 nm | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Orthotopic PDAC mouse model (CFPAC, BxPC-3, PANC-1) | After 24 h | Ability to visualize CA19.9+ PDAC after 24 h | Small tumors were virtually uni dentifiable under standard bright-field imaging but were clearly visible using fluorescence imaging. Administration of AlexaFluor 488-anti-CA19.9 facilitated visualization of experimental metastatic implants in the spleen, liver, and peritoneum at laparotomy. All metastatic lesions in the spleen, liver, and peritoneum were confirmed by histologic evaluation following whole-body imaging. | Proof-of-concept of in vivo fluorescence imaging of CA19.9+ PDAC with fluorescence imaging. Low expression/fluorescence on surrounding stromal tissue. Low background due to low CA19.9 expression in normal tissue. Additional evaluation is war ranted to address imaging characteristics and validate fluorescence signals. | [215] |
| 124I-anti-CA19-9 | Diabody | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC3: CA19.9+ Capan-2: CA19.9+ MiaPaCa-2: Ca19.9−) | After 4 h and 20 h | Tumor-to-background ratio (blood pool) | All cell lines: 3.0 BxPC3: 5.0 Capan-2: 2.0 BxPC3: 11.0 | Proof-of-concept PET/CT-imaging of CA19.9+ PDAC. The cys-diabody demonstrates target-specific binding of human pancreatic cancer cells allowing tumor visualization, and with the potential to deliver targeted treatment. Relative high uptake in the liver, which could interfere with imaging of the pancreas. | [216] | |
| Positive-to-negative tumor ratio | Capan-2: 6.0 BxPC3 (tumor+): 1.1 (0.5–1.8) | |||||||||
| Tumor uptake/biodistribution in % of injected dosage/g BW (range) | BxPC3 (tumor−): 0.1 (0.03–0.2) Capan-2 (tumor+): 0.5 (0.3–0.1) Capan-2 (tumor−): 0.1 (0.06–0.1) | |||||||||
| 124I-anti-CA19-9 | Cysteine-modified diabody | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC3: CA19.9+ MiaPaCa-2: Ca19.9-) | After 4 h and 20 h | Tumor-to-blood ratio | BxPC3: 2.7 BxPC3: 6.0 | Proof-of-concept PET/CT-imaging of CA19.9+ PDAC. High target binding affinity for CA19.9 allowing tumor visualization. Shorter half-life-time than diabody in comparison with study from Girgis et al. above. | [217] | |
| Positive-to-negative tumor ratio | BxPC3 (tumor+): 1.1 (0.4–1.7) | |||||||||
| Tumor uptake/biodistribution in % of injected dosage/g BW (range) | BxPC3 (tumor−): 0.2 (0.1–0.3) | |||||||||
| 89Zr(ss)DFO-5B1 | mAb, fully hu man | PET/CT, NIRF- 800 nm (dual-labeled) | In vitro/In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC3: CA19.9+ MiaPaca-2: Ca19.9−) | After 48 h and 120 h (PET/CT) | Tumor uptake/biodistribution in % of injected dosage/g BW (After 48 h; after 120 h) | DFO-5B1 BxPC3 (tumor+): 32; 40 MiaPaCa-2 (control): 8; 7 | Proof-of-concept with combined PET/NIRF imaging of CA19.9+ PDAC lesions with 89Zr-ssdual-5B1 to delineate the pancreatic tumor, distant metastases and positive lymph nodes using PET/CT and NIRF imaging. Dual-labeled imaging of CA19.9 with 89Zr(ss)dual-5B1 could serve as a guide for the staging, treatment planning, and resection of PDAC | [166] | |
| Dual-5B1 BxPC3 (tumor+): 36; 45 MiaPaCa-2 (control): 5; 4 | ||||||||||
| 89Zr(ss)FL-5B1 | ||||||||||
| Subcutaneous PDAC mouse model (SUIT-2) | After 120 h (single and dual-labeled) | Feasibility of in vivo NIRF-guided resection of tumor, metastases, and suspected lymph nodes | With NIRF-imaging, the tumor, (micro)metastases, and lymph nodes were clearly visible, due to extensive disease no complete resection could be achieved. | |||||||
| 89Zr(ss)dual-5B1 | ||||||||||
| Cathep-sin-E | Ala-Gly-Phe-Ser-Leu-Pro-Ala-Gly-Cys-CONH2-Cy5.5 | Peptide, linear | NIRF- 700 nm | In vitro/In vivo Preclinical activatable probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (MPanc96-E, CTSE+) | After 24 h, 48 h, 72 h | In vivo Tumor-to-background (SD) | 24 h: ±2 48 h: ±2.5 72 h: ±3 | Proof-of-concept of an activable NIRF-probe targeting cathepsin-E. Cathepsin-E+ PDAC showed fluorescent signals with subsequent TBR’s (>2) from 24–72 h post-injection. | [60] |
| Ex vivo Tumor-to-muscle | 16 | |||||||||
| Ala-Gly-Phe-Ser-Leu-Pro-Ala-Gly-Cys-CONH2-Cy5.5 | Peptide, linear | NIRF- 700 nm | In vitro/Ex vivo Preclinical activatable probe construction and target expression in mouse model | Orthotopic PDAC mouse model (MDA PATC-3, MPanc96-E) | After 48 h | Ex vivo Tumor-to-muscle | 5.5 | Proof-of-concept of an activable NIRF-probe targeting cathepsin-E. Cathepsin-E+ PDAC. Only Ex vivo quantification has been carried out. Abd-Elgaliel et al. [60] performed additional analysis of the same tracer/probe combination. Adequate signals with TBR’s (>2) from 24–72 h post-injection. | [58] | |
| CDCP-1 | 89Zr-DFO-4A06 | mAb, hu manized | PET/CT | In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (HPAC, HPAF II, Capan-1, Panc10.05, Panc2.03) | After 72 h | Tumor uptake/biodistribution in % of injected dosage/g BW (SD) | HPAC: 15.21 (±2.2) HPAF II: 7.78 (±4.8) Capan-1: 6.81 (±1.2) Panc10.05: 6.09 (±0.5) Panc2.03: 5.25 (±1.2) | Proof-of-concept, for in vivo PET/CT-imaging of CDCP-1+ PDAC in mice. | [63] |
| Biodistribution in % of injected dosage/g BW (range) | Blood: 2–2.5 Muscle: 0.75–1.0 | |||||||||
| 89Zr-DFO- 10D7 | mAb, mouse | PET/CT | In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous/Orthotopic PDAC mouse model (TKCC05) | After 24 h, 48 h, 72 h, 144 h | Tumor uptake/biodistribution in % of injected dosage/g BW (after 24 h) | Subcutaneous: PDAC: 53.0 Liver: 20.0 Spleen: 18.0 | Proof-of-concept, for in vivo PET/CT-imaging of CDCP-1+ PDAC in mice. | [146] | |
| Biodistribution in % of injected dosage/g BW (range) | Blood: 2–2.5 Muscle: 0.75–1.0 | |||||||||
| CEA | 124I- anti-CEA scFv-Fc(H310A) | Single-chain variable fragment (scFv-Fc) | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (Capan-1, HPAF-II, and BxPC3) | After 4 h and 20 h | Tumor-to-background ratio (blood pool) | All cell-lines: 4.0 CEA+ Capan-1: 3.7 CEA+ HPAF-II: 3.2 CEA+ BxPC3:5.2 | Proof-of-concept of in vivo PET/CT-imaging with a targeted anti-CEA-probe, high specific target binding. | [183] |
| Alexa Fluor 488-anti-CEA | mAb, hu manized | Fluorescence imaging- 500 nm | In vivo Preclinical target validation in mouse model | Subcutaneous PDAC mouse model (ASPC-1, BxPC-3, CFPAC, Panc-1, and Capan-1) | After 30 min, 1 h, 2 h, 6 h, 8 h, 24 h, 48 h, 192 h, 360 h | Ability to visualize orthotopic CEA+ pancreatic tumors after 24 h | In vivo fluorescence-imaging re vealed very small pancreatic tu mors which were difficult to visualize using standard brightfield illumination, furthermore extent of tumor invasion could be assessed. | Proof-of-concept of in vivo fluorescence imaging of CEA+ PDAC. Low background due to low CEA expression in normal parenchyma | [218] | |
| Orthotopic PDAC mouse model (BxPC-3) | ||||||||||
| Alexa Fluor 488-anti-CEA | mAb, hu manized | Fluorescence imaging- 500 nm | In vivo Preclinical CEA+ fluorescence-guided surgery in mouse model | Orthotopic PDAC mouse model (BxPC-3) | After 24 h | Ability to achieve complete resection compared to bright-light-surgery (BLS) | NIRF: 92% (23/25) BLS: 45.5% (10/22) | Proof-of-concept for fluorescence-guided surgery of PDAC, improving complete resection rate and 1-year survival. | [71] | |
| 1-year survival (proportion) | NIRF: 0% (0/22)BLS: 28% (7/25) | |||||||||
| hM5A-IR800 | mAb, hu manized | NIRF- 800 nm | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Orthotopic PDAC mouse model (BxPC-3) | After 6 h, 12 h, 24 h, 48 h, and 72 h | Tumor-to-background ratio (at all time points) | >5.0 | Proof-of-concept of in vivo NIRF-imaging of humanized antibody targeting CEA+ PDAC in mice, optimal window after 48 h. Low background fluorescence due to low CEA expression in normal parenchyma. Except for the liver parenchyma, possibly interfering with identification of CEA-positive primary liver or metastatic lesions. | [66] | |
| Maximum tumor-to-background ratio (at 48 h) | 16.6 | |||||||||
| EGFR | 64Cu-panitumumab-F(ab’)2 | Antibody fragment F(ab’)2 | PET/CT | In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (PANC-1, OCIP23) | After 24 h, 48 h, 72 h | Tumor uptake/biodistribution in % (SD) of injected dosage/g BW (after 24 h-72 h) | PANC-1: Subcutaneous: 11.8 (±0.9) OCIP23: Subcutaneous: 12.0 (±0.9) | Proof-of-concept, high target binding affinity using pani tumumab-F(ab′)2 fragments for EGFR+ PDAC allowing tumor visualization during in vivo imaging. | [173] |
| PDAC orthotopic tumor bearing mice (OCIP23) | OCIP23 Orthotopic: 6.1 (±1.1) Blood: 2.6 (±0.17) Muscle: 0.3 (±0.02) | |||||||||
| EGFR/ VEGF165 | Bi50-IRdye800 | Diabody | NIRF- 800 nm | In vitro/In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC-3) | After 8 h | Tumor-to-background Ratio (SD) | 4.32 (±0.1) | Proof-of-concept, simultane ous excellent target binding capacity to VEGFR and EGFR. Clear delineation of tumor and healthy tissue. Targeting tumor vasculature-rich areas (overexpression of VEGFR), as well as largely bonded the tumor parenchymal cells (EGFR overexpression) | [196] |
| FAP | 18F-FAPI-74 177Lu-FAPI-46 225Ac-FAPI-46 | SMI SMI SMI | PET/CT | In vitro/In vivo preclinical probe construction and target validation in mouse model In vivo preclinical targeted α-emitter therapy efficacy and monitoring validation in mouse model | Subcutaneous PDAC mouse model (PANC-1) | After 1 h, | SUVmean | Tumor: 0.24 (±0.04) Muscle: 0.05 (±0.01) Kidneys: 0.39 (±0.07) | Proof-of-concept, demonstrating the effectiveness of FAP-targeted PET-imaging and therapy in xenograft PDAC mouse model, observing rapid clearance from healthy tissue and high uptake in the tumors 3 h after injection. Tumor-suppressive effects were observed in both PANC-1 xenograft mice treated with [177Lu]FAPI-46 as well as 225Ac-FAPI-46, although 177Lu-FAPI-46 showed a mild but more prolonged therapeutic effects compared to 225Ac-FAPI-46. | [169] |
| After 3 h, 24 h | Biodistribution in % of injected dosage/g BW | 177Lu-FAPI-46 Tumor: 0.36; 0.10 Blood: 0.08; 0.01 | ||||||||
| After 40 d | Therapy effect as relative ratio of tumor size | 3 MBq: 0.62 10 MBq: 0.56 30 MBq: 0.27 | ||||||||
| After 3 h, 24 h | Biodistribution in % of injected dosage/g BW | 225Ac-FAPI-46 Tumor: 0.30; 0.10 Blood: 0.07; 0.01 | ||||||||
| After 30 d | Tumor-suppressive-effect versus control | 3 MBq: mild 10 MBq: strong (p = 0.05) 30 MBq: strong (p = 0.05) | ||||||||
| Fibronec-tin | 68Ga-NOTA-ZD2 | Peptide, linear | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC-3, Capan-1) | After 1 h | Tumor-to-muscle ratio (mouse-1/mouse-2) | BxPC-3: 5.4/5.6 Pacan-1: 10.0/11.0 | Proof-of-concept, for in vivo PET/CT-imaging of Fibronectin+ PDAC in mice. ZD2-(68Ga-NOTA) is able to clearly delineate the PDAC with a size of 10 mm or less with minimal background noise in normal tissue, including the liver. | [219] |
| BxPC-3: Biodistribution in % of injected dosage/g BW (range) | Tumor: 0.24 Liver: 0.15 Muscle: 0.05 | |||||||||
| Pacan-1: Biodistribution in % of injected dosage/g BW | Tumor: 0.32 Liver: 0.15 Muscle: 0.05 | |||||||||
| 64Cu-NJB2 | Nano-body | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Orthotopic PDAC mouse (BxPC-3, Capan-1) | After 2 h | Tumor-to-muscle ratio | PDAC: 10.0 LN+: 23.0 Liver metastases: 12.0 Muscle: 1.0 Liver: 7.0 | Proof-of-concept in a small cohort of mice with orthotopic PDAC, high-affinity target binding of fibronectin using nanobodies, NJB2, allowing for visualization of primary tumor, metastatic lymph nodes and liver metastasis. | [220] | |
| GRP-78 | 64Cu-DOTA-MAb159 | mAb, mouse | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC3) | After 1 h, 17 h, 48 h | Tumor uptake/biodistribution in % of injected dosage/g BW | 1 h: 4.3 (±1.2) 17 h: 15.4 (±2.6) 48 h: 18.3 (±1.0) | Proof-of-concept, high target binding affinity for GRP78+ PDAC, allowing tumor visual ization with targeted PET/CT-imaging. | [92] |
| Tumor-to-muscle ratio (SD) | 1 h: 1.40 (±0.30) 17 h: 7.4 (±4.6) 48 h: 11.5 (±7.2) | |||||||||
| Integrin αvβ3 | 68Ga-NODAGA-RGD | Peptide, linear | PET/CT | In vivo Preclinical feasibility and target validation in mouse model | Genetically engineered Orthotopic PDAC mouse model (Ptf1a+/Cre;Kras+/LSL-G12D;p53LoxP/LoxP) | After 75 min | Tumor uptake/biodistribution in % of injected dosage/g BW | PDAC: 5.9 Blood: 0.7 Muscle: 0.4 | Proof-of-concept, in vivo PET/CT-imaging with high target affinity for αvβ3+ PDAC lesions. | [172] |
| Tumor-to-muscle ratio | 14.8 | |||||||||
| Integrin αvβ3/αvβ5/αvβ6 | cRGD-ZW800-1 | Peptide, cyclic | NIRF- 800 nm | In vivo Preclinical feasibility and target validation in mouse model | Orthotopic PDAC mouse model (BxPC-3) | After 4 h | Tumor-to-background Ratio | PDAC (dose 0.1 nmol): 3.0 PDAC (dose 10 nmol): 3.4 PDAC (dose 30 nmol): 4.0 | Proof-of-concept, NIRF-imaging. Clear visualization of PDAC between 2 and 24 h post injection, non-selectively targeting integrins. | [190] |
| Integrin αvβ6 | R01-MG-IRDye800 | Peptide, cysteine knotted | NIRF- 800 nm | In vivo Preclinical feasibility and target validation in mouse model | Orthotopic PDAC mouse model (BxPC-3, MiaPaCa-2) | After 30 min-24 h | Tumor-to-Background Ratio (SD) | BxPC-3: 2.5 (±0.1) MiaPaCa-2: 1.2 (±0.1) Pdx1-Cretg/+; KRasLSL G12D/+; Ink4a/Arf−/−): 3.6 (±0.94) | Proof-of-concept, NIRF-imaging. High specific affinity for αvβ6, Fluorescent signal and tumor status corresponded well to αvβ6 expression as assessed by IHC. Renal clearance. Suitable for clinical validation in αvβ6+ PDAC. | [192] |
| Orthotopic PDAC transgenic mice (Pdx1-Cretg/+; KRasLSL G12D/+; Ink4a/Arf−/−) | ||||||||||
| 68Ga-DOTA-SFLAP3 | Peptide, cyclic | PET/CT | In vitro Preclinical probe construction and target validation in mouse model | Orthotopic PDAC mouse model (Capan-2) | N/A | N/A | N/A | High specific binding affinity to integrin αvβ6 on pancreatic cancer cell lines. No further data available. | [221]* Only abstract available | |
| 68Ga-cycratide | Peptide, cyclic | PET/CT | Combined pre-clinical probe construction and target validation/Experimental clinical study phase I | 2 PDAC patients Orthotopic PDAC mouse model (BxPC-3) | After 30 min | SUVmax | Patient 1: (diagnosis/staging): 4.86, histological confirmation of PDAC. Patient 2: (FU 7 m after surgery and ChemoTx): 1.6, no relapse, inflammatory response. | Proof-of-concept for 68Ga-cycratide as effective and selective αvβ6 targeting PET-probe and low-background signal with exclusive renal clearance. Although the clinical part of the study had a small sample size, further evaluation in a clinical setting is needed for the potential of 68Ga-cycratide imaging. | [95] | |
| After 2 h | Tumor uptake/biodistribution in % of injected dosage/g BW (SD) | 2.15 (±0.46) | ||||||||
| After 30 min | Tumor-to-muscle ratio (SD) | 4.77 (±1.62) | ||||||||
| MT1-MMP/MMP-14 | 89Zr-DFO-LEM2/15 68Ga-DOTA-AF7p | mAb, mouse Peptide, linear | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model, (Capan-2) | After 5 d, 7 days | Tumor-to-background (SD) of 89Zr-DFO-LEM2/15 | 5 days: 1.13 (±0.51) 7 days: 1.44 (±0.43) | Proof-of-concept of in vivo PET/CT-imaging of MT1-MMP/MMP-14+ PDAC, with high target specificity for 89Zr-DFO-LEM2/15. Low/Moderate specificity for 68Ga-DOTA-AF7p. Further evaluation of 89Zr-DFO-LEM2/15 is warranted to address its potential in humans. | [222] |
| Orthotopic PDX PDAC mouse model | After 90 min | Tumor-to-background of 68Ga-DOTA-AF7p | 90 min: 0.5 | |||||||
| After 1 d, 7 days | Tumor-to-blood (SD) of 89Zr-DFO-LEM2/15 | 1 days: 0.56 (±0.10) 7 days: 1.95 (±0.63) | ||||||||
| Mucin-1 | Anti-MUC1 (CT2)-DyLight550/650 | mAb, hamster | Fluorescence imaging- 600 nm | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous/Orthotopic PDAC mouse model (PANC-1, BxPC-3) | After 7–10 days | In vivo Tumor-to-background (Orthotopic tumors) | Panc-1: 6.70 BxPC-3: 2.39 | Proof-of-concept of in vivo fluorescence-imaging of MUC-1+ Subcutaneous/Orthotopic tumors. Biodistribution and further evaluation in pre-clinical is warranted before clinical studies could be initiated, furthermore humanized antibodies are preferred over animal antibodies. | [223] |
| NTSR1 | 64Cu-AmBaSar-NT, | Peptide, linear | PET/CT NIRF- 800 nm | In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (PANC-1, AsPC-1) | After 30 min, 1 h, 4 h | PET/CT: Tumor uptake/biodistribution in % of injected dosage/g BW (SD) | 1 h: 3.76 (±1.45) 4 h: 2.29 (±0.10) | Proof-of-concept, high target binding affinity for NTSR+ PDAC, moderate background in kidney uptake, low background in liver and intestines. Neurotensin peptide sequence could be used for adequate PDAC visualization with PET/CT and NIRF imaging. | [115] |
| IRDye800-NT | Peptide, linear | Orthotopic PDAC mouse model (PANC-1, AsPC-1) | NIRF: Tumor-to-background Ratio (SD) | 30 min: 8.09 (±0.38) 1 h: 6.67 (±0.43) | ||||||
| 68Ga-DOTA-NT-20.3 | Peptide, linear | PET/CT | In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (AsPC-1) | After 45 min | SUVmax (SD) | Subcutaneous: PDAC: 1 (±0.2) Background: 0.3 (±0.1) | Proof-of-concept, high target binding affinity for NTSR+ PDAC, adequate tumor-to-background ratio. Moderate background in kidney uptake, low background in liver and intestines. DOTA-NT-20.3 distinguishes PDAC from pancreatitis in orthotopic mouse model. NT-20.3 receptor targeting peptide sequence could be used for adequate PDAC visualization with PET/CT imaging. | [116] | |
| After 45 min | Tumor-to-no-tumor ratio (SD) | Subcutaneous: 3.5 (±0.8) | ||||||||
| Orthotopic PDAC mouse model (AsPC-1) | After 1 h | Tumor-to-blood ratio | Subcutaneous: 6.0 | |||||||
| After 1 h | Tumor uptake ratio (SD) | Orthoptic: 4.6 (±1.5) | ||||||||
| TF | 64Cu-NOTA-FVIIai | SMI | PET/CT | In vivo Preclinical feasibility and target validation in mouse model | Subcutaneous PDAC mouse model (PANC-1, AsPC-1, BxPC-3) | After 36 h | Maximum tumor uptake/biodistribution in % of injected dosage/g BW | PDAC: 3.7 Pancreas: 0.3 Liver: 8.0 Blood: 0.2 Muscle: 0.1 | Proof-of-concept, high accumulation in PDAC, suitable for PET/CT-imaging of TF+ PDAC. High accumulation in Liver, possibly interfering with imaging of the PDAC lesion due to high background. Delayed imaging of 64Cu-NOTA-FVIIai improved the tumor–to–background ratio, and subcutaneous tumors were clearly visible 15 h after injection. | [131] |
| After 15 h, 36 h | Tumor-to-muscle ratio | After 15 h: 20 | ||||||||
| Tumor-to-pancreas ratio | After 36 h: 36 After 15 h: 10 After 36 h: 13 | |||||||||
| Maximum tumor uptake/biodistribution in % (SD) of injected dosage/g BW | PANC-1 (low TF+): 2.2 (±0.1) AsPC-1 (intermed. TF+): 4.1 (±0.1) BxPC-3 (high TF+): 7.5 (±0.5) | |||||||||
| TF/ Endoglin (CD105) | 64Cu-NOTA-ALT-836/TRC105 | Dual- targeted antibody fragment | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BXPC-3, TF/CD105+/+) | After 30 h | Tumor uptake/biodistribution in % of injected dosage/g BW (SD) | Subcutaneous: PDAC: 17.1 (±4.9) Pancreas: <1 Liver: 8.5 | Proof-of-concept, high target binding affinity for dual-TF+/Endoglin+ PDAC, allowing tumor visualization with targeted PET/CT-imaging. Renally cleared. | [197] |
| Orthotopic PDAC mouse model (BXPC-3, TF/CD105+/+) | After 30 h | Tumor-to-muscle ratio (SD) | Orthotopic: 72.3 (±46.7) | |||||||
| Trans-ferrin receptor-1 (TfR1) | 89Zr-TSP-A01 | mAb, hu manized | PET/CT | In vitro/In vivo Preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (A4, MiaPaCa-2) | After 24 h; 6 days | Tumor-to-muscle ratio (Mouse 1/Mouse 2) | PDAC MiaPaCa-2: Day 1: 4.6/4.7 Day 6: 10.7/8.6 | Moderate/High target affinity, promising PET tracer to detect TfR1+ PDAC, although only tumors of MiaPaCa-2 cell line were clearly visualized. Moderate uptake in healthy liver parenchyma. | [224] |
| PDAC A4: Day 1: 2.2/2.4 Day 6: 2.1/2.6 | ||||||||||
| After 6 days | Maximum tumor uptake/biodistribution in % of injected dosage/g BW | PDAC MiaPaCa-2: 12.0 PDAC A4: 4.0 Blood: 4.0 Muscle: <1.0 | ||||||||
| uPA/ uPAR system | 89Zr-Df-ATN-291 | mAb, hu manized | PET/CT | In vitro/In vivo preclinical probe construction and target validation in mouse model | Subcutaneous PDAC mouse model (BxPC-3) | After 2, 24, 72 and 120 h | Tumor-to-muscle Ratio (after 24, 72 h) | PDAC: 7.4–21.3 | Proof-of-concept, high target affinity for uPAR+ PDAC, useful imaging tool for cancer (metastasis) detection and evaluation of a given uPA/uPAR-targeted treatment. | [174] |
| Tumor uptake/biodistribution in % (SD) of injected dosage/g BW (after 24 h-72 h) | PDAC: 9.4 (±0.6)–18.9 (±1.9) | |||||||||
| Glu-Glu-AE105-ICG | Peptide, linear | NIRF- 800 nm | In vivo preclinical target validation and NIRF-guided surgery in mouse model | Orthotopic PDAC mouse model (BxPC-3) | After 15 h | Tumor-to-background Ratio (95% CI) | PDAC: 3.5 (3.3–3.7) Metastases: 3.4 (3.1–4.0) | Clear localization of primary PDAC and metastases with NIRF imaging Glu-Glu-AE105-ICG. Identification of additional fluorescent lesions, resulting in resection. | [193] | |
| Identification and removal of additional metastases only on NIRF compared (%) | Mice: 4 out of 8 (50%) Metastases: 6/35 (14%) |
References
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- System, E.-E.C.I. Incidence and Mortality Estimates 2020. Available online: https://ecis.jrc.ec.europa.eu (accessed on 21 September 2021).
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Milan, M.; Diaferia, G.R.; Natoli, G. Tumor cell heterogeneity and its transcriptional bases in pancreatic cancer: A tale of two cell types and their many variants. EMBO J. 2021, 40, e107206. [Google Scholar] [CrossRef]
- Tummers, W.S.; Groen, J.V.; Mulder, B.G.S.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; Van De Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. BJS 2019, 106, 1055–1065. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef]
- Vachiranubhap, B.; Kim, Y.H.; Balci, N.C.; Semelka, R.C. Magnetic Resonance Imaging of Adenocarcinoma of the Pancreas. Top. Magn. Reson. Imaging 2009, 20, 3–9. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef]
- Schmocker, R.K.; Delitto, D.; Wright, M.J.; Ding, D.; Cameron, J.L.; Lafaro, K.J.; Burns, W.R.; Wolfgang, C.L.; Burkhart, R.A.; He, J. Impact of Margin Status on Survival in Patients with Pancreatic Ductal Adenocarcinoma Receiving Neoadjuvant Chemotherapy. J. Am. Coll. Surg. 2020, 232, 405–413. [Google Scholar] [CrossRef]
- Maeda, S.; Moore, A.M.; Yohanathan, L.; Hata, T.; Truty, M.J.; Smoot, R.L.; Cleary, S.; Nagorney, D.M.; Grotz, T.E.; Park, E.J.; et al. Impact of resection margin status on survival in pancreatic cancer patients after neoadjuvant treatment and pancreatoduodenectomy. Surgery 2020, 167, 803–811. [Google Scholar] [CrossRef]
- Ghaneh, P.; Kleeff, J.; Halloran, C.M.; Raraty, M.; Jackson, R.; Melling, J.; Jones, O.; Palmer, D.H.; Cox, T.F.; Smith, C.J.; et al. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 520–529. [Google Scholar] [CrossRef]
- Verbeke, C.S. Resection margins and R1 rates in pancreatic cancer—Are we there yet? Histopathology 2008, 52, 787–796. [Google Scholar] [CrossRef]
- Do, Z.J.B.; Cloyd, J.M. Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2021, 123, 1432–1440. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III Preopanc Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy with Folfirinox Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Han, Y.; Lee, H.; Kim, S.-W.; Kwon, W.; Lee, K.-H.; Oh, D.-Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Pentheroudakis, G. Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann. Oncol. 2019, 30, 1395–1397. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Wang, W.-Q.; Zhang, S.-R.; Xu, H.-X.; Wu, C.-T.; Qi, Z.-H.; Gao, H.-L.; Li, S.; Ni, Q.-X.; Yu, X.-J.; et al. Neoadjuvant Therapy is Essential for Resectable Pancreatic Cancer. Curr. Med. Chem. 2020, 26, 7196–7211. [Google Scholar] [CrossRef]
- Truty, M.J.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Cleary, S.; Graham, R.; Goenka, A.H.; Hallemeier, C.L.; Haddock, M.G.; Harmsen, W.S.; et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann. Surg. 2019, 273, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; O’Reilly, E.M.; Van Eijck, C.H.J.; Koerkamp, B.G. Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front. Oncol. 2020, 10, 41. [Google Scholar] [CrossRef]
- Janssen, Q.P.; for the Dutch Pancreatic Cancer Group; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Coene, P.P.L.O.; van Eijck, C.H.J.; de Hingh, I.H.J.T.; Karsten, T.M.; et al. Total neoadjuvant Folfirinox versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): Study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021, 21, 300. [Google Scholar] [CrossRef]
- Wang, Z.J.; Arif-Tiwari, H.; Zaheer, A.; Ameli, S.; Bhosale, P.R.; Do, R.K.; Goenka, A.H.; Guimares, A.R.; Sangster, G.P.; Soloff, E.V.; et al. Therapeutic response assessment in pancreatic ductal adenocarcinoma: Society of abdominal radiology review paper on the role of morphological and functional imaging techniques. Abdom. Radiol. 2020, 45, 4273–4289. [Google Scholar] [CrossRef]
- Baliyan, V.; Kordbacheh, H.; Parakh, A.; Kambadakone, A. Response assessment in pancreatic ductal adenocarcinoma: Role of imaging. Abdom. Radiol. 2017, 43, 435–444. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the Recist Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated With First-line Folfirinox vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mourad, A.F.; Hassan, R.A.; Ibrahim, M.A.E.; Soliman, A.; Aboeleuon, E.; Elbadee, O.M.A.; Hetta, H.F.; Jabir, M.A. Preoperative CT staging of borderline pancreatic cancer patients after neoadjuvant treatment: Accuracy in the prediction of vascular invasion and resectability. Abdom. Radiol. 2020, 46, 280–289. [Google Scholar] [CrossRef]
- Vuijk, F.A.; De Muynck, L.D.A.N.; Franken, L.C.; Busch, O.R.; Wilmink, J.W.; Besselink, M.G.; Bonsing, B.A.; Bhairosingh, S.S.; Kuppen, P.J.K.; Mieog, J.S.D.; et al. Molecular targets for diagnostic and intraoperative imaging of pancreatic ductal adenocarcinoma after neoadjuvant Folfirinox treatment. Sci. Rep. 2020, 10, 16211. [Google Scholar] [CrossRef]
- Cassinotto, C.; Cortade, J.; Belleannée, G.; Lapuyade, B.; Terrebonne, E.; Vendrely, V.; Laurent, C.; Cunha, A.S. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur. J. Radiol. 2013, 82, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and Surgical Implications of Neoadjuvant Treatment With Folfirinox for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Willmann, J.K.; Bonsing, B.A.; Vahrmeijer, A.L.; Gambhir, S.S.; Swijnenburg, R.-J. Advances in Diagnostic and Intraoperative Molecular Imaging of Pancreatic Cancer. Pancreas 2018, 47, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Antunes, C.; Pietrasz, D.; Cassinotto, C.; Zappa, M.; Cunha, A.S.; Lucidarme, O.; Bachet, J.-B. CT evaluation after neoadjuvant Folfirinox chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur. Radiol. 2016, 27, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Garg, I.; Truty, M.J.; Kline, T.L.; Johnson, M.P.; Ehman, E.C.; Suman, G.; Anaam, D.A.; Kemp, B.J.; Johnson, G.B.; et al. Borderline Resectable and Locally Advanced Pancreatic Cancer: FDG PET/MRI and CT Tumor Metrics for Assessment of Pathologic Response to Neoadjuvant Therapy and Prediction of Survival. Am. J. Roentgenol. 2021, 217, 730–740. [Google Scholar] [CrossRef]
- Zimmermann, C.; Distler, M.; Jentsch, C.; Blum, S.; Folprecht, G.; Zöphel, K.; Polster, H.; Troost, E.G.C.; Abolmaali, N.; Weitz, J.; et al. Evaluation of response using FDG-PET/CT and diffusion weighted MRI after radiochemotherapy of pancreatic cancer: A non-randomized, monocentric phase II clinical trial—PaCa-DD-041 (Eudra-CT 2009-011968-11). Strahlenther. Onkol. 2020, 197, 19–26. [Google Scholar] [CrossRef]
- Ben-Haim, S.; Ell, P. 18F-FDG PET and PET/CT in the Evaluation of Cancer Treatment Response. J. Nucl. Med. 2009, 50, 88–99. [Google Scholar] [CrossRef]
- Wang, L.; Dong, P.; Shen, G.; Hou, S.; Zhang, Y.; Liu, X.; Tian, B. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Treatment Efficacy and Clinical Outcome for Patients With Pancreatic Carcinoma. Pancreas 2019, 48, 996–1002. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; Van Der Vorst, J.R.; Van De Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.-J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2021, 1–14. [Google Scholar] [CrossRef]
- Tummers, W.S.; Farina-Sarasqueta, A.; Boonstra, M.C.; Prevoo, H.A.; Sier, C.F.; Mieog, J.S.; Morreau, J.; Van Eijck, C.H.; Kuppen, P.J.; Van De Velde, C.J.; et al. Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 56816–56828. [Google Scholar] [CrossRef]
- Kramer-Marek, G.; Gore, J.; Korc, M. Molecular imaging in pancreatic cancer—A roadmap for therapeutic decisions. Cancer Lett. 2013, 341, 132–138. [Google Scholar] [CrossRef]
- De Geus, S.W.L.; Boogerd, L.S.F.; Swijnenburg, R.-J.; Mieog, J.S.D.; Tummers, W.S.F.J.; Prevoo, H.A.J.M.; Sier, C.F.M.; Morreau, H.; Bonsing, B.A.; Van De Velde, C.J.H.; et al. Selecting Tumor-Specific Molecular Targets in Pancreatic Adenocarcinoma: Paving the Way for Image-Guided Pancreatic Surgery. Mol. Imaging Biol. 2016, 18, 807–819. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Yang, F.; Song, W.; Han, D.; Wen, W.; Qin, W. Quicker, deeper and stronger imaging: A review of tumor-targeted, near-infrared fluorescent dyes for fluorescence guided surgery in the preclinical and clinical stages. Eur. J. Pharm. Biopharm. 2020, 152, 123–143. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Cornelissen, B.; Knight, J.C.; Mukherjee, S.; Evangelista, L.; Xavier, C.; Caobelli, F.; Del Vecchio, S.; Rbah-Vidal, L.; Barbet, J.; De Jong, M.; et al. Translational molecular imaging in exocrine pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2442–2455. [Google Scholar] [CrossRef]
- England, C.G.; Hernandez, R.; Eddine, S.B.Z.; Cai, W. Molecular Imaging of Pancreatic Cancer with Antibodies. Mol. Pharm. 2015, 13, 8–24. [Google Scholar] [CrossRef]
- Van Oosten, M.; Crane, L.M.; Bart, J.; van Leeuwen, F.W.; van Dam, G.M. Selecting Potential Targetable Biomarkers for Imaging Purposes in Colorectal Cancer Using TArget Selection Criteria (TASC): A Novel Target Identification Tool. Transl. Oncol. 2011, 4, 71–82. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodríguez, E.; Van Kooyk, Y.; Van Vliet, S.J. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The Clinical Utility of CA 19-9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Ni, X.G.; Bai, X.F.; Mao, Y.L.; Shao, Y.F.; Wu, J.X.; Shan, Y.; Wang, C.F.; Wang, J.; Tian, Y.T.; Liu, Q.; et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur. J. Surg. Oncol. EJSO 2005, 31, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Loy, T.S.; Sharp, S.C.; Andershock, C.J.; Craig, S.B. Distribution of CA 19-9 in Adenocarcinomas and Transitional Cell Carcinomas: An Immunohistochemical Study of 527 Cases. Am. J. Clin. Pathol. 1993, 99, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, L.; Yu, Z.; Zheng, J.; Yang, D.; Bouvet, M.; Hoffman, R.M. Marker Expression in Circulating Cancer Cells of Pancreatic Cancer Patients. J. Surg. Res. 2011, 171, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Haglund, C.; Lindgren, J.; Roberts, P.J.; Nordling, S. Gastrointestinal cancer-associated antigen CA 19-9 in histological specimens of pancreatic tumours and pancreatitis. Br. J. Cancer 1986, 53, 189–195. [Google Scholar] [CrossRef]
- Houvast, R.D.; Vankemmelbeke, M.; Durrant, L.G.; Wuhrer, M.; Baart, V.M.; Kuppen, P.J.K.; De Geus-Oei, L.-F.; Vahrmeijer, A.L.; Sier, C.F.M. Targeting Glycans and Heavily Glycosylated Proteins for Tumor Imaging. Cancers 2020, 12, 3870. [Google Scholar] [CrossRef]
- Pontious, C.; Kaul, S.; Hong, M.; Hart, P.A.; Krishna, S.G.; Lara, L.F.; Conwell, D.L.; Cruz-Monserrate, Z. Cathepsin E expression and activity: Role in the detection and treatment of pancreatic cancer. Pancreatology 2019, 19, 951–956. [Google Scholar] [CrossRef]
- Cruz-Monserrate, Z.; Abd-Elgaliel, W.R.; Grote, T.; Deng, D.; Ji, B.; Arumugam, T.; Wang, H.; Tung, C.-H.; Logsdon, C.D. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut 2011, 61, 1315–1322. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Cui, L.; Wang, B.; Cui, W.; Li, M.; Cheng, Y. Monitoring Pancreatic Carcinogenesis by the Molecular Imaging of Cathepsin E In Vivo Using Confocal Laser Endomicroscopy. PLoS ONE 2014, 9, e106566. [Google Scholar] [CrossRef]
- Abd-Elgaliel, W.R.; Cruz-Monserrate, Z.; Logsdon, C.D.; Tung, C.-H. Molecular imaging of Cathepsin E-positive tumors in mice using a novel protease-activatable fluorescent probe. Mol. BioSyst. 2011, 7, 3207–3213. [Google Scholar] [CrossRef]
- Lwin, T.M.; Hoffman, R.M.; Bouvet, M. The development of fluorescence guided surgery for pancreatic cancer: From bench to clinic. Expert Rev. Anticancer. Ther. 2018, 18, 651–662. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Uekita, T.; Hiraoka, N.; Fujii, S.; Kosuge, T.; Kanai, Y.; Nojima, Y.; Sakai, R. CUB Domain–Containing Protein 1, a Prognostic Factor for Human Pancreatic Cancers, Promotes Cell Migration and Extracellular Matrix Degradation. Cancer Res. 2010, 70, 5136–5146. [Google Scholar] [CrossRef]
- Moroz, A.; Wang, Y.-H.; Sharib, J.M.; Wei, J.; Zhao, N.; Huang, Y.; Chen, Z.; Martinko, A.J.; Zhuo, J.; Lim, S.A.; et al. Theranostic Targeting of CUB Domain Containing Protein 1 (CDCP1) in Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3608–3615. [Google Scholar] [CrossRef]
- Khan, T.; Kryza, T.; Lyons, N.J.; He, Y.; Hooper, J.D. The CDCP1 Signaling Hub: A Target for Cancer Detection and Therapeutic Intervention. Cancer Res. 2021, 81, 2259–2269. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Enjoji, M.; Tsuneyoshi, M. Pancreatoduodenal carcinoma: A clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. J. Surg. Oncol. 1991, 47, 148–154. [Google Scholar] [CrossRef]
- Lwin, T.M.; Murakami, T.; Miyake, K.; Yazaki, P.J.; Shivley, J.E.; Hoffman, R.M.; Bouvet, M. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann. Surg. Oncol. 2018, 25, 1079–1085. [Google Scholar] [CrossRef]
- Bouvet, M.; Hoffman, R.M. Toward Curative Fluorescence-Guided Surgery of Pancreatic Cancer. Hepatogastroenterology 2015, 62, 715–722. [Google Scholar]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Mulder, B.G.S.; Mieog, J.S.D.; Swijnenburg, R.J.; Van De Velde, C.J.H.; Farina Sarasqueta, A.; Bonsing, B.A.; Framery, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef]
- Wong, P.; Li, L.; Chea, J.; Hu, W.; Poku, E.; Ebner, T.; Bowles, N.; Wong, J.Y.C.; Yazaki, P.J.; Sligar, S.G.; et al. Antibody Targeted PET Imaging of 64Cu-DOTA-Anti-CEA PEGylated Lipid Nanodiscs in CEA Positive Tumors. Bioconjugate Chem. 2020, 31, 743–753. [Google Scholar] [CrossRef]
- Boonstra, M.C.; Tolner, B.; Schaafsma, B.E.; Boogerd, L.S.; Prevoo, H.A.; Bhavsar, G.; Kuppen, P.; Sier, C.; Bonsing, B.A.; Frangioni, J.V.; et al. Preclinical evaluation of a novel CEA-targeting near-infrared fluorescent tracer delineating colorectal and pancreatic tumors. Int. J. Cancer 2015, 137, 1910–1920. [Google Scholar] [CrossRef]
- Metildi, C.A.; Kaushal, S.; Pu, M.; Messer, K.A.; Luiken, G.A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Fluorescence-guided Surgery with a Fluorophore-conjugated Antibody to Carcinoembryonic Antigen (CEA), that Highlights the Tumor, Improves Surgical Resection and Increases Survival in Orthotopic Mouse Models of Human Pancreatic Cancer. Ann. Surg. Oncol. 2014, 21, 1405–1411. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Avan, A.; Manian, M.; Salehi, R.; Ghayour-Mobarhan, M. EGFR as a potential target for the treatment of pancreatic cancer: Dilemma and controversies. Curr. Drug Targets 2014, 15, 1293–1301. [Google Scholar] [CrossRef]
- Oliveira-Cunha, M.; Newman, W.G.; Siriwardena, A.K. Epidermal Growth Factor Receptor in Pancreatic Cancer. Cancers 2011, 3, 1513–1526. [Google Scholar] [CrossRef]
- Troiani, T.; Martinelli, E.; Capasso, A.; Morgillo, F.; Orditura, M.; De Vita, F.; Ciardiello, F. Targeting EGFR in pancreatic cancer treatment. Curr. Drug Targets 2012, 13, 802–810. [Google Scholar] [CrossRef]
- Oliveira, S.; Van Dongen, G.A.; Walsum, M.S.-V.; Roovers, R.; Stam, J.C.; Mali, W.; Van Diest, P.J.; Henegouwen, P.M.V.B.E. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-Infrared Fluorescent Anti–Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef]
- Duff, S.E.; Li, C.; Garland, J.M.; Kumar, S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, L.; Ding, G.; Chen, W.; Zheng, S.; Cao, L. Overexpressions of DLL4 and CD105 are Associated with Poor Prognosis of Patients with Pancreatic Ductal Adenocarcinoma. Pathol. Oncol. Res. 2015, 21, 1141–1147. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Kobayashi, S.; Ohtsuka, M.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Miyazaki, M. Specific Expression of Endoglin (CD105) in Endothelial Cells of Intratumoral Blood and Lymphatic Vessels in Pancreatic Cancer. Pancreas 2008, 37, 275–281. [Google Scholar] [CrossRef]
- Yonaiyama, S.; Toyoki, Y.; Morohashi, S.; Sakuraba, S.; Yoshizawa, T.; Suzuki, T.; Wu, Y.; Kijima, H.; Hakamada, K. Epithelial cell adhesion molecule (EpCAM) overexpression is correlated with malignant potentials of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. Biomed. Res. 2013, 34, 87–95. [Google Scholar] [CrossRef]
- Fong, D.; Steurer, M.; Obrist, P.; Barbieri, V.; Margreiter, R.; Amberger, A.; Laimer, K.; Gastl, G.; Tzankov, A.; Spizzo, G. Ep-CAM expression in pancreatic and ampullary carcinomas: Frequency and prognostic relevance. J. Clin. Pathol. 2007, 61, 31–35. [Google Scholar] [CrossRef]
- Boogerd, L.S.; Boonstra, M.C.; Prevoo, H.A.; Handgraaf, H.J.; Kuppen, P.J.; van de Velde, C.J.; Fish, A.; Cordfunke, R.A.; Valentijn, A.R.P.; van Scheltinga, A.T.; et al. Fluorescence-guided tumor detection with a novel anti-EpCAM targeted antibody fragment: Preclinical validation. Surg. Oncol. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Shi, M.; Yu, D.-H.; Chen, Y.; Zhao, C.-Y.; Zhang, J.; Liu, Q.-H.; Ni, C.-R.; Zhu, M.-H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Roselt, P.J.; Kallur, K.G.; Tothill, R.W.; Mileshkin, L. FAPI PET/CT: Will It End the Hegemony of 18F-FDG in Oncology? J. Nucl. Med. 2020, 62, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Topalovski, M.; Brekken, R.A. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2015, 381, 252–258. [Google Scholar] [CrossRef]
- Hu, D.; Ansari, D.; Zhou, Q.; Sasor, A.; Hilmersson, K.S.; Andersson, R. Stromal fibronectin expression in patients with resected pancreatic ductal adenocarcinoma. World J. Surg. Oncol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Amrutkar, M.; Aasrum, M.; Verbeke, C.S.; Gladhaug, I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 2019, 19, 596. [Google Scholar] [CrossRef]
- Dauer, P.; Sharma, N.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A.; Banerjee, S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019, 10, 132. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Y.; Lee, A.S. Beyond the endoplasmic reticulum: Atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 2011, 434, 181–188. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, M.; Zhou, L.; Yao, L.; Liao, Q.; Zhao, Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015, 5, 16067. [Google Scholar] [CrossRef]
- Sahni, S.; Nahm, C.; Krisp, C.; Molloy, M.P.; Mehta, S.; Maloney, S.; Itchins, M.; Pavlakis, N.; Clarke, S.; Chan, D.; et al. Identification of Novel Biomarkers in Pancreatic Tumor Tissue to Predict Response to Neoadjuvant Chemotherapy. Front. Oncol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Liu, S.; Liu, R.; Yuan, H.; Krasnoperov, V.; Shan, H.; Conti, P.S.; Gill, P.S.; Li, Z. Small-Animal PET Imaging of Pancreatic Cancer Xenografts Using a 64Cu-Labeled Monoclonal Antibody, MAb159. J. Nucl. Med. 2015, 56, 908–913. [Google Scholar] [CrossRef]
- Hosotani, R.; Kawaguchi, M.; Masui, T.; Koshiba, T.; Ida, J.; Fujimoto, K.; Wada, M.; Doi, R.; Imamura, M. Expression of Integrin αVβ3 in Pancreatic Carcinoma: Relation to MMP-2 Activation and Lymph Node Metastasis. Pancreas 2002, 25, e30–e35. [Google Scholar] [CrossRef]
- Steiger, K.; Schlitter, A.-M.; Weichert, W.; Esposito, I.; Wester, H.-J.; Notni, J. Perspective of αvβ6-Integrin Imaging for Clinical Management of Pancreatic Carcinoma and Its Precursor Lesions. Mol. Imaging 2017, 16, 1536012117709384. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Lu, D.; Xu, X.; Zhou, X.; Zhang, H.; Zhang, T.; Zhu, H.; Yang, Z.; Wang, F.; et al. Clinical Translation of a 68Ga-Labeled Integrin αvβ6–Targeting Cyclic Radiotracer for PET Imaging of Pancreatic Cancer. J. Nucl. Med. 2020, 61, 1461–1467. [Google Scholar] [CrossRef]
- Reader, C.S.; Vallath, S.; Steele, C.W.; Haider, S.; Brentnall, A.; Desai, A.; Moore, K.M.; Jamieson, N.; Chang, D.; Bailey, P.; et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 2019, 249, 332–342. [Google Scholar] [CrossRef]
- Turaga, R.C.; Sharma, M.; Mishra, F.; Krasinskas, A.; Yuan, Y.; Yang, J.J.; Wang, S.; Liu, C.; Li, S.; Liu, Z.-R. Modulation of Cancer-Associated Fibrotic Stroma by An Integrin αvβ3 Targeting Protein for Pancreatic Cancer Treatment. Cell. Mol. Gastroenterol. Hepatol. 2020, 11, 161–179. [Google Scholar] [CrossRef]
- Sipos, B.; Hahn, D.; Carceller, A.; Piulats, J.; Hedderich, J.; Kalthoff, H.; Goodman, S.L.; Kosmahl, M.; Klöppel, G. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 2004, 45, 226–236. [Google Scholar] [CrossRef]
- Haubner, R.; Maschauer, S.; Prante, O. PET Radiopharmaceuticals for Imaging Integrin Expression: Tracers in Clinical Studies and Recent Developments. BioMed Res. Int. 2014, 2014, 871609. [Google Scholar] [CrossRef]
- Kimura, R.H.; Cheng, Z.; Gambhir, S.S.; Cochran, J.R. Engineered Knottin Peptides: A New Class of Agents for Imaging Integrin Expression in Living Subjects. Cancer Res. 2009, 69, 2435–2442. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Kessler, H.; Wester, H.-J.; Schwaiger, M.; Beer, A.J. Radiolabelled RGD peptides for imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. 1), 126–138. [Google Scholar] [CrossRef]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.; Kluth, M.; Hube-Magg, C.; Blessin, N.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9, 397. [Google Scholar] [CrossRef]
- Le, K.; Wang, J.; Zhang, T.; Guo, Y.; Chang, H.; Wang, S.; Zhu, B. Overexpression of Mesothelin in Pancreatic Ductal Adenocarcinoma (PDAC). Int. J. Med. Sci. 2020, 17, 422–427. [Google Scholar] [CrossRef]
- Argani, P.; Iacobuzio-Donahue, C.; Ryu, B.; Rosty, C.; Goggins, M.; E Wilentz, R.; Murugesan, S.R.; Leach, S.D.; Jaffee, E.; Yeo, C.J.; et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 2001, 7, 3862–3868. [Google Scholar]
- Hassan, R.; Laszik, Z.G.; Lerner, M.; Raffeld, M.; Postier, R.; Brackett, D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am. J. Clin. Pathol. 2005, 124, 838–845. [Google Scholar] [CrossRef]
- Seiki, M. Membrane-type 1 matrix metalloproteinase: A key enzyme for tumor invasion. Cancer Lett. 2003, 194, 1–11. [Google Scholar] [CrossRef]
- Itoh, Y. MT1-MMP: A key regulator of cell migration in tissue. IUBMB Life 2006, 58, 589–596. [Google Scholar] [CrossRef]
- Ottaviano, A.J.; Sun, L.; Ananthanarayanan, V.; Munshi, H.G. Extracellular Matrix–Mediated Membrane-Type 1 Matrix Metalloproteinase Expression in Pancreatic Ductal Cells Is Regulated by Transforming Growth Factor-β1. Cancer Res. 2006, 66, 7032–7040. [Google Scholar] [CrossRef]
- Slapak, E.J.; Duitman, J.; Tekin, C.; Bijlsma, M.F.; Spek, C.A. Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression? Biology 2020, 9, 80. [Google Scholar] [CrossRef]
- Suh, H.; Pillai, K.; Morris, D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am. J. Cancer Res. 2017, 7, 1372–1383. [Google Scholar]
- Matsuyama, M.; Kondo, F.; Ishihara, T.; Yamaguchi, T.; Ito, R.; Tsuyuguchi, T.; Tawada, K.; Yokosuka, O. Evaluation of pancreatic intraepithelial neoplasia and mucin expression in normal pancreata. J. Hepato-Biliary-Pancreat. Sci. 2011, 19, 242–248. [Google Scholar] [CrossRef]
- Qu, C.F.; Li, Y.; Song, Y.J.; Rizvi, S.M.A.; Raja, C.; Zhang, D.; Samra, J.; Smith, R.; Perkins, A.C.; Apostolidis, C.; et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by 213Bi-C595 radioimmunoconjugate. Br. J. Cancer 2004, 91, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-T.; Pantazopouos, P.; Medarova, Z.; Moore, A. Presentation of underglycosylated mucin 1 in pancreatic adenocarcinoma (PDAC) at early stages. Am. J. Cancer Res. 2016, 6, 1986–1995. [Google Scholar] [PubMed]
- Takahashi, K.; Ehata, S.; Miyauchi, K.; Morishita, Y.; Miyazawa, K.; Miyazono, K. Neurotensin receptor 1 signaling promotes pancreatic cancer progression. Mol. Oncol. 2020, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, M.; Wang, H.; Deng, H.; He, T.; Tan, Y.; Zhu, Z.; Wu, Z.; Hu, S.; Li, Z. Evaluation of neurotensin receptor 1 as a potential imaging target in pancreatic ductal adenocarcinoma. Amino Acids 2017, 49, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Prignon, A.; Provost, C.; Alshoukr, F.; Wendum, D.; Couvelard, A.; Barbet, J.; Forgez, P.; Talbot, J.-N.; Gruaz-Guyon, A. Preclinical Evaluation of 68Ga-DOTA-NT-20.3: A Promising PET Imaging Probe To Discriminate Human Pancreatic Ductal Adenocarcinoma from Pancreatitis. Mol. Pharm. 2019, 16, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Dancer, P.-A.; Portal, C.; Denat, F.; Prignon, A.; Goncalves, V. Design of Bimodal Ligands of Neurotensin Receptor 1 for Positron Emission Tomography Imaging and Fluorescence-Guided Surgery of Pancreatic Cancer. J. Med. Chem. 2019, 63, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Hodolic, M.; Wu, W.-Y.; Zhao, Z.; Yu, F.; Virgolini, I.; Wang, F. Safety and tolerability of 68Ga-NT-20.3, a radiopharmaceutical for targeting neurotensin receptors, in patients with pancreatic ductal adenocarcinoma: The first in-human use. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1229–1234. [Google Scholar] [CrossRef]
- Stock, K.; Steinestel, K.; Wiesch, R.; Mikesch, J.-H.; Hansmeier, A.; Trautmann, M.; Beller, N.; Rehkämper, J.; Wardelmann, E.; Heitkötter, B.; et al. Neovascular Prostate-Specific Membrane Antigen Expression Is Associated with Improved Overall Survival under Palliative Chemotherapy in Patients with Pancreatic Ductal Adenocarcinoma. BioMed Res. Int. 2017, 2017, 2847303. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Wang, X.; Liu, J.; Yuan, Z.; Hao, J. Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med. Oncol. 2014, 31, 857. [Google Scholar] [CrossRef]
- Chang, S.S.; E Reuter, V.; Heston, W.D.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef]
- Lütje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; Broek, S.A.M.W.V.D.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2020, 31, 2199–2208. [Google Scholar] [CrossRef]
- Fragomeni, R.A.S.; Amir, T.; Sheikhbahaei, S.; Harvey, S.C.; Javadi, M.S.; Solnes, L.B.; Kiess, A.P.; Allaf, M.E.; Pomper, M.G.; Gorin, M.A.; et al. Imaging of Nonprostate Cancers Using PSMA-Targeted Radiotracers: Rationale, Current State of the Field, and a Call to Arms. J. Nucl. Med. 2018, 59, 871–877. [Google Scholar] [CrossRef]
- Derks, Y.H.; Löwik, D.W.P.M.; Sedelaar, J.P.M.; Gotthardt, M.; Boerman, O.C.; Rijpkema, M.; Lütje, S.; Heskamp, S. PSMA-targeting agents for radio- and fluorescence-guided prostate cancer surgery. Theranostics 2019, 9, 6824–6839. [Google Scholar] [CrossRef]
- Haas, S.L.; Jesnowski, R.; Steiner, M.; Hummel, F.; Ringel, J.; Burstein, C.; Nizze, H.; Liebe, S.; Löhr, J.M. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J. Gastroenterol. 2006, 12, 4843–4849. [Google Scholar] [CrossRef]
- Khorana, A.A.; Ahrendt, S.; Ryan, C.K.; Francis, C.W.; Hruban, R.H.; Hu, Y.C.; Hostetter, G.; Harvey, J.; Taubman, M.B. Tissue Factor Expression, Angiogenesis, and Thrombosis in Pancreatic Cancer. Clin. Cancer Res. 2007, 13, 2870–2875. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Lemoine, N.R.; Scully, M.F.; Tebbutt, S.; Williamson, R.C.N. Tissue factor expression correlates with histological grade in human pancreatic cancer. BJS 1995, 82, 1101–1104. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Nayak, T.R.; Engle, J.W.; Wong, H.C.; Liu, B.; Barnhart, T.E.; Cai, W. Immuno-PET of Tissue Factor in Pancreatic Cancer. J. Nucl. Med. 2012, 53, 1748–1754. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Jeppesen, T.E.; Kristensen, L.K.; Jensen, M.M.; El Ali, H.H.; Madsen, J.; Wiinberg, B.; Petersen, L.C.; Kjaer, A. PET Imaging of Tissue Factor in Pancreatic Cancer Using 64Cu-Labeled Active Site–Inhibited Factor VII. J. Nucl. Med. 2016, 57, 1112–1119. [Google Scholar] [CrossRef]
- Hernandez, R.; England, C.G.; Yang, Y.; Valdovinos, H.; Liu, B.; Wong, H.C.; Barnhart, T.E.; Cai, W. ImmunoPET imaging of tissue factor expression in pancreatic cancer with 89Zr-Df-ALT-836. J. Control. Release 2017, 264, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.C.; Brown, G.; Trowbridge, I.S.; Woolston, R.E.; Mason, D.Y. Transferrin receptors in human tissues: Their distribution and possible clinical relevance. J. Clin. Pathol. 1983, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Hwang, S.; Seong, R. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem. Biophys. Res. Commun. 2016, 471, 373–379. [Google Scholar] [CrossRef]
- Ryschich, E.; Huszty, G.; Knaebel, H.; Hartel, M.; Büchler, M.; Schmidt, J. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur. J. Cancer 2004, 40, 1418–1422. [Google Scholar] [CrossRef]
- Di Mauro, C.; Pesapane, A.; Formisano, L.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Monteleone, F.; et al. Urokinase-type plasminogen activator receptor (uPAR) expression enhances invasion and metastasis in RAS mutated tumors. Sci. Rep. 2017, 7, 9388. [Google Scholar] [CrossRef]
- De Geus, S.W.; Baart, V.M.; Boonstra, M.C.; Kuppen, P.J.; Prevoo, H.A.; Mazar, A.P.; Bonsing, B.A.; Morreau, H.; Van De Velde, C.J.; Vahrmeijer, A.L.; et al. Prognostic Impact of Urokinase Plasminogen Activator Receptor Expression in Pancreatic Cancer: Malignant Versus Stromal Cells. Biomark. Insights 2017, 12, 1177271917715443. [Google Scholar] [CrossRef]
- Hildenbrand, R.; Niedergethmann, M.; Marx, A.; Belharazem, D.; Allgayer, H.; Schleger, C.; Ströbel, P. Amplification of the Urokinase-Type Plasminogen Activator Receptor (uPAR) Gene in Ductal Pancreatic Carcinomas Identifies a Clinically High-Risk Group. Am. J. Pathol. 2009, 174, 2246–2253. [Google Scholar] [CrossRef]
- Costache, M.; Ioana, M.; Iordache, S.; Ene, D.; Costache, C.A.; Săftoiu, A. VEGF expression in pancreatic cancer and other malignancies: A review of the literature. Rom. J. Intern. Med. 2015, 53, 199–208. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Liang, Q.-L.; Wang, B.-R.; Chen, G.-Q.; Li, G.-H.; Xu, Y.-Y. Clinical significance of vascular endothelial growth factor and connexin43 for predicting pancreatic cancer clinicopathologic parameters. Med. Oncol. 2009, 27, 1164–1170. [Google Scholar] [CrossRef]
- Cannon, A.; Thompson, C.; Hall, B.R.; Jain, M.; Kumar, S.; Batra, S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer 2018, 9, 78–86. [Google Scholar] [CrossRef]
- Wood, L.D.; Hruban, R.H. Pathology and Molecular Genetics of Pancreatic Neoplasms. Cancer J. 2012, 18, 492–501. [Google Scholar] [CrossRef]
- Kryza, T.; Khan, T.; Puttick, S.; Li, C.; Sokolowski, K.A.; Tse, B.W.; Cuda, T.; Lyons, N.; Gough, M.; Yin, J.; et al. Effective targeting of intact and proteolysed CDCP1 for imaging and treatment of pancreatic ductal adenocarcinoma. Theranostics 2020, 10, 4116–4133. [Google Scholar] [CrossRef]
- Allum, W.H.; Stokes, H.J.; Macdonald, F.; Fielding, J.W. Demonstration of carcinoembryonic antigen (CEA) expression in normal, chronically inflamed, and malignant pancreatic tissue by immunohistochemistry. J. Clin. Pathol. 1986, 39, 610–614. [Google Scholar] [CrossRef]
- Seeberger, K.L.; Eshpeter, A.; Rajotte, R.V.; Korbutt, G.S. Epithelial cells within the human pancreas do not coexpress mesenchymal antigens: Epithelial–mesenchymal transition is an artifact of cell culture. Lab. Investig. 2008, 89, 110–121. [Google Scholar] [CrossRef]
- Hsing, J.; McConkey, D.; Logsdon, C. GRP78 and HSP90 are overexpressed in pancreatic cancer. In Proceedings of the 98th AACR Annual Meeting, Los Angeles, CA, USA, 14–18 April 2007; Volume 67, p. 708. [Google Scholar]
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M.; et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 1999, 85, 14–20. [Google Scholar] [CrossRef]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin expression profile in pancreatic cancer and the precursor lesions. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 243–254. [Google Scholar] [CrossRef]
- Saftoiu, A.; Angelescu, R.; Burada, F.; Angelescu, C.; Gheonea, D.; Iordache, S.; Mixich, F.; Ioana, M. Expression of vascular endothelial growth factor and epidermal growth factor receptor in pancreatic ductal adenocarcinomas, neuroendocrine tumours and chronic pancreatitis. Endosc. Ultrasound 2013, 2, 86–91. [Google Scholar] [CrossRef]
- Arnone, A.; Laudicella, R.; Caobelli, F.; Guglielmo, P.; Spallino, M.; Abenavoli, E.; Martini, A.L.; Filice, R.; Comis, A.D.; Cuzzocrea, M.; et al. Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics 2020, 10, 1042. [Google Scholar] [CrossRef]
- Ghaneh, P.; Hanson, R.; Titman, A.; Lancaster, G.; Plumpton, C.; Lloyd-Williams, H.; Yeo, S.T.; Edwards, R.T.; Johnson, C.; Abu Hilal, M.; et al. PET-PANC: Multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol. Assess. 2018, 22, 1–114. [Google Scholar] [CrossRef]
- Yeh, R.; Dercle, L.; Garg, I.; Wang, Z.J.; Hough, D.M.; Goenka, A.H. The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2018, 43, 415–434. [Google Scholar] [CrossRef]
- Yokose, T.; Kitago, M.; Matsusaka, Y.; Masugi, Y.; Shinoda, M.; Yagi, H.; Abe, Y.; Oshima, G.; Hori, S.; Endo, Y.; et al. Usefulness of18F-fluorodeoxyglucose positron emission tomography/computed tomography for predicting the prognosis and treatment response of neoadjuvant therapy for pancreatic ductal adenocarcinoma. Cancer Med. 2020, 9, 4059–4068. [Google Scholar] [CrossRef]
- Dalah, E.; Tai, A.; Oshima, K.; A Hall, W.; Erickson, B.; Li, X.A. PET-based Treatment Response Assessment for Neoadjuvant Chemoradiation in Pancreatic Adenocarcinoma: An Exploratory Study. Transl. Oncol. 2018, 11, 1104–1109. [Google Scholar] [CrossRef]
- Heinrich, S.; Schäfer, M.; Weber, A.; Hany, T.F.; Bhure, U.; Pestalozzi, B.C.; Clavien, P.-A. Neoadjuvant Chemotherapy Generates a Significant Tumor Response in Resectable Pancreatic Cancer Without Increasing Morbidity. Ann. Surg. 2008, 248, 1014–1022. [Google Scholar] [CrossRef]
- Yoshioka, M.; Sato, T.; Furuya, T.; Shibata, S.; Andoh, H.; Asanuma, Y.; Hatazawa, J.; Shimosegawa, E.; Koyama, K.; Yamamoto, Y. Role of positron emission tomography with 2-deoxy-2-[18 F]fluoro-d-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J. Gastroenterol. 2004, 39, 50–55. [Google Scholar] [CrossRef]
- Quon, A.; Chang, S.T.; Chin, F.; Kamaya, A.; Dick, D.W.; Loo, B.W.; Gambhir, S.S.; Koong, A.C. Initial evaluation of 18F-fluorothymidine (FLT) PET/CT scanning for primary pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 527–531. [Google Scholar] [CrossRef][Green Version]
- Wieder, H.; Beer, A.J.; Siveke, J.; Schuster, T.; Buck, A.K.; Herrmann, K.; Stollfuss, J.C. 18F-fluorothymidine PET for predicting survival in patients with resectable pancreatic cancer. Oncotarget 2018, 9, 10128–10134. [Google Scholar] [CrossRef]
- Segard, T.; Robins, P.D.; Yusoff, I.F.; Ee, H.; Morandeau, L.; Campbell, E.M.; Francis, R.J. Detection of Hypoxia With 18F-Fluoromisonidazole (18F-FMISO) PET/CT in Suspected or Proven Pancreatic Cancer. Clin. Nucl. Med. 2013, 38, 1–6. [Google Scholar] [CrossRef]
- Yamane, T.; Aikawa, M.; Yasuda, M.; Fukushima, K.; Seto, A.; Okamoto, K.; Koyama, I.; Kuji, I. [18F]FMISO PET/CT as a preoperative prognostic factor in patients with pancreatic cancer. EJNMMI Res. 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Metran-Nascente, C.; Yeung, I.; Vines, D.C.; Metser, U.; Dhani, N.C.; Green, D.; Milosevic, M.; Jaffray, D.; Hedley, D. Measurement of Tumor Hypoxia in Patients with Advanced Pancreatic Cancer Based on 18F-Fluoroazomyin Arabinoside Uptake. J. Nucl. Med. 2016, 57, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, C.; O’Reilly, E.M.; O’Donoghue, J.A.; Pandit-Taskar, N.; Carrasquillo, J.A.; Lyashchenko, S.K.; Ruan, S.; Teng, R.; Scholz, W.; Maffuid, P.W.; et al. Retooling a blood-based biomarker: Phase I assessment of the high-affinity CA19-9 antibody HuMAB-5B1 for immuno-PET imaging of pancreatic cancer. Clin. Cancer Res. 2019, 25, 7014–7023. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Aggeler, R.; Sawada, R.; Agnew, B.J.; Scholz, W.W.; Lewis, J.S. Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 15850–15855. [Google Scholar] [CrossRef]
- Röhrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2020, 62, 779–786. [Google Scholar] [CrossRef]
- Liermann, J.; Syed, M.; Ben-Josef, E.; Schubert, K.; Schlampp, I.; Sprengel, S.D.; Ristau, J.; Weykamp, F.; Röhrich, M.; Koerber, S.A.; et al. Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers 2021, 13, 796. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2021, 1–9. [Google Scholar] [CrossRef]
- Kimura, R.H.; Wang, L.; Shen, B.; Huo, L.; Tummers, W.; Filipp, F.V.; Guo, H.H.; Haywood, T.; Abou-Elkacem, L.; Baratto, L.; et al. Evaluation of integrin αvβ6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat. Commun. 2019, 10, 4673. [Google Scholar] [CrossRef]
- Nakamoto, R.; Duan, H.; Ferri, V.; HATAMI, N.; Goel, M.; Kimura, R.; Wardak, M.; Haywood, T.; Shen, B.; Park, W.; et al. Biodistribution and Safety of 18-F-FP-R01-MG-F2 Knottin PET Tracer in Patients with Pancreatic Cancer. J. Nucl. Med. 2021, 62, 1008. [Google Scholar]
- Trajkovic-Arsic, M.; Mohajerani, P.; Sarantopoulos, A.; Kalideris, E.; Steiger, K.; Esposito, I.; Ma, X.; Themelis, G.; Burton, N.; Michalski, C.W.; et al. Multimodal Molecular Imaging of Integrin αvβ3 for In Vivo Detection of Pancreatic Cancer. J. Nucl. Med. 2014, 55, 446–451. [Google Scholar] [CrossRef]
- Boyle, A.; Cao, P.-J.; Hedley, D.; Sidhu, S.S.; Winnik, M.A.; Reilly, R.M. MicroPET/CT imaging of patient-derived pancreatic cancer xenografts implanted subcutaneously or orthotopically in NOD-scid mice using 64Cu-NOTA-panitumumab F(ab’)2 fragments. Nucl. Med. Biol. 2015, 42, 71–77. [Google Scholar] [CrossRef]
- Yang, D.; Severin, G.W.; Dougherty, C.A.; Lombardi, R.; Chen, D.; Van Dort, M.E.; Barnhart, T.E.; Ross, B.D.; Mazar, A.P.; Hong, H. Antibody-based PET of uPA/uPAR signaling with broad applicability for cancer imaging. Oncotarget 2016, 7, 73912–73924. [Google Scholar] [CrossRef]
- Verbeek, F.P.R.; van der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Bonsing, B.A.; van Leeuwen, F.W.B.; Frangioni, J.V.; van de Velde, C.J.H.; Swijnenburg, R.-J.; Vahrmeijer, A.L. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J. Hepato-Biliary-Pancreat. Sci. 2012, 19, 626–637. [Google Scholar] [CrossRef]
- Lwin, T.M.; Hoffman, R.M.; Bouvet, M. The future of tumour-specific fluorescence-guided surgery for pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 715–717. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 35. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Hutteman, M.; Van Der Vorst, J.; Mieog, J.; Bonsing, B.; Hartgrink, H.; Kuppen, P.; Löwik, C.; Frangioni, J.; Van De Velde, C.; Vahrmeijer, A. Near-Infrared Fluorescence Imaging in Patients Undergoing Pancreaticoduodenectomy. Eur. Surg. Res. 2011, 47, 90–97. [Google Scholar] [CrossRef]
- Van Der Vorst, J.R.; Vahrmeijer, A.L.; Hutteman, M.; Bosse, T.; Smit, V.T.H.B.M.; Van De Velde, C.J.H.; Frangioni, J.V.; A Bonsing, B. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J. Gastrointest. Surg. 2012, 4, 180–184. [Google Scholar] [CrossRef]
- Majlesara, A.; Golriz, M.; Hafezi, M.; Saffari, A.; Stenau, E.; Maier-Hein, L.; Müller-Stich, B.P.; Mehrabi, A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagn. Photodyn. Ther. 2017, 17, 208–215. [Google Scholar] [CrossRef]
- Girgis, M.D.; Olafsen, T.; Kenanova, V.; E McCabe, K.; Wu, A.M.; Tomlinson, J.S. Targeting CEA in Pancreas Cancer Xenografts with a Mutated scFv-Fc Antibody Fragment. EJNMMI Res. 2011, 1, 24. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Kulbersh, B.D.; King, T.; Chaudhuri, T.R.; Zinn, K. Use of fluorescent labeled anti–epidermal growth factor receptor antibody to image head and neck squamous cell carcinoma xenografts. Mol. Cancer Ther. 2007, 6, 1230–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.-R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Berg, N.S.V.D.; Hasan, A.; Longacre, T.A.; Fisher, G.A.; Bonsing, B.A.; Vahrmeijer, A.L.; Gambhir, S.S.; et al. Detection of visually occult metastatic lymph nodes using molecularly targeted fluorescent imaging during surgical resection of pancreatic cancer. HPB 2019, 21, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Koch, M.; De Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; Van Scheltinga, A.G.T.; Jansen, L.; De Vries, J.; Hooge, M.N.L.-D.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab–IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef]
- Handgraaf, H.; Boonstra, M.C.; Prevoo, H.A.; Kuil, J.; Bordo, M.W.; Boogerd, L.S.; Mulder, B.G.S.; Sier, C.; Slooten, M.L.V.-V.; Valentijn, A.R.P.; et al. Real-time near-infrared fluorescence imaging using cRGD-ZW800-1 for intraoperative visualization of multiple cancer types. Oncotarget 2017, 8, 21054–21066. [Google Scholar] [CrossRef]
- De Valk, K.S.; Deken, M.M.; Handgraaf, H.J.M.; Bhairosingh, S.S.; Bijlstra, O.D.; Van Esdonk, M.J.; Van Scheltinga, A.G.T.; Valentijn, A.R.P.; March, T.L.; Vuijk, J.; et al. First-in-Human Assessment of cRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin. Cancer Res. 2020, 26, 3990–3998. [Google Scholar] [CrossRef]
- Tummers, W.S.; Kimura, R.H.; Abou-Elkacem, L.; Beinat, C.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; Willmann, J.K.; Gambhir, S.S. Development and Preclinical Validation of a Cysteine Knottin Peptide Targeting Integrin αvβ6 for Near-infrared Fluorescent-guided Surgery in Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 1667–1676. [Google Scholar] [CrossRef]
- Juhl, K.; Christensen, A.; Rubek, N.; Karnov, K.K.S.; Von Buchwald, C.; Kjaer, A. Improved surgical resection of metastatic pancreatic cancer using uPAR targeted in vivo fluorescent guidance: Comparison with traditional white light surgery. Oncotarget 2019, 10, 6308–6316. [Google Scholar] [CrossRef]
- Zettlitz, K.A.; Tsai, W.-T.K.; Knowles, S.M.; Kobayashi, N.; Donahue, T.R.; Reiter, R.E.; Wu, A.M. Dual-Modality Immuno-PET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using an Anti–Prostate Stem Cell Antigen Cys-Diabody. J. Nucl. Med. 2018, 59, 1398–1405. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-Targeted Molecular Imaging of Cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, H.; Wang, Z.; Li, Z.; Li, D.; Li, Z.; Wang, K.; Tian, J.; Zhao, X. Construction of a novel bispecific fusion protein to enhance targeting for pancreatic cancer imaging. Biomaterials 2020, 255, 120161. [Google Scholar] [CrossRef]
- Luo, H.; England, C.G.; Shi, S.; Graves, S.A.; Hernandez, R.; Liu, B.; Theuer, C.P.; Wong, H.C.; Nickles, R.J.; Cai, W. Dual Targeting of Tissue Factor and CD105 for Preclinical PET Imaging of Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 3821–3830. [Google Scholar] [CrossRef]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Rousseau, C.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Barbet, J. Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm. Chem. 2017, 2, 6. [Google Scholar] [CrossRef]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J. Nucl. Med. 2015, 57, 453–459. [Google Scholar] [CrossRef]
- Du, J.; Gu, J.; Li, J. Mechanisms of drug resistance of pancreatic ductal adenocarcinoma at different levels. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Mucciolo, G.; Roux, C.; Scagliotti, A.; Brugiapaglia, S.; Novelli, F.; Cappello, P. The dark side of immunotherapy: Pancreatic cancer. Cancer Drug Resist. 2020, 3, 419–520. [Google Scholar] [CrossRef]
- Yang, E.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Caputo, D.; Pozzi, D.; Farolfi, T.; Passa, R.; Coppola, R.; Caracciolo, G. Nanotechnology and pancreatic cancer management: State of the art and further perspectives. World J. Gastrointest. Oncol. 2021, 13, 231–237. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Dawson, K.M.; Binz, H.K. Beyond Antibodies: The DARPin® Drug Platform. BioDrugs 2020, 34, 423–433. [Google Scholar] [CrossRef]
- Shilova, O.N.; Deyev, S.M. DARPins: Promising Scaffolds for Theranostics. Acta Nat. 2019, 11, 42–53. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, F.; Brismar, T.B.; Li, X.; He, R.; Heuchel, R.; El-Sayed, R.; Feliu, N.; Zheng, W.; Oerther, S.; et al. Multimodal Imaging of Pancreatic Ductal Adenocarcinoma Using Multifunctional Nanoparticles as Contrast Agents. ACS Appl. Mater. Interfaces 2020, 12, 53665–53681. [Google Scholar] [CrossRef]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Montemagno, C.; Cassim, S.; De Leiris, N.; Durivault, J.; Faraggi, M.; Pagès, G. Pancreatic Ductal Adenocarcinoma: The Dawn of the Era of Nuclear Medicine? Int. J. Mol. Sci. 2021, 22, 6413. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, L.E.; Menke-van der Houven, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with Anti-Mesothelin Antibody in Patients with Pancreatic and Ovarian Cancer before Anti-Mesothelin Antibody–Drug Conjugate Treatment. Clin. Cancer Res. 2016, 22, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- McElroy, M.; Kaushal, S.; Luiken, G.A.; Talamini, M.A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Imaging of Primary and Metastatic Pancreatic Cancer Using a Fluorophore-Conjugated Anti-CA19-9 Antibody for Surgical Navigation. World J. Surg. 2008, 32, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Girgis, M.D.; Kenanova, V.; Olafsen, T.; McCabe, K.E.; Wu, A.M.; Tomlinson, J.S. Anti-CA19-9 Diabody as a PET Imaging Probe for Pancreas Cancer. J. Surg. Res. 2011, 170, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Girgis, M.D.; Federman, N.; Rochefort, M.M.; McCabe, K.E.; Wu, A.M.; Nagy, J.O.; Denny, C.; Tomlinson, J.S. An engineered anti-CA19-9 cys-diabody for positron emission tomography imaging of pancreatic cancer and targeting of polymerized liposomal nanoparticles. J. Surg. Res. 2013, 185, 45–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaushal, S.; McElroy, M.K.; Luiken, G.A.; Talamini, M.A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J. Gastrointest. Surg. 2008, 12, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Qin, J.; Sergeeva, O.; Sergeev, M.; Qiao, P.; Roelle, S.; Avril, N.; Lee, Z.; Li, Y.; Lu, Z. Synthesis and assessment of ZD2-(68Ga-NOTA) specific to extradomain B fibronectin in tumor microenvironment for PET imaging of pancreatic cancer. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 216–229. [Google Scholar]
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [Google Scholar] [CrossRef]
- Müller, M.; Altmann, A.; Sauter, M.; Lindner, T.; Jäger, D.; Rathke, H.; Herold-Mende, C.; Marmé, F.; Babich, J.; Mier, W.; et al. Preclinical evaluation of peptide-based radiotracers for integrin αvβ6-positive pancreatic carcinoma. Nuklearmedizin 2019, 58, 309–318. [Google Scholar] [CrossRef]
- Morcillo, M.Á.; de Lucas, G.; Oteo, M.; Romero, E.; Magro, N.; Ibañez, M.; Martinez, A.; Garaulet, G.; Arroyo, A.G.; Lopez-Casas, P.P.; et al. MT1-MMP as a PET imaging biomarker for pancreas cancer management. Contrast Media Mol. Imaging 2018, 2018, 8382148. [Google Scholar] [CrossRef]
- Park, J.Y.; Hiroshima, Y.; Lee, J.Y.; Maawy, A.A.; Hoffman, R.M.; Bouvet, M. MUC1 Selectively Targets Human Pancreatic Cancer in Orthotopic Nude Mouse Models. PLoS ONE 2015, 10, e0122100. [Google Scholar] [CrossRef]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.-R.; Kurosawa, Y.; Saga, T. Preclinical evaluation of 89Zr-labeled human antitransferrin receptor monoclonal antibody as a PET probe using a pancreatic cancer mouse model. Nucl. Med. Commun. 2015, 36, 286–294. [Google Scholar] [CrossRef]
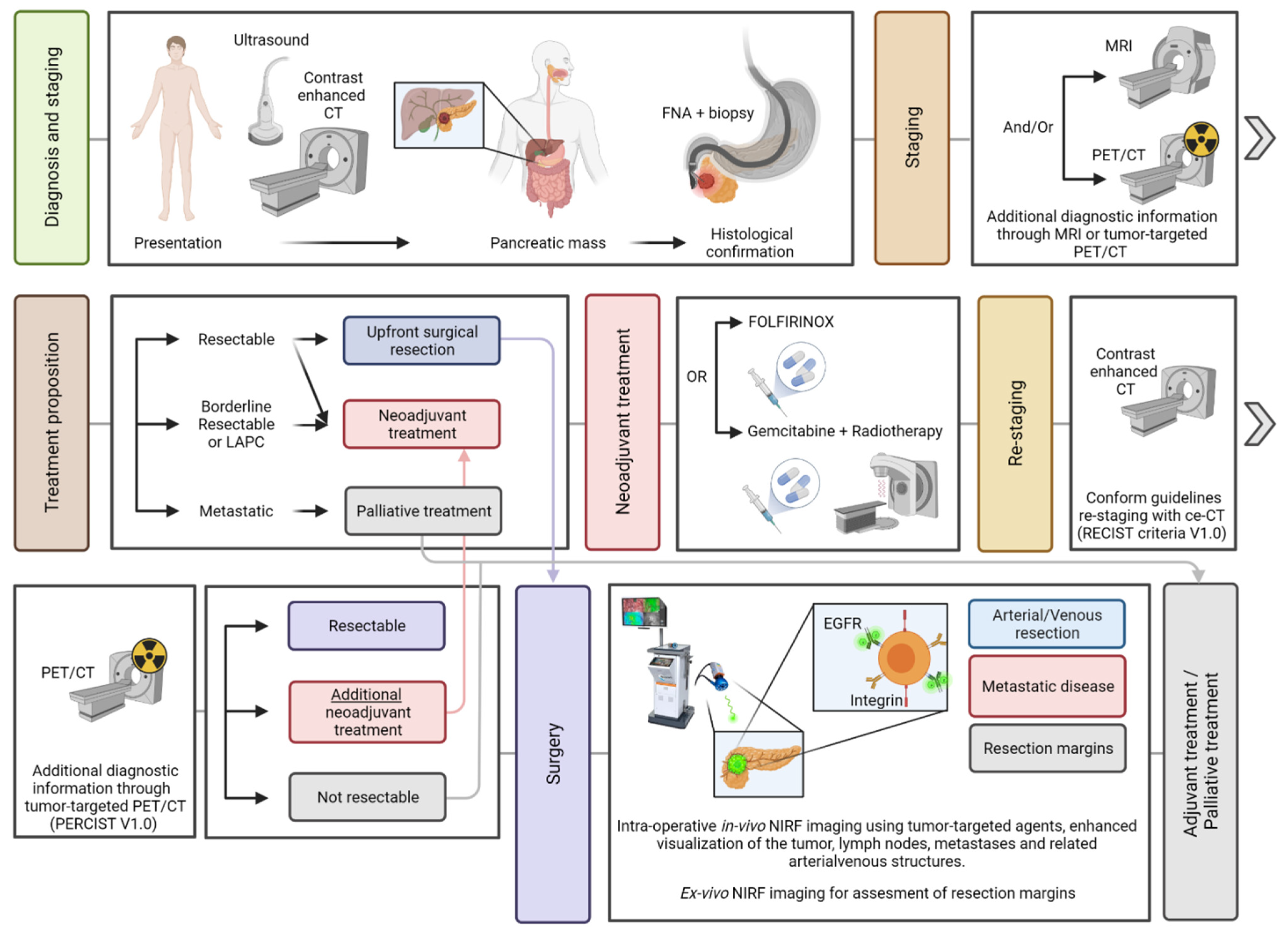
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Dam, M.A.; Vuijk, F.A.; Stibbe, J.A.; Houvast, R.D.; Luelmo, S.A.C.; Crobach, S.; Shahbazi Feshtali, S.; de Geus-Oei, L.-F.; Bonsing, B.A.; Sier, C.F.M.; et al. Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy. Cancers 2021, 13, 6088. https://doi.org/10.3390/cancers13236088
van Dam MA, Vuijk FA, Stibbe JA, Houvast RD, Luelmo SAC, Crobach S, Shahbazi Feshtali S, de Geus-Oei L-F, Bonsing BA, Sier CFM, et al. Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy. Cancers. 2021; 13(23):6088. https://doi.org/10.3390/cancers13236088
Chicago/Turabian Stylevan Dam, Martijn A., Floris A. Vuijk, Judith A. Stibbe, Ruben D. Houvast, Saskia A. C. Luelmo, Stijn Crobach, Shirin Shahbazi Feshtali, Lioe-Fee de Geus-Oei, Bert A. Bonsing, Cornelis F. M. Sier, and et al. 2021. "Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy" Cancers 13, no. 23: 6088. https://doi.org/10.3390/cancers13236088
APA Stylevan Dam, M. A., Vuijk, F. A., Stibbe, J. A., Houvast, R. D., Luelmo, S. A. C., Crobach, S., Shahbazi Feshtali, S., de Geus-Oei, L.-F., Bonsing, B. A., Sier, C. F. M., Kuppen, P. J. K., Swijnenburg, R.-J., Windhorst, A. D., Burggraaf, J., Vahrmeijer, A. L., & Mieog, J. S. D. (2021). Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy. Cancers, 13(23), 6088. https://doi.org/10.3390/cancers13236088









